Magnetic resonance imaging in heart failure, including coronary imaging: numbers, facts, and challenges
Correspondence to: Marcus Makowski, Department of Radiology, Charité – Universitätsmedizin Berlin, Campus Mitte, Charitéplatz 1, D-10117 Berlin, Germany. Tel: +49(0)30450627345; Fax: +49(0)304507527911.
Email: [email protected]
Abstract
Coronary artery disease (CAD) is a major risk factor for the incidence and progression of heart failure (HF). HF is characterized by a substantial morbidity and mortality and its lifetime risk is estimated at approximately 20% for men and women. As patients are in most cases identified only after developing overt clinical symptoms, detecting early stages of CAD and HF is of paramount importance. Due to its non-invasiveness, excellent soft-tissue contrast, high spatial resolution, and multiparametric nature, cardiovascular magnetic resonance (CMR) imaging has emerged as a promising radiation-free technique to assess a wide range of cardiovascular diseases such as CAD or HF, enabling a comprehensive evaluation of myocardial anatomy, regional and global function, and viability with the additional benefit of in vivo tissue characterization. CMR has the potential to enhance our understanding of coronary atherosclerosis and the aetiology of HF on functional and biological levels, to identify patients at risk for CAD or HF, and to enable individualized patient management and improved outcomes. Even though larger-scale studies on the different applications of CMR for the assessment of heart failure are scarce, recent research highlighted new possible clinical applications for CMR in the evaluation of CAD and HF.
Introduction
Coronary artery disease (CAD) is the most common cause of heart failure (HF) and the leading cause of death and disability in both developed and developing countries, accounting for >17 million deaths per year.2, 3 HF has a mortality of up to 50% within 5 years and is associated with an additional substantial socio-economic burden.4 Despite the advances in diagnostics and treatments over the past decades, acute cardiovascular events remain unpredictable and are often the first manifestation of underlying coronary atherosclerosis.5 Plaque assessment provides a powerful diagnostic information on the probability of individual cardiac events, having the potential of modulating and maybe even preventing early stages of coronary atherosclerosis.6
HF, which often is the consequence of CAD but can also evolve from non-ischaemic origin, is a clinical syndrome referring to the inability of the heart to fill or eject blood.7, 8 It can be differentiated between clinical presentations with reduced left ventricular ejection fraction (≤ 40%; HFrEF), with preserved ejection fraction (LVEF >50%; HFpEF), and with mid-range ejection fraction (40-49%, HFmrEF).4, 8 Since patients are currently only identified, when they already have clinical symptoms, detecting early stages of HF is of paramount importance.
Due to its non-invasiveness, excellent soft-tissue contrast, high spatial resolution, and multiparametric nature, cardiovascular magnetic resonance (CMR) has emerged as a promising radiation-free technique to assess a wide range of cardiovascular diseases such as CAD or HF.9 It enables a comprehensive evaluation of myocardial anatomy, regional and global function, and viability with the additional benefit of in vivo tissue characterization,10, 11 providing information about acute tissue injury such as oedema or necrosis and myocardial perfusion deficits, and can also be used to predict the necessity of revascularization in patients with suspected CAD.12, 13 In non-ischaemic HF, CMR enables the assessment of fibrosis, infiltration, and iron overload. Up to now, computed tomography (CT) is the modality of choice for the assessment of CAD. CMR plays a central role in the diagnosis of HF such as in the assessment of ventricular global and regional dysfunction, the visualization of injured myocardium and its transmural extent,13 or the evaluation of the underlying aetiology, and provides insights into disease progression.14 CMR techniques might not only enhance our understanding of the pathophysiology of coronary plaque formation and of the aetiology of dysfunction in HF, but also improve individual risk assessment, and they guide therapeutic interventions in individuals at highest risk for acute cardiovascular events and chronic HF.15, 16
This editorial focuses on the current knowledge, challenges, and future prospects for CMR in imaging HF and coronary atherosclerosis.
Numbers
CAD is a leading cause of death, leading to ~20% of deaths in the European Union and accounting for one in every seven deaths in the USA.17, 18 The lifetime risk of developing HF is estimated at ~20% for men and women,1 and HF is also characterized by substantial morbidity and mortality. Timely diagnosis and effective management of CAD and HF are, therefore, of high importance. In recent years, magnetic resonance imaging (MRI) and CT have emerged as the most promising non-invasive imaging techniques in the primary diagnosis of CAD and HF. While a meta-analysis published in 2010 suggested that CT was superior to MRI, recent studies directly comparing CT and MRI or X-ray coronary angiography and MRI indicated that there was no significant difference when assessing presence or absence of >50% coronary artery stenosis.19-22 For the visualization of myocardial ischaemia in HF, CMR was demonstrated to be superior to single-photon emission CT in a large prospective trial with 752 recruited patients.9 The establishment of T1, T2, and T2* mapping sequences provides a unique possibility to characterize myocardial tissue and also to determine the aetiology of non-ischaemic causes of HF.23
To date, larger-scale studies on the different applications of CMR for the assessment of HF are scarce (Table 1). In a study published in 2015, Bohnen et al. demonstrated that CMR T2 mapping enables a more accurate assessment of active myocarditis in patients with recent-onset HF with a sensitivity of 94%.24 For HFpEF, a recent study by Dusch et al. including 80 patients who were subjected to CMR within 6 months after transthoracic echocardiography showed that diastolic dysfunction may be reliably identified by CMR by the use of midwall longitudinal fractional shortening as a correlate for long-axis systolic function.25 Another recent study by Rommel et al.26 found that CMR T1 mapping can reliably quantify diffuse myocardial fibrosis as extracellular volume fraction, which can be used as a reliable predictor of left ventricular stiffness (β = 0.75; P < 0.01).
The treatment of HF also depends on the underlying cause of the disease.27 To differentiate, if HF is related to CAD, coronary angiography is routinely performed at many centres. McCrohon et al. demonstrated in 90 patients and 15 control subjects (0% vs. 100% enhancement) that gadolinium-enhanced CMR enables a reliable non-invasive differentiation between HF related to dilated cardiomyopathy and HF resulting from CAD.27 Furthermore, they calculated that 13% of the patients would have received an incorrect diagnosis of dilated cardiomyopathy if they had not received CMR.27
With regard to a comparison of echocardiography, radionuclide ventriculography, and CMR, Bellenger et al. examined 52 patients and concluded that CMR should be the preferred technique for the estimation of volume and ejection fraction in HF patients.28
| Type of heart failure | Study | Patients | Main findings |
|---|---|---|---|
| Recent-onset heart failure with active myocarditis | Bohnen et al. 201524 | 31 | Cardiovascular magnetic resonance (CMR) T2 mapping enables a more accurate assessment of active myocarditis with a sensitivity of 94% |
| Diastolic heart failure | Dusch et al. 201425 | 80 | Transthoracic echocardiography-evidenced diastolic dysfunction can be reliably identified by CMR with use of midwall longitudinal fractional shortening |
| Heart failure with preserved ejection fraction | Rommel et al. 201626 | 24 | CMR T1 mapping can correctly assess myocardial fibrosis, which independently predicts invasively measured stiffness of the left ventricle |
| Differentiation of heart failure related to dilated cardiomyopathy or coronary artery disease | McCrohon et al. 200327 | 90 | Contrast-enhanced CMR enables a differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease |
| Comparison of ejection fraction measurements in patients with heart failure using different modalities (echocardiography, radionuclide ventriculography, and CMR) | Bellenger et al. 200028 | 52 | CMR is the preferred technique for volume and ejection fraction estimation in heart failure patients |
Facts
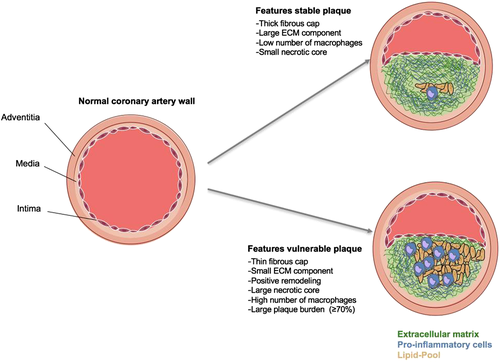
Typically, CAD occurs when the coronary arteries develop atherosclerosis. Atherosclerotic lesions can cause progressive vascular remodelling, which may result in sudden plaque rupture. While atherosclerotic lesions with a low-risk of rupture are linked to a thick fibrotic cap and a high proportion of fibrotic tissue, plaques that rupture often show a thin fibrous cap, a necrotic core, positive remodelling, inflammation, angiogenesis, and plaque haemorrhage.15 The identification of such vulnerable plaques can help in identifying patients with an active state of disease who are at an increased risk of acute cardiovascular events.29 Due to its superior soft-tissue contrast, CMR has the potential to provide detailed imaging of the coronary vessel wall.
Plaque assessment with non-contrast-enhanced and contrast-enhanced magnetic resonance imaging
With non-contrast-enhanced MRI, direct assessment of the coronary vessel wall, remodelling, and plaque instability have been demonstrated in patients with subclinical or stable CAD, type 1 diabetes, and hyper-IgE syndrome and in an asymptomatic multiethnic population cohort.29-34 A recent study investigating 568 stable patients demonstrated a significantly higher risk of adverse cardiac events for patients with high-intensity coronary plaques on T1-weighted images (26% vs. 3%).29 More recent advances have led to the development of targeted contrast agents, aimed at cell surface receptors or proteins. Fibrin and elastin represent an essential component of atherosclerotic plaques, play a key role during their development and progression, and have already been established as promising targets.35-37 With targeted molecular probes, contrast-enhanced CMR can visualize and quantify proteins and cells of the atherosclerotic vessel wall, which, given clinical translation is successful, may improve assessment of vulnerable plaques and allow for better-tailored invasive and non-invasive therapies (Figure 1).
Heart failure with reduced ejection fraction
Evaluation of cardiomyopathy with reduced ejection fraction by CMR is usually performed through a pharmacologic stress test, whereby information on wall motion, rest and stress myocardial perfusion, and delayed gadolinium enhancement are collected.38 Apart from diagnosing ischaemia, CMR stress test results have been demonstrated to provide accurate estimates of cardiovascular prognosis.39 For example, Steel et al. showed the prognostic implication and usefulness of stress CMR myocardial perfusion and late gadolinium enhancement in a population of 254 patients, whereby patients with neither perfusion deficits nor late gadolinium enhancement had a 98.1% negative annual event rate for death and myocardial infarction.40
Heart failure with preserved ejection fraction
Approximately half of HF patients have abnormalities in diastolic function with preserved LVEF.41 Even though transthoracic echocardiography is currently the modality of choice for the diagnosis of diastolic dysfunction, this method is prone to relatively poor acoustic windows, a limited field of view, calculation errors relative to flow direction, and inferior spatial resolution.42
Another common cause of HFpEF is cardiac hypertrophy. By use of CMR, important information on wall thickness and location of asymmetric hypertrophy can be obtained, and it is also useful in distinguishing HF linked to hypertensive heart disease from hypertrophic cardiomyopathy. Based on CMR, Maron et al. identified mitral valve abnormalities such as elongation of mitral valve leaflets as a new biomarker for assessment of hypertrophic cardiomyopathy.43
Challenges
CMR of coronary arteries has several advantages over CT, showing a superior soft-tissue contrast for the visualization of plaque morphology, not being affected by calcium blooming, and most importantly not involving ionizing radiation, which add to its attractiveness as a screening tool.15 However, there are still technical challenges regarding cardiac motion and spatial resolution, as the heart shows a rapid movement with the cardiac and breathing cycle. With its relatively long scanning and acquisition times, these requirements are especially challenging for CMR. The development and validation of new CMR techniques, for example, for quantification of fibrosis, plaque characterization, and CMR-targeted intervention, represent the leading research challenges for the future. From a clinical perspective, another important challenge is to increase the availability of the modality for patients.
Outlook
CMR has made great advances in the past decade, with a significant reduction in imaging time, and holds the promise as a non-invasive and radiation-free multifaceted assessment of coronary atherosclerosis and HF with information on disease activity and prognosis. Especially with regard to the evaluation of HF patients, it is expected that the applications of CMR will expand rapidly. New techniques aiming at the identification and quantification of diffuse fibrosis will improve the in vivo assessment of pathology, novel contrast agents will enable to target specific tissue types for both diagnosis and treatment, and interventional CMR will open up guidance of therapeutic procedures.14 CMR has the potential to enhance our understanding of coronary atherosclerosis and HF on functional and biological levels, to identify patients at risk for heart disease, and to enable individualized patient management and improved outcomes.
Conflict of interest
None declared.




