Clinical characteristics and outcomes of black African heart failure patients with preserved, mid-range, and reduced ejection fraction: a post hoc analysis of the THESUS-HF registry
Abstract
Aims
Limited data are available on clinical characteristics and prognosis of heart failure (HF) in black African populations especially with respect to current classifications and HF management guidelines.
Methods and results
In this post hoc analysis, African patients admitted with acute HF and enrolled in the THESUS-HF registry in one of 12 hospitals in 9 countries were classified as having preserved left ventricular ejection fraction (LVEF) (HFpEF), mid-range LVEF (HFmrEF), and reduced LVEF (HFrEF) based on echocardiography performed close to the time of admission. Sociodemographic and clinical characteristics, management, and 60 and 180 day outcomes were compared between the groups. Of 888 patients with LVEF available, there were 472 (53.2%) with HFrEF, 174 (19.6%) with HFmrEF, and 243 (27.3%) with HFpEF. History of atrial fibrillation was higher in patients with HFmrEF (28.5%) than in patients with HFrEF (14.5%). Patients with HFrEF had a larger mean LV systolic diameter (54.1 ± 9.67 mm) than patients with HFmrEF (42.9 ± 8.47 mm), who had a larger mean LV diameter than patients with HFpEF (32.6 ± 8.64 mm); a similar pattern with LV diastolic diameter was observed. The mean posterior diastolic wall thickness (10.2 ± 2.94 mm) was lower in patients with HFrEF than in those with HFmrEF (11.1 ± 2.59 mm) and HFpEF (11.2 ± 2.90 mm). Patients with HFpEF were less likely to use angiotensin-converting enzyme inhibitor/angiotensin receptor blockers, and aldosterone inhibitors, and more likely to use beta-blockers than those with HFrEF at either admission or discharge/Day 7. Death or readmission rates through Day 60 and 180 day death rates did not differ significantly among the groups; unadjusted hazard ratios relative to patients with HFrEF were 1.32 [95% confidence interval (CI) 0.84–2.08] and 1.24 (95% CI 0.82–1.89) for 60 day death or readmission and 0.92 (95% CI 0.59–1.43) and 0.78 (95% CI 0.51–1.20) for 180 day death in patients with HFmrEF and HFpEF, respectively.
Conclusions
Classification by LVEF according to European Society of Cardiology guidelines revealed some differences in clinical presentation but similar mortality and rehospitalization rates across all EF groups in Africans admitted for HF.
Introduction
Heart failure (HF) is a global challenge of the 21st century. It is a complex clinical syndrome characterized by the reduced ability of the heart to pump and/or fill with blood. Large epidemiological studies have shown that left ventricular ejection fraction (LVEF) is a predictive marker of adverse outcomes even in the absence of symptomatic HF.1 In 2013, the American Heart Association/American College of Cardiology Foundation (ACCF/AHA) guidelines first classified HF into three subtypes, namely, HF with reduced ejection fraction (HFrEF), HF with preserved ejection fraction (HFpEF), and HF with mid-range ejection fraction (HFmrEF), according to the LVEF measurement at diagnosis, natriuretic peptide levels, and the presence of structural heart disease and diastolic dysfunction.2 In 2016, the European Society of Cardiology (ESC) also specified three subgroups of HF based on LVEF.3 Notably, the strong rationale for these classifications as well as guidelines for the global management of HF derived from large epidemiological studies was conducted in western countries over the past four decades.4-6 While 17% of the world's population live in Africa,7 and despite HF being a major burden in this region with existing data suggesting differences in HF aetiologies, therapies, and outcomes,8, 9 very little information has been published on these HF patients phenotype, particularly with regard to the echocardiographic subtypes. The Sub-Saharan Africa Survey of Heart Failure (THESUS-HF) is the largest documented study of HF in the region. Publications8, 10, 11 from THESUS-HF data have been largely cited by the scientific community owing to its rigorous methodology and internal and external validity including over nine participating countries making it a unique evidence resource in the region.
The need for further post hoc analysis on THESUS-HF data to provide insight on subtypes of HF cannot be overemphasized. While HF in Africa is managed per ACCF/AHA or ESC guidelines, to date, there are no data on HF in black Africans based on the current classification of HF.
This report aimed to determine the differences in clinical presentation, management, and outcome in black Africans with HF based on their LVEF using the 2016 ESC guidelines.3
Methods
THESUS-HF was a prospective, multicentre, international observational survey conducted in 12 hospitals from 9 countries in the southern, eastern, central, and western regions of sub-Saharan Africa (SSA). Centres were selected for participation if they had a physician trained in clinical cardiology and echocardiography. The methodology of the study has been described in detail elsewhere.10 In brief, from July 2007 to June 2010, all patients were recruited during admission for acute HF (AHF) and were followed for 6 months. Patients admitted with dyspnoea and diagnosed with AHF based on symptoms and signs (including dyspnoea, orthopnoea, dyspnoea on exercise, rales, oedema, jugular venous pulse, and oxygen saturation), and who provided written informed consent, were enrolled into the study. A detailed systematic transthoracic echocardiography assessment of left ventricular function, valvular structure, and function, as well as regional wall motion abnormalities, was performed by trained physicians. All measurements were undertaken according to the American Society of Echocardiography guidelines.12 The most likely aetiology of HF was provided by the attending physician, based on the 2012 ESC guidelines.13 LVEF was assessed using the conventional apical two-chamber and four-chamber views and the modified Simpson's method. In this post hoc analysis, patients were classified according to the terminology of the 2016 ESC guidelines for the diagnosis and treatment of AHF and chronic HF3 as HFpEF (LVEF ≥50%), HFmrEF (LVEF 40–49%), and HFrEF (LVEF <40%). Information on readmissions and death, with respective reasons and cause, was collected over the 6 month follow-up. Outcomes of interest were readmission or death within 60 days and death within 180 days. Detailed data collected on standardized case report forms at admission included medical history, medication use, laboratory values, and physical examination with symptoms and signs of HF. Human immunodeficiency virus testing was performed as clinically indicated. Patients were followed by either clinic visit or telephone contact over 6 months for the occurrence of readmissions and death.
Written informed consent was obtained from each subject before enrolment into the study. Approval was obtained from the ethics committee of each participating institution, and the study conformed to the principles of the Declaration of Helsinki.
Statistical analysis
Eight hundred and eighty-eight participants were included in this post hoc analysis. Results are presented as counts and percentages for categorical variables and means, standard deviation, median, and 25th to 75th percentiles for continuous variables. Comparison between participants with HFrEF, HFmrEF, and HFpEF for demographics, baseline clinical characteristics, medical history, echocardiographic parameters, and baseline medication use was tested using χ2 tests for qualitative variables, Kruskal–Wallis test for ordinal variables, and one-way ANOVA for quantitative variables. No continuous variable was found to have a highly skewed distribution, which would have required ANOVA based on log-transformed values.
The associations between LVEF and clinical outcomes were examined. Length of initial hospital stay was compared between groups using t-test. A logistic regression model was used for the comparison between groups on in-hospital mortality with the odds ratio, 95% confidence interval (CI), and Wald χ2 P-value presented. For time-to-event outcomes, Kaplan–Meier estimates are presented with the log-rank test used for comparison between groups (HFrEF, HFmrEF, and HFpEF); hazard ratios and 95% CIs from Cox regression models are also given. Adjusted hazard ratios are also presented for the outcomes of death or readmission to Day 60 and all-cause death to Day 180 adjusting for predictors known to be associated with each of these outcomes.11 These predictors include history of hyperlipidaemia, malignancy, and cor pulmonale and baseline rales, ejection fraction, blood urea nitrogen, and systolic blood pressure for death or readmission to Day 60 and history of malignancy, cor pulmonale, and HIV, gender, smoking status, and baseline values for systolic blood pressure, creatinine, haemoglobin, heart rate, orthopnoea, rales, and oedema for death to Day 180. To account for missing predictors in the adjusted analyses, multiple imputation methods were used to impute missing values. Seven imputation datasets were used with parameter estimates averaged across these datasets using Rubin's algorithm (SAS PROC MIANALYZE). Plots of Kaplan–Meier curves for time to all-cause death and/or HF readmission according to the EF (HFrEF, HFmrEF, and HFpEF) are shown. Data were analysed with the use of SAS software version 9.4 (SAS Institute, Cary, NC, USA). A P-value <0.05 was considered statistically significant.
Results
Participants
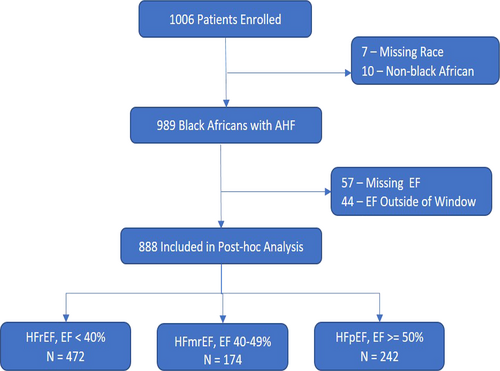
A total of 1006 patients with AHF from 9 countries in Africa were enrolled in the THESUS-HF cohort study. Of these, 989 (98.3%) were black Africans. We excluded 101 patients for missing ejection fraction (57 cases) and patients whose ejection fraction was measured more than 4 months before or 2 months after admission (44). The median time of the echocardiogram from admission was 1.0 (interquartile range 0.0–2.0) day. Of the 888 patients with AHF included in this post hoc analysis, 472 (53.2%) had an ejection fraction <40%, 174 (19.6%) had an EF 40–49%, and 242 (27.3%) had an EF ≥50% (Figure 1).
Comparison of sociodemographic and risk factor profile by ejection fraction group
There were 438 (49.4%) male patients, and the mean age was 52.5 (18.19) years. The sociodemographic and risk factors profile are shown in Table 1. There were more males in patients with HFrEF (53.4%) and more females in patients with HFpEF (58.1%). History of cor pulmonale was more prevalent in patients with HFpEF (12.4%) and HFmrEF (9.2%) than in patients with HFrEF (3.6%). History of atrial fibrillation was higher in patients with HFmrEF (28.5%) than in patients with HFrEF (14.5%). As previously reported, the most common aetiology in this patient population was hypertensive cardiomyopathy.10
| Parameter | Observed n (% missing) | Statistic | HFrEF (N = 472) | HFmrEF (N = 174) | HFpEF (N = 242) | P-valuea | Total (N = 888) |
|---|---|---|---|---|---|---|---|
| Age (years) | 880 (0.9%) | Mean (SD) | 51.4 (17.49) | 54.6 (18.01) | 53.2 (19.52) | 0.11 | 52.5 (18.19) |
| Median (Q1, Q3) | 53.0 (39.0, 65.0) | 57.0 (41.0, 69.0) | 56.5 (39.0, 70.0) | 55.0 (40.0, 67.0) | |||
| Male sex | 887 (0.1%) | n (%) | 252 (53.4%) | 85 (48.9%) | 101 (41.9%)* | 0.015 | 438 (49.4%) |
| Hx of atrial fibrillation prior to admission | 881 (0.8%) | n (%) | 68 (14.5%) | 49 (28.5%)* | 51 (21.3%) | 0.0002 | 168 (19.1%) |
| # AHF admission in prior 12 months | 820 (7.7%) | Mean (SD) | 0.4 (0.76) | 0.3 (0.63) | 0.4 (0.80) | 0.64 | 0.4 (0.75) |
| Median (Q1, Q3) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | |||
| Hx hyperlipidaemia | 870 (2.0%) | n (%) | 33 (7.2%) | 20 (11.7%) | 23 (9.7%) | 0.17 | 76 (8.7%) |
| Hx of smoking | 884 (0.5%) | n (%) | 46 (9.8%) | 14 (8.1%) | 17 (7.1%) | 0.46 | 77 (8.7%) |
| Hx hypertension | 884 (0.5%) | n (%) | 259 (55.2%) | 105 (60.3%) | 138 (57.3%) | 0.50 | 502 (56.8%) |
| Hx of diabetes | 886 (0.2%) | n (%) | 52 (11.1%) | 18 (10.3%) | 26 (10.7%) | 0.97 | 96 (10.8%) |
| Hx of malignancy | 885 (0.3%) | n (%) | 10 (2.1%) | 0 | 3 (1.2%) | 0.13 | 13 (1.5%) |
| Hx of cor pulmonale | 882 (0.7%) | n (%) | 17 (3.6%) | 16 (9.2%)* | 30 (12.4%)* | <0.0001 | 63 (7.1%) |
| Hx of myocardial infarction | 854 (3.8%) | n (%) | 16 (3.5%) | 8 (4.9%) | 7 (3.0%) | 0.60 | 31 (3.6%) |
| Valvular disease | 883 (0.6%) | n (%) | 93 (19.8%) | 69 (40.1%)* | 89 (36.9%)* | <0.0001 | 251 (28.4%) |
| Mitral stenosis | 883 (0.6%) | n (%) | 13 (2.8%) | 19 (11.0%)* | 22 (9.1%)* | <0.0001 | 54 (6.1%) |
| Mitral regurgitation | 883 (0.6%) | n (%) | 69 (14.7%) | 49 (28.5%)* | 65 (27.0%)* | <0.0001 | 183 (20.7%) |
| Aortic stenosis | 883 (0.6%) | n (%) | 9 (1.9%) | 7 (4.1%) | 9 (3.7%) | 0.21 | 25 (2.8%) |
| Aortic regurgitation | 883 (0.6%) | n (%) | 25 (5.3%) | 22 (12.8%)* | 26 (10.8%)* | 0.0024 | 73 (8.3%) |
| Other | 883 (0.6%) | n (%) | 41 (8.7%) | 19 (11.0%) | 37 (15.4%)* | 0.028 | 97 (11.0%) |
| Heart failure aetiology | 865 (2.6%) | ||||||
| Hypertensive cardiomyopathy | n (%) | 199 (42.7%) | 75 (43.9%) | 86 (37.7%) | 360 (41.6%) | ||
| Idiopathic dilated cardiomyopathy | n (%) | 103 (22.1%) | 16 (9.4%) | 2 (0.9%) | 121 (14.0%) | ||
| Rheumatic heart disease | n (%) | 34 (7.3%) | 41 (24.0%) | 54 (23.7%) | 129 (14.9%) | ||
| Ischaemic heart disease | n (%) | 37 (7.9%) | 15 (8.8%) | 10 (4.4%) | 62 (7.2%) | ||
| Peripartum cardiomyopathy | n (%) | 55 (11.8%) | 3 (1.8%) | 2 (0.9%) | 60 (6.9%) | ||
| Other | n (%) | 10 (2.1%) | 13 (7.6%) | 35 (15.4%) | 58 (6.7%) | ||
| Pericardial effusion/tamponade | n (%) | 18 (3.9%) | 4 (2.3%) | 23 (10.1%) | 45 (5.2%) | ||
| HIV cardiomyopathy | n (%) | 10 (2.1%) | 2 (1.2%) | 8 (3.5%) | 20 (2.3%) | ||
| Endomyocardial fibroelastosis | n (%) | 0 | 2 (1.2%) | 8 (3.5%) | 10 (1.2%) | ||
| Body mass index | 863 (2.8%) | Mean (SD) | 24.6 (5.52) | 25.2 (5.80) | 24.7 (6.10) | 0.51 | 24.8 (5.74) |
| Median (Q1, Q3) | 23.7 (20.8, 27.8) | 24.2 (21.3, 28.1) | 23.9 (20.4, 28.1) | 23.9 (20.8, 27.9) |
- AHF, acute heart failure; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; Q1, 25th percentile; Q3, 75th percentile; SD, standard deviation.
- a P-value from ANOVA (F-test) for continuous variables or from χ2 test for categorical variables.
- * P < 0.05 vs. HFrEF (adjusted P-value using Bonferroni step-down).
- ** P < 0.05 vs. HFmrEF (adjusted P-value using Bonferroni step-down).
Comparison of clinical presentation and echocardiographic characteristics by ejection fraction group
The clinical presentation is shown in Table 2. Patients with HFrEF had a lower mean systolic blood pressure (125.8 ± 30.82 mmHg) than those with HFmrEF (133.1 ± 34.84 mmHg) or HFpEF (137.3 ± 36.38 mmHg). The mean heart rate was higher in those with HFrEF (106.0 ± 19.63 b.p.m.) than in patients with HFpEF (101.1 ± 22.72 b.p.m.). Patients with HFrEF were more likely to have 3+ oedema than those with HFmrEF and HFpEF (P = 0.013). The echocardiographic characteristics are shown in Table 3. Patients with HFrEF had a larger mean LV systolic diameter (54.1 ± 9.67 mm) than patients with HFmrEF (42.9 ± 8.47 mm), who had a larger mean LV diameter than patients with HFpEF (32.6 ± 8.64 mm); a similar pattern with LV diastolic diameter was observed. The mean posterior diastolic wall thickness (10.2 ± 2.94 mm) was lower in patients with HFrEF than in those with HFmrEF (11.1 ± 2.59 mm) and HFpEF (11.2 ± 2.90 mm). The transmitral inflow velocities were lowest in patients with HFrEF.
| Parameter | Observed n (% missing) | Statistic | HFrEF (N = 472) | HFmrEF (N = 174) | HFpEF (N = 242) | P-valuea | Total (N = 888) |
|---|---|---|---|---|---|---|---|
| NYHA class 1 month prior to admission | 574 (35.4%) | ||||||
| I | n (%) | 46 (15.6%) | 18 (16.1%) | 41 (24.6%)* | 0.11 | 105 (18.3%) | |
| II | n (%) | 137 (46.4%) | 54 (48.2%) | 73 (43.7%) | 264 (46.0%) | ||
| III | n (%) | 94 (31.9%) | 36 (32.1%) | 50 (29.9%) | 180 (31.4%) | ||
| IV | n (%) | 18 (6.1%) | 4 (3.6%) | 3 (1.8%) | 25 (4.4%) | ||
| Systolic BP (mmHg) | 878 (1.1%) | Mean (SD) | 125.8 (30.82) | 133.1 (34.84)* | 137.3 (36.38)* | <0.0001 | 130.4 (33.56) |
| Median (Q1, Q3) | 120.0 (102.0, 143.0) | 130.0 (108.0, 153.0) | 130.0 (110.0, 160.0) | 128.0 (107.0, 150.0) | |||
| Diastolic BP (mmHg) | 877 (1.2%) | Mean (SD) | 84.0 (20.47) | 83.6 (20.45) | 85.5 (22.08) | 0.57 | 84.3 (20.91) |
| Median (Q1, Q3) | 80.0 (70.0, 100.0) | 81.5 (70.0, 100.0) | 80.0 (70.0, 100.0) | 80.0 (70.0, 100.0) | |||
| Heart rate (b.p.m.) | 882 (0.7%) | Mean (SD) | 106.0 (19.63) | 103.1 (23.97) | 101.1 (22.72)* | 0.011 | 104.1 (21.49) |
| Median (Q1, Q3) | 108.0 (92.0, 120.0) | 100.0 (88.0, 120.0) | 100.0 (86.0, 113.0) | 104.0 (90.0, 116.0) | |||
| Peripheral oedema | 875 (1.5%) | Mean (SD) | 2.0 (1.01) | 1.7 (1.03)* | 1.7 (1.09)* | 0.0008 | 1.8 (1.04) |
| 0 | n (%) | 58 (12.5%) | 31 (17.9%) | 47 (19.7%) | 0.013 | 136 (15.5%) | |
| 1 | n (%) | 70 (15.1%) | 33 (19.1%) | 47 (19.7%) | 150 (17.1%) | ||
| 2 | n (%) | 168 (36.3%) | 67 (38.7%) | 77 (32.2%) | 312 (35.7%) | ||
| 3+ | n (%) | 167 (36.1%) | 42 (24.3%) | 68 (28.5%) | 277 (31.7%) | ||
| Rales | 797 (10.2%) | Mean (SD) | 1.7 (0.86) | 1.7 (0.88) | 1.6 (1.00) | 0.15 | 1.7 (0.91) |
| 0 | n (%) | 44 (10.1%) | 18 (12.5%) | 44 (20.3%) | 0.013 | 106 (13.3%) | |
| 1 | n (%) | 111 (25.5%) | 28 (19.4%) | 43 (19.8%) | 182 (22.8%) | ||
| 2 | n (%) | 208 (47.7%) | 75 (52.1%) | 92 (42.4%) | 375 (47.1%) | ||
| 3 | n (%) | 73 (16.7%) | 23 (16.0%) | 38 (17.5%) | 134 (16.8%) | ||
| Creatinine (mg/dL) | 848 (4.5%) | Mean (SD) | 1.4 (1.00) | 1.4 (0.99) | 1.3 (1.07) | 0.80 | 1.4 (1.02) |
| Median (Q1, Q3) | 1.1 (0.9, 1.5) | 1.2 (0.9, 1.6) | 1.0 (0.8, 1.5) | 1.1 (0.9, 1.5) | |||
| BUN (mg/dL) | 846 (4.7%) | Mean (SD) | 34.5 (29.55) | 36.8 (29.53) | 36.0 (38.41) | 0.69 | 35.4 (32.18) |
| Median (Q1, Q3) | 27.4 (17.6, 40.6) | 28.0 (18.0, 42.0) | 25.8 (15.0, 45.0) | 27.6 (17.0, 42.0) |
- BP, blood pressure; b.p.m., beats per minute; BUN, blood urea nitrogen; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; NYHA, New York Heart Association; Q1, 25th percentile; Q3, 75th percentile; SD, standard deviation.
- a P-value from ANOVA (F-test) for continuous variables or from χ2 test for categorical variables.
- * P < 0.05 vs. HFrEF (adjusted P-value using Bonferroni step-down).
- ** P < 0.05 vs. HFmrEF (adjusted P-value using Bonferroni step-down).
| Parameter | Observed n (% missing) | Statistic | HFrEF (N = 472) | HFmrEF (N = 174) | HFpEF (N = 242) | P-valuea | Total (N = 888) |
|---|---|---|---|---|---|---|---|
| Heart rate (b.p.m.) | 690 (22.3%) | Mean (SD) | 96.1 (15.21) | 92.9 (20.20) | 92.7 (20.37) | 0.057 | 94.5 (17.87) |
| Median (Q1, Q3) | 96.0 (86.5, 104.0) | 94.0 (81.5, 102.0) | 90.0 (82.0, 104.0) | 94.0 (84.0, 104.0) | |||
| Dimensions and LV function | |||||||
| LV systole diameter (mm) | 879 (1.0%) | Mean (SD) | 54.1 (9.67) | 42.9 (8.47)* | 32.6 (8.64)*,** | <0.0001 | 46.0 (13.05) |
| Median (Q1, Q3) | 54.0 (48.6, 60.0) | 44.0 (37.0, 48.0) | 32.0 (26.0, 38.0) | 47.0 (37.0, 55.0) | |||
| LV diastole diameter (mm) | 882 (0.7%) | Mean (SD) | 63.1 (9.60) | 55.9 (10.16)* | 49.1 (10.47)*,** | <0.0001 | 57.9 (11.62) |
| Median (Q1, Q3) | 63.0 (57.1, 68.0) | 57.0 (50.0, 62.0) | 49.0 (41.8, 56.0) | 58.1 (50.0, 65.0) | |||
| IV septum (diastole) (mm) | 863 (2.8%) | Mean (SD) | 10.6 (3.15) | 11.9 (3.03)* | 12.1 (3.45)* | <0.0001 | 11.2 (3.29) |
| Median (Q1, Q3) | 10.0 (8.7, 12.4) | 11.7 (10.0, 14.0) | 12.0 (9.0, 14.0) | 11.0 (9.0, 13.0) | |||
| Posterior wall (diastole) (mm) | 840 (5.4%) | Mean (SD) | 10.2 (2.94) | 11.1 (2.59)* | 11.2 (2.90)* | <0.0001 | 10.7 (2.90) |
| Median (Q1, Q3) | 10.0 (8.0, 12.0) | 11.0 (9.4, 13.0) | 11.0 (9.0, 13.0) | 10.0 (9.0, 12.9) | |||
| Diastolic function | |||||||
| Left atrial diameter (mm) | 845 (4.8%) | Mean (SD) | 47.3 (7.58) | 47.0 (9.60) | 47.0 (11.35) | 0.89 | 47.2 (9.15) |
| Median (Q1, Q3) | 47.0 (42.0, 52.0) | 46.5 (41.0, 53.0) | 46.0 (38.4, 54.0) | 47.0 (41.0, 53.0) | |||
| Left atrial area (mm2) | 475 (46.5%) | Mean (SD) | 2759.4 (712.87) | 2793.9 (995.43) | 2867.9 (1190.44) | 0.54 | 2800.6 (942.68) |
| Median (Q1, Q3) | 2620.0 (2285.0, 3200.0) | 2750.0 (2115.0, 3404.5) | 2685.0 (2030.0, 3410.0) | 2657.0 (2200.0, 3300.0) | |||
| Mitral E wave (cm/s) | 731 (17.7%) | Mean (SD) | 47.6 (41.54) | 54.3 (54.06) | 58.3 (56.69)* | 0.031 | 51.8 (48.75) |
| Median (Q1, Q3) | 41.3 (8.7, 81.0) | 36.7 (10.1, 83.0) | 45.8 (10.6, 90.5) | 44.0 (9.4, 83.0) | |||
| E wave deceleration time (ms) | 699 (21.3%) | Mean (SD) | 127.1 (67.63) | 168.0 (114.92)* | 171.0 (88.73)* | <0.0001 | 146.5 (86.28) |
| Median (Q1, Q3) | 120.0 (96.0, 142.0) | 146.5 (120.0, 192.0) | 160.0 (120.0, 210.0) | 130.0 (100.0, 170.0) | |||
| Mitral A wave (cm/s) | 606 (31.8%) | Mean (SD) | 29.0 (27.31) | 43.4 (37.40)* | 42.5 (38.62)* | <0.0001 | 35.2 (33.30) |
| Median (Q1, Q3) | 22.0 (5.0, 46.0) | 32.0 (7.6, 67.0) | 36.5 (6.9, 69.0) | 26.9 (5.9, 55.0) | |||
| Mitral A wave duration time (ms) | 483 (45.6%) | Mean (SD) | 117.0 (39.61) | 131.0 (37.66)* | 141.4 (56.69)* | <0.0001 | 126.2 (45.89) |
| Median (Q1, Q3) | 114.0 (94.0, 140.0) | 127.0 (110.0, 156.0) | 137.0 (107.0, 161.0) | 121.0 (100.0, 150.0) | |||
- HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LV, left ventricle; Q1, 25th percentile; Q3, 75th percentile; SD, standard deviation.
- a P-value from ANOVA (F-test) for continuous variables or from χ2 test for categorical variables.
- * P < 0.05 vs. HFrEF (adjusted P-value using Bonferroni step-down).
- ** P < 0.05 vs. HFmrEF (adjusted P-value using Bonferroni step-down).
Comparison of management and outcome by ejection fraction group
The treatment of AHF is shown in Table 4. There were significant differences in the prescription of HF medicines across LVEF groups. Patients with HFpEF were less likely prescribed angiotensin-converting enzyme inhibitor/angiotensin receptor blockers and aldosterone inhibitors than those with HFrEF. Patients with HFpEF were more likely prescribed beta-blockers than those with HFrEF.
| Parameter | Observed n (% missing) | Statistic | HFrEF (N = 472) | HFmrEF (N = 174) | HFpEF (N = 242) | P-valuea | Total (N = 888) |
|---|---|---|---|---|---|---|---|
| Admission | |||||||
| ACEi/ARBs | 879 (1.0%) | n (%) | 372 (79.5%) | 125 (72.7%) | 163 (68.2%)* | 0.0033 | 660 (75.1%) |
| Loop diuretics | 863 (2.8%) | n (%) | 59 (12.9%) | 22 (13.0%) | 42 (17.7%) | 0.20 | 123 (14.3%) |
| Beta-blockers | 874 (1.6%) | n (%) | 64 (13.8%) | 34 (19.9%) | 50 (21.0%)* | 0.028 | 148 (16.9%) |
| Digoxin | 873 (1.7%) | n (%) | 240 (51.9%) | 83 (48.3%) | 79 (33.1%)*,** | <0.0001 | 402 (46.0%) |
| Hydralazine | 866 (2.5%) | n (%) | 2 (0.4%) | 2 (1.2%) | 5 (2.1%) | 0.12 | 9 (1.0%) |
| Nitrates | 875 (1.5%) | n (%) | 23 (5.0%) | 11 (6.4%) | 11 (4.6%) | 0.69 | 45 (5.1%) |
| Aldosterone inhibitors | 878 (1.1%) | n (%) | 351 (75.5%) | 113 (65.3%)* | 145 (60.4%)* | <0.0001 | 609 (69.4%) |
| Statins | 866 (2.5%) | 40 (8.8%) | 16 (9.4%) | 27 (11.3%) | 0.57 | 83 (9.6%) | |
| Aspirin | 876 (1.4%) | n (%) | 176 (38.1%) | 62 (35.8%) | 83 (34.4%) | 0.62 | 321 (36.6%) |
| Anticoagulants | 877 (1.2%) | n (%) | 164 (35.3%) | 69 (39.9%) | 70 (29.2%) | 0.068 | 303 (34.5%) |
| Discharge or Day7 | |||||||
| ACEi/ARBs | 861 (3.0%) | n (%) | 397 (86.5%) | 136 (81.0%) | 177 (75.6%)* | 0.0015 | 710 (82.5%) |
| Loop diuretics | 855 (3.7%) | n (%) | 368 (80.3%) | 133 (79.6%) | 169 (73.5%) | 0.11 | 670 (78.4%) |
| Beta-blockers | 853 (3.9%) | n (%) | 115 (25.2%) | 52 (31.0%) | 78 (34.2%)* | 0.037 | 245 (28.7%) |
| Digoxin | 853 (3.9%) | n (%) | 318 (69.6%) | 109 (65.7%) | 108 (47.0%)*,** | <0.0001 | 535 (62.7%) |
| Hydralazine | 845 (4.8%) | n (%) | 10 (2.2%) | 5 (3.0%) | 4 (1.7%) | 0.70 | 19 (2.2%) |
| Nitrates | 858 (3.4%) | n (%) | 38 (8.3%) | 18 (10.7%) | 10 (4.3%)* | 0.050 | 66 (7.7%) |
| Aldosterone inhibitors | 861 (3.0%) | n (%) | 369 (80.4%) | 126 (74.1%) | 156 (67.2%)* | 0.0006 | 651 (75.6%) |
| Statins | 850 (4.3%) | n (%) | 56 (12.5%) | 27 (16.1%) | 38 (16.3%) | 0.30 | 121 (14.2%) |
| Aspirin | 860 (3.2%) | n (%) | 289 (63.4%) | 91 (53.5%)* | 114 (48.7%)* | 0.0006 | 494 (57.4%) |
| Anticoagulants | 860 (3.2%) | n (%) | 83 (18.1%) | 37 (21.9%) | 45 (19.3%) | 0.57 | 165 (19.2%) |
- ACEi, angiotensin-converting enzyme inhibitor; ARBs, angiotensin receptor blockers; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
- a P-value from ANOVA (F-test) for continuous variables or from χ2 test for categorical variables.
- * P < 0.05 vs. HFrEF (adjusted P-value using Bonferroni step-down).
- ** P < 0.05 vs. HFmrEF (adjusted P-value using Bonferroni step-down).
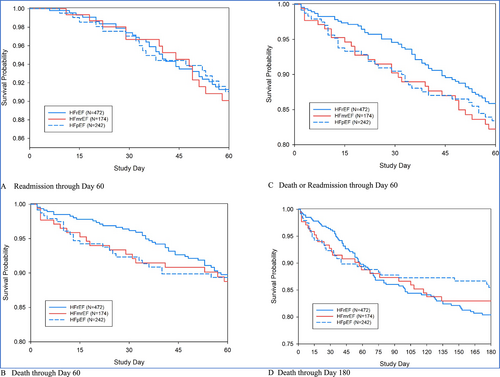
Major outcomes of death or readmission through Days 60 and 180 are shown in Table 5 and Figure 2. There was no statistically significant difference in the outcomes across the EF groups (all P > 0.05), even after covariate adjustment. Death or readmission rates through Day 60 and 180 day death rates did not differ significantly among the groups; unadjusted hazard ratios relative to patients with HFrEF were 1.32 (95% CI 0.84–2.08) and 1.24 (95% CI 0.82–1.89) for 60 day death or readmission and 0.92 (95% CI 0.59–1.43) and 0.78 (95% CI 0.51–1.20) for 180 day death in patients with HFmrEF and HFpEF, respectively.
| Parameter | Observed n (% missing) | Statistic | HFrEF (N = 472) | HFmrEF (N = 174) | HFpEF (N = 242) | P-valuea | Total (N = 888) |
|---|---|---|---|---|---|---|---|
| Length of initial hospital stay (days) | 764 (14.0%) | Mean (SD) | 9.7 (9.74) | 10.1 (13.36) | 8.3 (6.90) | 0.14 | 9.4 (9.93) |
| Median (Q1, Q3) | 8.0 (6.0, 11.0) | 8.0 (6.0, 10.0) | 7.0 (5.0, 10.0) | 8.0 (6.0, 10.0) | |||
| Initial hospitalization mortality | n (%) | 13 (2.8%) | 10 (5.7%) | 13 (5.4%) | 0.11 | 36 (4.1%) | |
| Readmission to Day 60 | n (KM%) | 34 (8.7%) | 14 (9.9%) | 17 (9.0%) | 0.93 | 65 (9.0%) | |
| Death to Day 60 | n (KM%) | 42 (10.2%) | 18 (11.3%) | 23 (10.7%) | 0.83 | 83 (10.5%) | |
| Death or readmission to Day 60 | |||||||
| Unadjusted | n (KM%) | 58 (14.1%) | 28 (17.8%) | 35 (16.6%) | 0.39 | 121 (15.5%) | |
| HR (95% CI)a | [REF] | 1.32 (0.84, 2.08) | 1.24 (0.82, 1.89) | 0.40 | |||
| Covariate-adjustedb, complete casesc | N | 406 | 136 | 204 | |||
| HR (95% CI)a | [REF] | 1.06 (0.62, 1.80) | 1.07 (0.65, 1.76) | 0.96 | |||
| Covariate-adjustedb, multiply imputed | N | 472 | 174 | 242 | |||
| HR (95% CI)a | [REF] | 1.29 (0.82, 2.03) | 1.35 (0.87, 2.08) | 0.33 | |||
| Death to Day 180 | |||||||
| Unadjusted | n (KM%) | 76 (19.6%) | 26 (17.0%) | 30 (14.5%) | 0.52 | 132 (17.7%) | |
| HR (95% CI)a | [REF] | 0.92 (0.59, 1.43) | 0.78 (0.51, 1.20) | 0.53 | |||
| Covariate-adjustedd, complete casesc | N | 440 | 163 | 220 | |||
| HR (95% CI)a | [REF] | 0.94 (0.58, 1.51) | 0.90 (0.57, 1.44) | 0.90 | |||
| Covariate-adjustedb, multiply imputed | N | 472 | 174 | 242 | |||
| HR (95% CI)a | [REF] | 0.94 (0.59, 1.52) | 0.90 (0.57, 1.43) | 0.91 | |||
- HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; Q1, 25th percentile; Q3, 75th percentile; SD, standard deviation.
- a P-value from ANOVA (F-test) for continuous variables, log-rank test for time-to event outcomes or from χ2 test for categorical variables; hazard ratios (HRs), and associated confidence intervals (CIs) and P-values from Cox regression.
- b Adjusted for rales, cor pulmonale, blood urea nitrogen, and systolic blood pressure.
- c Complete cases are subjects with non-missing values for all the covariates.
- d Adjusted for oedema, creatinine, cor pulmonale, and systolic blood pressure.
Discussion
The current post hoc analysis of the THESUS-HF registry describes the characteristics and outcomes of patients admitted for AHF in SSA according to the current guidelines of HF definition and classification.
In 2016, a new version of the ESC guidelines for the management of AHF and chronic HF was released. The guideline introduced a new category of HF–HFmrEF.3 The guideline also updated recommendations about the diagnosis and treatment of HF.
To the best of our knowledge, no major registries of HF patients have been published in SSA after publication of the 2016 ESC guidelines especially regarding HFmrEF prevalence, characteristics, treatment, and outcome. Thus, there is a gap in evidence for patients with HFmrEF in SSA. The THESUS-HF registry, the largest registry to date on HF in SSA, was published in 2012,10 and since then, none of the secondary papers has focused on echocardiographic subtypes of HF. In this post hoc analysis to compare the clinical characteristics, treatment, and outcome across the LVEF categories (HFrEF, HFmrEF, and HFpEF) based on the 2016 ESC guidelines, we reclassified participants in the THESUS-HF registry into these three categories. More than half of participants (53%) had HFrEF while 20% were ascribed to HFmrEF. To our knowledge, this is one of the rare descriptions of HFmrEF in SSA. Our prevalence of HFmrEF was in accordance with the 17–20% reported in other registries and cohort studies in the majority of high-income countries14-16 but lower than the 10% reported by Nelson et al. in Denmark. That Nelson et al. included all acute cases of dyspnoea and classified severe valve disease before other echocardiographic subtypes of HF may account for the lower prevalence of HFmrEF in their study. Thus, HFmrEF is common in clinical practice with a prevalence that might be similar in high-income and low-income countries. When compared with previous LVEF classification, the prevalence of participants with HFrEF was similar.17 Reports from registries and cohort studies have shown HFrEF to be the most prevalent category of HF.14, 18 HFmrEF is an intermediate between HFrEF and HFpEF. Data from registries and cohort studies are inconsistent as to whether clinical characteristics in HFmrEF might be similar to those in HFrEF or HFpEF or intermediate.17-21 In the THESUS-HF registry, patients with HFmrEF were similar to those with HFrEF and HFpEF with respect to some characteristics and intermediate between HFrEF and HFpEF with respect to others. But patients with HFmrEF and HFpEF were more likely to have atrial fibrillation, and patients with HFpEF had the highest prevalence of cor pulmonale. Several studies from high-income countries have reported that the clinical characteristics of HFmrEF patients are generally intermediate between HFrEF and HFpEF.14 Hypertension that is the most common cause of HF in SSA was higher in HFmrEF, though not significant. On the contrary, it was most frequently associated with HFpEF in some studies from high-income countries.15, 17 Our patients with HFpEF were more often female, a finding that is in accordance with some studies from high-income countries.15, 17 Clinical presentation with respect to symptoms and signs was similar among groups, except for participants with HFpEF who had a higher mean systolic blood pressure and a lower heart rate. Also, HFrEF patients had more severe oedema compared with the other HF categories. A recent study in Europe did not show any differences in signs and symptoms between the three categories of HF.17 When comparing echocardiographic parameters, patients with HFrEF had larger heart chambers and lower wall thicknesses than those with HFmrEF and HFpEF. This was a similar finding in other studies.17
In our study, there was no significant age difference between the three categories of HF. This is in contrast with some studies that reported a significantly older age in HFmrEF compared with HFrEF.17 Generally, HF occurs at a younger age in SSA compared with high-income countries. The mean age of HFmrEF in this study was 54.6 years, lower than reported in developed countries.14, 22 In this report, the mean ages across all EF categories were lower than the mean age across corresponding EF categories in high-income countries.14 This may reflect differences in HF aetiologies between low-income and high-income countries.
In the current analysis, patients with HFrEF were more likely prescribed inhibitors of the renin–angiotensin system and mineralocorticoid receptor antagonists (MRAs) compared with patients with HFmrEF and HFpEF. This is not surprising as several studies have shown that patients with HFrEF are more likely to be prescribed guideline-directed medical treatment.15 MRAs were commonly prescribed in the THESUS-HF registry. The impact of MRA on outcome in HFmrEF is less well known. A recent study showed that the MRA spironolactone was independently associated with a significant reduction in mortality and HF rehospitalization in HFmrEF.22 Although patients with HFpEF in our study had lower heart rates, they were more likely to be prescribed beta-blockers. This finding is in contrast with the guideline suggested treatment for patients with HF where the use of beta-blockers is mandated in patients with HFrEF regardless of their EF. Finally, many more patients in the current registry regardless of EF were prescribed digoxin.
Patients with HFmrEF had a non-significant longer mean hospitalization duration compared with HFrEF and HFpEF. A total of 4% of the participants experienced the endpoint of death on initial hospitalization without any significant difference between groups of HFrEF, HFmrEF, and HFpEF although it was higher in patients with HFrEF. During the follow-up period of 6 months, there was no statistically significant difference in the short-term outcomes between the groups of HFrEF, HFmrEF, and HFpEF. This was in accordance with findings by some authors in high-income countries.17, 23 Previous studies in patients with stable, chronic HF have described an association between outcome and LVEF.17, 23
Strengths and limitations
Prospective clinical cohort studies in the African setting are challenging with a lot of pitfalls including lack of infrastructure, limited funding and resources, and high rates of loss to follow-up, but it is the only way to characterize real-world African patients who may present with features that are not similar to what has been described in high-income countries. Our results are of relevance and generate data to describe the new phenotype, HFmrEF, in SSA, which deserves further investigation and may be of clinical importance.
The advantages of the present study include that all HF cases were rigorously diagnosed and followed up in a large hospital-based registry with multiple centres in Africa. All AHF cases underwent a systematic and comprehensive echocardiogram evaluation by certified cardiologists. Although this was a post hoc analysis of the THESUS-HF registry and HFmrEF was not predefined, the comprehensive cardiologist echocardiographic evaluation of our population permitted the assessment of criteria of diastolic function to diagnose HFpEF rather than relying only on the incomplete criterion ‘lack of an echocardiogram showing a low LVEF’.
Some limitations are noteworthy. First, natriuretic peptides were not available for confirmation of HF in several centres and should be acknowledged as a limitation. A second limitation is that the EF was assessed once close to admission and not longitudinally throughout follow-up, and hence, changes in EF during follow-up were not captured in the current analysis. Finally, the data presented are a post hoc analysis of the THESUS-HF registry, which enrolled patients between 2007 and 2010 and may not be entirely representative of current African patients with HF.
Conclusions
Our study showed that the proportions of HFrEF, HFmrEF, and HFpEF occurrences among SSA hospitalized HF patients were 53.2%, 19.6%, and 27.3%, respectively. The clinical characteristics and the medication prescription of these patients were slightly different, with the new phenotype of HFmrEF sharing intermediate characteristics with HFrEF and HFpEF. Despite the differences in clinical characteristics between patients with AHF in SSA and other regions of the world, the current analysis suggests that similarly to data from other regions, there was no difference in short-term outcomes of patients admitted with AHF by admission EF.
Acknowledgement
The authors thank all the physicians, nurses, and patients who participated in the registry.
Conflict of interest
Drs Cotter and Davison report grants from Abbott Laboratories, Amgen Inc., Celyad, Cirius Therapeutics Inc., Sanofi, Roche Diagnostics Inc., Trevena Inc., Ventrix, and Windtree Therapeutics, Inc. None reported for all other authors.




