Transient heart rate reduction improves acute decompensated heart failure-induced left ventricular and coronary dysfunction
Abstract
Aims
Acute decompensated heart failure (ADHF), a live-threatening complication of heart failure (HF), associates a further decrease of the already by HF-impaired cardiac function with an increase in heart rate. We evaluated, using a new model of ADHF, whether heart rate reduction (HRR) opposes the acute decompensation-related aggravation of cardiovascular dysfunction.
Methods and results
Cardiac output (echocardiography), cardiac tissue perfusion (magnetic resonance imaging), pulmonary wet weight, and in vitro coronary artery relaxation (Mulvany) were assessed 1 and 14 days after acute decompensation induced by salt-loading (1.8 g/kg, PO) in rats with well-established HF due to coronary ligation. HRR was induced by administration of the If current inhibitor S38844, 12 mg/kg PO twice daily for 2.5 days initiated 12 h or 6 days after salt-loading (early or delayed treatment, respectively). After 24 h, salt-loading resulted in acute decompensation, characterized by a reduction in cardiac output (HF: 130 ± 5 mL/min, ADHF: 105 ± 8 mL/min; P < 0.01), associated with a decreased myocardial perfusion (HF: 6.41 ± 0.53 mL/min/g, ADHF: 4.20 ± 0.11 mL/min/g; P < 0.01), a slight increase in pulmonary weight (HF: 1.68 ± 0.09 g, ADHF: 1.81 ± 0.15 g), and impaired coronary relaxation (HF: 55 ± 1% of pre-contraction at acetylcholine 4.5 10−5 M, ADHF: 27 ± 7 %; P < 0.01). Fourteen days after salt-loading, cardiac output only partially recovered (117 ± 5 mL/min; P < 0.05), while myocardial tissue perfusion (4.51 ± 0.44 mL/min; P < 0.01) and coronary relaxation (28 ± 4%; P < 0.01) remained impaired, but pulmonary weight further increased (2.06 ± 0.15 g, P < 0.05). Compared with untreated ADHF, HRR induced by S38844 improved cardiac output (125 ± 1 mL/min; P < 0.05), myocardial tissue perfusion (6.46 ± 0.42 mL/min/g; P < 0.01), and coronary relaxation (79 ± 2%; P < 0.01) as soon as 12 h after S38844 administration. These effects persisted beyond S38844 administration, illustrated by the improvements in cardiac output (130 ± 6 mL/min; P < 0.05), myocardial tissue perfusion (6.38 ± 0.48 mL/min/g; P < 0.01), and coronary relaxation (71 ± 4%; P < 0.01) at Day 14. S38844 did not modify pulmonary weight at Day 1 (1.78 ± 0.04 g) but tended to decrease pulmonary weight at Day 14 (1.80 ± 0.18 g). While delayed HRR induced by S38844 never improved cardiac function, early HRR rendered less prone to a second acute decompensation.
Conclusions
In a model mimicking human ADHF, early, but not delayed, transient HRR induced by the If current inhibitor S38844 opposes acute decompensation by preventing the decompensated-related aggravation of cardiovascular dysfunction as well as the development of pulmonary congestion, and these protective effects persist beyond the transient treatment. Whether early transient HRR induced by If current inhibitors or other bradycardic agents, i.e. beta-blockers, exerts beneficial effects in human ADHF warrants further investigation.
Introduction
Exacerbation of chronic heart failure (HF), better known as acute decompensated heart failure (ADHF), has become a major medical problem.1 Indeed, ADHF is associated with high morbi-mortality, despite the large variety of medical treatment including positive pressure ventilation, ultrafiltration, veno/arterial dilators, and inotropic drugs.2, 3
This inefficacy of treatments is, at least in part, related with the incomplete recovery of cardiac function in patients with ADHF. Moreover, after the first decompensation, patients become prone for subsequent decompensation and are at risk for recurrent hospitalizations.4 Thus, reducing early-term and middle-term mortality and readmission for HF are important goals in ADHF therapy, but no treatment has yet been shown to improve prognosis.
Interestingly, the lowest rate of in-hospital mortality in patient hospitalized for ADHF occurs in patients with heart rates of 70–75 bpm, with increased mortality both below and above this range.5, 6 Moreover, after hospitalization for ADHF, elevated heart rate at 1 or 4 weeks after discharge is associated with an increased risk of mortality during subsequent follow-up.7 Thus, early heart rate reduction (HRR) could represent an attractive target to improve both early-term and middle-term outcomes in ADHF patients.8
Selective If current blockers induce in humans and animals a dose-dependent HRR and exert cardiovascular protective effects both in chronic HF9-12 and in experimental models of metabolic syndrome.13 However, whether the HRR induced by selective If current blockade exerts beneficial effects in ADHF has, until now, not been evaluated. Using a new rodent model of ADHF, we assessed the cardiovascular effects of the If current blocker S38844 following cardiac decompensation and investigated the pathophysiological mechanism(s) involved.
Methods and materials
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved (authorization number 01955.01) by a certified review board (CENOMEXA n°54) according to French and EU legislation.
Animals and treatment
Myocardial infarction was induced by coronary artery ligation in 10-week-old male Wistar rats.12 Three months after ligation, and thus after infarct healing and development of overt well-established chronic HF, acute decompensation was induced by a single gavage with NaCl (1.8 g/kg in 2 mL of tap water by gavage), and three separated protocols were performed.
A first protocol evaluated the effect of early but transient HRR induced by the If current inhibitor S38844 on acute decompensation. In brief, ADHF rats were treated with vehicle or with S38844 (12 mg/kg bid PO for 2.5 days) initiated 12 h after salt-loading. Left ventricular (LV) haemodynamics and structure as well as LV coronary function were assessed either 24 h (D1) or 14 days (D14) after salt-loading.
A second protocol evaluated the effect of delayed but transient HRR induced by the If current inhibitor S38844 on acute decompensation. In this protocol, transient S38844 administration (12 mg/kg bid PO for 2.5 days) was initiated 6 days after salt-loading, and LV haemodynamics and structure as well as LV coronary function were assessed 14 days after salt-loading.
A third protocol evaluated the effect of early but transient HRR induced by the If current inhibitor S38844 on repeated acute decompensations. In this protocol, three sequential episodes of acute decompensations were induced at 2 week intervals by acute gavage with NaCl (1.8 g/kg as above), and ADHF rats were treated with vehicle or with S38844 (12 mg/kg bid by gavage for 2.5 days), starting 12 h after each salt-loading.
Systemic haemodynamics
Arterial blood pressure and heart rate were measured in conscious rats by telemetry (Dataquest ART system, Data Sciences Inc, USA) before and during the 14 days following decompensation, as previously described.14
Left ventricular parameters
Left ventricular haemodynamics was assessed by LV pressure–volume curves at Day 1 (i.e. 24 h after salt-loading and for S3884 12 h after the first administration) as well as at 14 days (i.e. 11.5 days after the last administration of S38844), and LV end-systolic and end-diastolic pressures, LV relaxation constant Tau, and LV end-systolic as well as LV end-diastolic pressure–volume relations were measured/calculated in anaesthetized rats (Brietal™; 50 mg/kg, IP) as previously described.11
Left ventricular echocardiographic studies were performed in anaesthetized rats (Brietal™; 50 mg/kg, IP) before and at 1, 6, and 14 days after salt-loading using an echocardiographic system (HDI 5000, ATL, USA) equipped with an 8–5 MHz transducer. LV diameters were measured using the American Society of Echocardiology leading-edge method, while LV outflow velocity was measured by pulsed-wave Doppler, and cardiac output calculated as CO = aortic VTI ● [π ● (LV outflow diameter/2)2] ● heart rate, where VTI is velocity–time integral, as described previously.11
Left ventricular tissue perfusion was evaluated in anaesthetized animals (Brietal™, 50 mg/kg IP) before and 1 and 14 days after salt-loading, using magnetic resonance imaging (Bruker Biospec 4.7 Tesla, France) with arterial spin labelling technique, as previously described.15 Perfusion images were analysed with ParaVision 5.0 software (Bruker AG Germany).
Left ventricular reactive oxygen species (ROS) production was determined by electron paramagnetic resonance spectroscopy in tissue LV samples of animals sacrificed 1 and 14 days after salt-loading, as previously described.13
Plasma biology: plasma nitrite concentrations, an indicator of nitric oxide (NO) bioavailability as nitrite is a stable, non-volatile end-product of NO, were determined, by a triiodide/ozone-based chemiluminescence assay, as previously described,13 while syndecan-1 plasma level was measured by ELISA as previously described.16
Left ventricular tissue histology: after assessment of cardiac haemodynamics, the heart was dissected; the atria as well as the ventricles were weighted separately, and sections of the LV were either immersed in fixative solution or snap frozen in liquid nitrogen for subsequent assessment of LV collagen or LV immunohistochemistry.
Left ventricular infarct size and LV collagen density were determined on Sirius-red stained slices as previously described,17 while cardiomyocyte sizes (wheat germ agglutinin stain, 1:100), LV capillary density (mouse antirat CD31 antibody 1:100; BD #555026), and LV macrophage subpopulations (mouse antirat CD68 antibody 1:400 Bio-Rad # MCA341R; rabbit antirat CD206 antibody 1:250, Abcam # ab64693) were evaluated by immunohistochemistry in frozen sections, as previously described.17 Images, acquired on a Zeiss epifluorescence microscope (AxioImager J1) equipped with an apotome, were analysed using ImageJ software.
Coronary vascular function
Left coronary artery endothelial function was assessed either at 1 or 14 days after salt-loading, as previously described.18 In brief, after obtaining haemodynamic parameters, the heart was removed and placed in cold oxygenated Krebs buffer. A 1.5 to 2 mm segment of the left coronary artery was dissected and mounted in a small vessel myograph (JP Trading; Aarhus, Denmark). Normalization procedure was performed after an equilibration period, as previously described.18 Concentration–response curves to acetylcholine (10−9 to 3.10−5 mol/L) were obtained in serotonin (10−5 mol/L) pre-contracted segments.
Statistical analysis
All results are given as mean ± SEM.
Left ventricular function, that is, cardiac output and LV haemodynamic parameters, was assessed as a primary endpoint, whereas all other parameters, that is, myocardial perfusion, coronary function, and cellular and biochemical alterations, were assessed as secondary endpoints. Based upon historical data obtained with ivabradine and S38884 in rats with chronic HF on remodelling and haemodynamics,11-13 we made a simulation for each parameter obtained in untreated animals necessary to demonstrate a statistical significance (p < 0.05) with a minimal power of 80 %. The minimal expected effect size or difference between untreated and treated groups was fixed to 10 % and 30 %, with the coefficient of variation to 25 % and 15 % for echocardiographic and haemodynamic studies, respectively. For all significant differences concerning primary endpoints, a posteriori power higher than 80% was also checked.
In order to evaluate the effect of salt-loading, all parameters obtained in control salt-loaded chronic HF rats vs. tap water-loaded chronic HF rats were compared by Student's unpaired two-tailed t-test. In order to evaluate the effects of S38884, all parameters obtained in S3884 treated salt-loaded rats were compared with time-matched salt-loaded control HF rats using Student's unpaired two-tailed t-test.
Before applying parametric tests, the Gaussian distribution of data was assessed by Shapiro–Wilk normality test and Kolmogorov–Smirnov test and graphically by QQplot and normal probability plot.
Results
Systemic haemodynamics
Salt-loading provoked an immediate increase in systolic and diastolic blood pressures, which returned progressively to pre-salt-loading values over the next 3 days following salt-loading. Moreover, the increase in blood pressure was associated with an increase in heart rate, which also returned progressively reaching pre-salt-loading values over the 2 days following salt-loading. Treatment with S38844, starting 12 h after salt-loading, induced an immediate reduction in heart rate during the 2.5 day treatment period, and this was associated with a limitation of the increase in systolic and diastolic blood pressures induced by salt-loading (Figure 1).
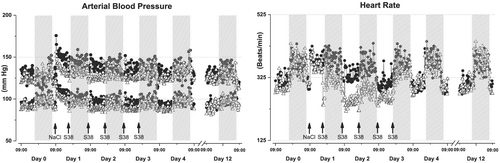
Echocardiographic assessment
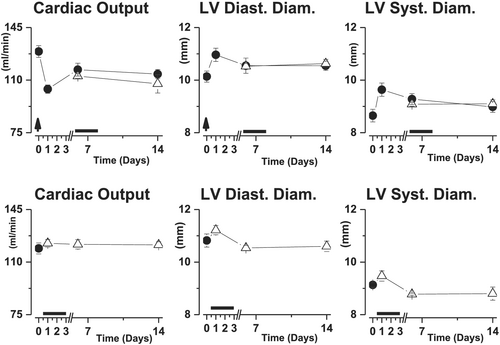
In rats with well-established chronic HF, cardiac output was stably reduced during the 14 day experimental period as compared with healthy sham rats. In contrast, salt-loading induced an immediate aggravation of the cardiac output, which only partially recovered during the 14 days of study period (Figure 2). S38844 administered 12 h after salt-loading increased cardiac output to levels observed in chronic HF animals as soon as 12 h after S38844 administration, and this effect persisted even beyond the 2.5 day S38844 treatment period (Figure 2).
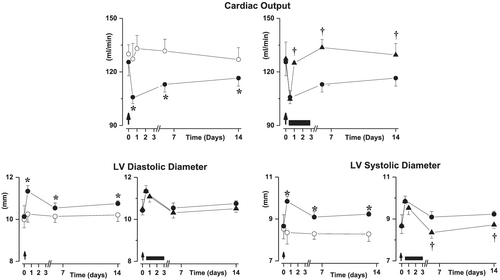
The impairment of cardiac output provoked by salt-loading was due to immediate increases in both LV diastolic and systolic diameters, but only LV diastolic diameter partially recovered over time (Figure 2). The improvement in cardiac output induced by S38884 was related to a smaller increase in LV systolic diameter, while LV diastolic diameter was not modified (Figure 2).
Left ventricular haemodynamics
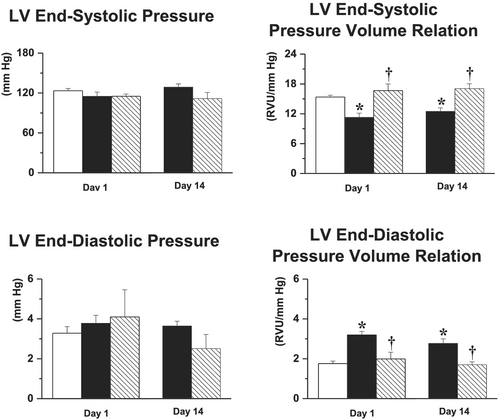
Salt-loading did not alter LV end-systolic or end-diastolic pressures, but LV end-systolic pressure–volume relation and end-diastolic pressure–volume relation were significantly altered as soon as 1 day after salt-loading, and these effects persisted over time (Figure 3). While neither LV end-systolic or end-diastolic pressures were modified by S38844, the treatment restored both LV end-systolic pressure–volume relation and LV end-diastolic pressure–volume relation to levels observed in chronic HF animals. These beneficial effects persisted throughout the 14 day experimental period, that is, beyond the 2.5 day S38844 treatment period (Figure 3).
Cardiac perfusion and coronary relaxation
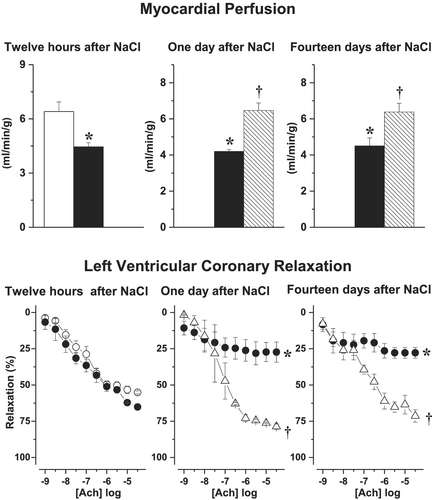
While cardiac perfusion and coronary dysfunction were impaired in chronic HF rats, as compared with healthy sham, acute salt-loading further reduced both LV tissue perfusion and LV coronary relaxation at Day 1 and Day 14 (Figure 4). S38844 significantly increased myocardial tissue perfusion and improved LV coronary artery relaxation, as soon as 12 h after administration, and this normalization close to values observed in chronic HF animals persisted over time (Figure 4). Neither salt-loading nor S38844 modified LV capillary density over the 14 day study period.
Cardio-pulmonary remodelling
Left ventricular infarct size was similar in all groups (Table 1). Compared with the chronic HF control group, LV weight was slightly increased at 1 and 14 days after salt-loading, reaching statistical significance at Day 14. Moreover, myocyte density tended to increase, while myocyte sizes were significantly reduced at 1 day after salt-loading, indicating cell shrinkage. However, by 14 days after salt-loading, myocyte size returned to pre-salt levels. S38844 did not modify myocyte density or sizes but significantly reduced salt-induced increase in LV weight at Day 14 (Table 1). LV collagen density was not modified by neither salt-loading nor S38844 throughout the study (Table 1).
| Time | CHF | Decompensated CHF | ||
|---|---|---|---|---|
| Untreated | S38844 | |||
| Infarct size (%) | D1 | 27.4 ± 3.2 | 28.0 ± 2.4 | 26.7 ± 3.1 |
| D14 | 26.4 ± 2.3 | 27.5 ± 3.2 | ||
| LV wet weight (g) | D1 | 1.09 ± 0.03 | 1.17 ± 0.05 | 1.16 ± 0.06 |
| D14 | 1.22 ± 0.04* | 1.09 ± 0.02† | ||
| LV myocyte number (nb/field) | D1 | 92.8 ± 1.8 | 100.9 ± 4.0 | 93.1 ± 2.4 |
| D14 | 85.9 ± 2.9* | 95.0 ± 3.3(6%) | ||
| LV myocyte surface (μm2) | D1 | 284 ± 6 | 235 ± 14* | 261 ± 3(11%) |
| D14 | 293 ± 6 | 308 ± 11 | ||
| LV collagen density (%) | D1 | 2.90 ± 0.07 | 2.60 ± 0.13 | 2.82 ± 0.08 |
| D14 | 3.13 ± 0.25 | 2.85 ± 0.25 | ||
| Pulmonary weight (g) | D1 | 1.68 ± 0.09 | 1.81 ± 0.15 | 1.78 ± 0.04 |
| D14 | 2.09 ± 0.15* | 1.80 ± 0.18 | ||
| Plasma nitrite (nM) | D1 | 373 ± 46 | 236 ± 31* | 453±† |
| D14 | 315 ± 58 | 501 ± 133 | ||
| Plasma syndecan-1 (nM) | D1 | 22.8 ± 1.5 | 27.7 ± 1.5* | 23.5 ± 1.1† |
| D14 | 26.5 ± 3.3 | 23.1 ± 1.0† | ||
| LV ROS production (AU/g/min) | D1 | 48.5 ± 2.1 | 59.8 ± 4.9* | 46.6 ± 3.79† |
| D14 | 56.7 ± 2.7* | 46.7 ± 2.24† | ||
| LV eNOS (AU) | D1 | 1 | 1.07 ± 0.14 | 0.99 ± 0.19 |
| D14 | 1.15 ± 0.14 | 0.98 ± 0.14 | ||
| LV iNOS (AU) | D1 | 1 | 1.06 ± 0.20 | 0.94 ± 0.20 |
| D14 | 1.54 ± 0.44* | 0.95 ± 0.15 | ||
| Macrophage Type-1 (nb/field) | D1 | 4.75 ± 0.40 | 6.79 ± 0.31* | 5.31 ± 0.89 |
| D14 | 7.21 ± 0.60* | 5.67 ± 0.26† | ||
| Macrophage Type-2 (nb/field) | D1 | 13.75 ± 0.59 | 13.09 ± 1.15 | 12.34 ± 0.34 |
| D14 | 13.12 ± 0.92 | 16.70 ± 0.37† | ||
| Ratio Type-1/Type-2 | D1 | 0.35 ± 0.03 | 0.51 ± 0.04* | 0.44 ± 0.08 |
| D14 | 0.57 ± 0.05 | 0.37 ± 0.04† |
- CHF, chronic heart failure; eNOS, endothelial nitric oxide synthase; iNOS, inducible nitric oxide synthase; LV, left ventricular; ROS, reactive oxygen species.
- * p < 0.05 vs. CHF.
- † p < 0.05 vs. CHF + salt-loading.
Concerning pulmonary congestion, pulmonary wet weight was significantly increased at 14 days after salt-loading. S38844 did not modify pulmonary wet weight at Day 1, but a statistically non-significant reduction, as compared with untreated salt-loaded HF rats, was observed at Day 14 (Table 1).
Plasma biology
Plasma nitrite levels, used as a proxy for NO bioavailability, were transiently decreased at 1 day after salt-loading, as compared with chronic HF rats. S38844 prevented the salt-induced decrease in plasma nitrite levels (Table 1).
Furthermore, plasma syndecan-1 levels, used as a biomarker of glycocalyx shedding, were increased vs. chronic HF controls, at both 1 and 14 days after salt-loading, reaching statistical significance at Day 1. S38844 reduced the salt-induced increase in plasma syndecan-1 levels (Table 1).
Left ventricular endothelial nitric oxide synthase and inducible nitric oxide synthase expression
While LV endothelial nitric oxide synthase (eNOS) expression was unaltered after salt-loading, inducible NOS (iNOS) expression significantly increased at 14 days as compared with chronic HF controls. S38844 did not modify either LV eNOS or iNOS expression, except for a slight, non-significant reduction in iNOS at 14 days (Table 1).
Left ventricular inflammation
Salt-loading was associated with an increase in LV classical, pro-inflammatory M1 macrophage levels at both 1 and 14 days, with no change observed in alternative CD206+ M2-like macrophages. This led to a transient increase in the M1 to M2 macrophage ratio at Day 1, but not Day 14, after salt-loading (Table 1). S38844 prevented the increase in M1 macrophage levels, reaching significance at Day 14, with a concomitant increase in M2-like macrophages, leading to a reduction in M1 to M2 macrophage ratios as compared with untreated NaCl group (Table 1).
Left ventricular reactive oxygen species production
Salt-loading increased LV ROS production at both 1 and 14 days, and S38844 prevented this increase.
Delayed S38844 administration
Neither delayed transient S38844 administration after salt-loading, nor transient S38844 administration to HF animals modified cardiac output or LV diastolic or systolic diameters. (Figure 5)
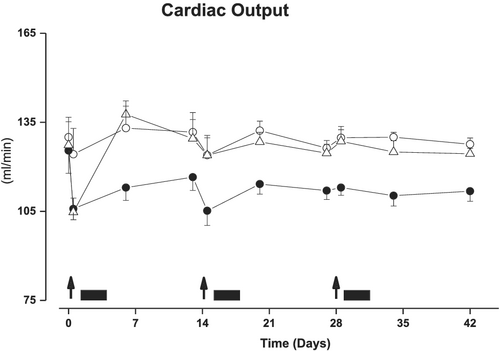
Repeated decompensation
In control rats with well-established stable chronic HF, cardiac output was stable during the 42 days of experimental period. In contrast, repeated salt-loading induced, at each salt-loading, an immediate further reduction of cardiac output, which only partially recovered during the 14 days following each salt-loading (Figure 6). S38844 treatment administered 12 h after each salt-loading opposed the reduction of cardiac output. Notably, S38844 administered for 2.5 days after first and second salt-loading also limited the decrease in cardiac output induced by the second and third salt-loading at Days 14 and 28.
Discussion
Our data show that in rats with well-established stable chronic HF, salt-loading provokes an immediate further aggravation of the already by HF-impaired cardiac function. This decline in LV systolic and diastolic function was associated with acute and persistent reduction in myocardial tissue perfusion and coronary endothelium-dependent relaxation. Furthermore, LV function only partially recovered over time, while myocardial tissue perfusion and coronary function remained reduced, and pulmonary congestion developed over time. The data also clearly demonstrate the key role of heart rate during the initial phase of acute decompensation, because transient HRR, induced by the If current inhibitor S38844 initiated 12 h after salt-loading, opposed the salt-induced aggravation of LV and coronary dysfunctions, with protective effects persisting beyond the treatment period.
To the best of our knowledge, this study is the first description of an experimental rat model mimicking ADHF in humans, that is, a rapid further decrease of the by HF already reduced CO followed by a partial recovery over time. This clearly differs from the time course of aggravation of cardiac function induced by myocardial infarction or embolization of coronary arteries by microspheres, both experimental models in which CO progressively declines and never recovers over time, thus mimicking the progressive development of cardiac dysfunction/remodelling leading to chronic HF in humans.
Moreover, our results suggest that the acute aggravation of the existing cardiac dysfunction results from multiple mechanisms triggered by salt-loading. For example, the reduced myocardial tissue perfusion (provoked by the tachycardia) will reduce O2/nutrient supply, while the augmented cardiac work (provoked by the tachycardia per se) and the increased LV wall tension (provoked by the increases in LV diameter as well as LV end-diastolic pressure) will enhance O2 consumption. This modification of the balance between O2 supply and O2 demand is likely to trigger LV hypoxia and sets off deleterious mechanisms, such as enhanced LV inflammation (illustrated by the increases in LV M1 macrophages and iNOS levels) and local myocardial oxidative stress (illustrated by the increase in LV ROS production), resulting in a persistent aggravation of cardiac dysfunction. Furthermore, the increase in LV ROS will also decrease NO bioavailability (illustrated by the decrease in plasma nitrite), which per se not only impairs LV diastolic dysfunction by altering Ca2+ handling and myocyte resting tension19, 20 but also may explain the persistent aggravation of coronary endothelial dysfunction. This aggravation of coronary endothelial dysfunction likely contributes to the persistent reduction of LV tissue perfusion despite normalization of heart rate/blood pressure during the ‘recovery’ phase. Furthermore, the persistent reduction in LV tissue perfusion and/or inflammation/oxidative stress will also render LV tissue prone to hypoxia and ischaemia/reperfusion injury and thus for subsequent acute decompensations. This hypothesis is strengthened by the facts that (i) in humans, ADHF is mostly observed in patients with cardiac co-morbidities (HF, diabetes, metabolic syndrome, etc.) characterized by low-grade systemic inflammation and endothelial dysfunction, and (ii) salt-loading in healthy rats did not provoke acute decompensation (data not shown).
It must be pointed out that the vascular endothelium might be involved in acute decompensation via its role in the regulation of natremia via the endothelial glycocalyx,20 which selectively buffers sodium ions with its negatively charged proteoglycans. Given that endothelial dysfunction is associated with glycocalyx damage,15 acute salt-loading in a pathophysiological state associated with glycocalyx damage/dysfunction, as illustrated in our study by the increase in plasma syndecan-1 levels, may lead to insufficient buffering of NaCl by the endothelial glycocalyx, leading to a slower sodium excretion rate and higher natremia. This might provoke oedema, that is, cardiac oedema as indicated by the increased LV weight observed in our experiments, which will contribute to the decrease of cardiac output, because even a minor increase in cardiac water content provokes major reduction of cardiac output.21
The second major finding of our study is the demonstration of the central role of heart rate during the acute phase of decompensation, given the fact that as soon as 12 h after administration of the If current inhibitor S38844 cardiac output was increased to pre-NaCl levels, and this beneficial effect persisted beyond the early short-term treatment. In contrast, neither delayed administration nor administration in chronic HF rats of S38844 increased cardiac output. This clearly shows that the mechanisms involved during the initial phase of decompensation differ from those involved in the ‘late’ stage. This observation might explain the ‘inefficacy’ of treatments used in human ADHF, because the delay between the ‘unknown’ trigger initiating decompensation and hospitalization is often unknown.
Furthermore, this divergent efficacy between early and delayed S38844 administration might be due to indirect effects related with the immediate effects induced by the transient If current inhibition. Indeed, the immediate increase in myocardial perfusion and reduction in LV oxygen consumption related with the HRR will likely reduce LV tissue hypoxia, which in turn will limit the vicious circle of increased LV oxidative stress, enhanced LV inflammation, reduced NO bio-availability/coronary relaxation reduced LV tissue perfusion, because hypoxia is known to induce oxidative stress/inflammation, all observed in acute decompensation rats. Moreover, the fact that only early but not delayed S38844 administration exerts protective effects clearly shows that the early mechanisms involved in decompensation become either irreversible over time or are followed by other irreversible cellular mechanisms, leading to incomplete recovery of cardiac function over time and/or rendering prone for subsequent decompensation. This hypothesis is strengthened by the fact that S38844 administered for 2.5 days but initiated 12 h after first and second salt-loading and also limited the decrease in cardiac output induced by the second and third salt-loading.
Study limitations
While our new rat model of acute decompensation induced by salt-loading in rats with chronic HF clearly mimics major characteristics of ADHF in humans, that is, an immediate aggravation of existing cardiac dysfunction with only partially recovery over time, several aspects need further investigation.
For example, in this model, only a progressive increase in pulmonary oedema was observed, suggesting only progressive development of pulmonary congestion. In contrast, humans hospitalized for ADHF show signs of pulmonary congestion/dyspnoea upon arrival in the emergency department. This might be important, because only HRR induced by If current inhibition during the early phase opposed acute decompensation (and also limited the magnitude of the second decompensation), revealing a therapeutic window.
It must be pointed out that this study clearly shows that an immediate but transient HRR of about 10% induced by the If current inhibitor S38844 is a therapeutic target in ADHF, but whether HRR with smaller or more marked magnitudes will exert similar protective effects remains to be evaluated. Moreover, in this study, HRR is induced by If current inhibition lacking negative inotropic properties, which might be an advantage over other HRR drugs, that is, beta-blockers, some Ca2+ channel blockers, for which its use might be hindered by their negative inotropic properties.
Furthermore, the cellular mechanism(s) other than increased oxidative stress/inflammation/reduced coronary relaxation such as humoral (des)activation, altered protein folding due to ER stress or epigenetic modifications, explaining the discrepancy between early and delayed HRR, remain to be elucidated. This might be of importance because it could give an explanation of the disappointing results of clinical trials in ADHF. Indeed, it might explain, at least in part, the neutral results with ularitide and serelaxin in terms of survival or functional benefit in ADHF.22-24
Conclusions
Our results, obtained in an original rat model mimicking ADHF in humans, clearly show the role of heart rate, reduced myocardial perfusion, and impaired coronary endothelial dysfunction in the progression of cardiac decompensation. Moreover, immediate but not delayed transient HRR, induced by the If current inhibitor S38844, immediately and persistently re-establishes cardiac and coronary vascular function to pre-decompensation levels suggesting (i) a therapeutic window and (ii) multiple mechanisms involved in the immediate and delayed phases of acute decompensation.
Conflict of interest
N.P., N.H., M.S., M.L., L.N., I.R.J., J.P.H., A.D., S.R., E.B., A.O.P., C.T., V.R., and P.M. have nothing to declare. F.F. and J.R. are employee of Institut de Recherche International Servier.
Funding
This work was funded by a research grant from the Institut de Recherche International Servier, France, and co-supported by the European Union and the Région Normandie [Europe gets involved in Normandy with European Regional Development Fund (ERDF), PACT-CBS], the European Fibro-Targets Project (FP7#602904), the FP7-funded COST ADMIRE network (BM1301), and the Fédération Française de Cardiology. N.P. was a recipient of a research grant from the Société Française de Médecine d'Urgence.




