Recent Advances on Polyoxometalate-Based Ion-Conducting Electrolytes for Energy-Related Devices
Abstract
Solid-state electrolytes have attracted considerable attention in new energy-related devices due to their high safety and broad application platform. Polyoxometalates (POMs) are a kind of molecular-level cluster compounds with unique structures. In recent years, owing to their abundant physicochemical properties (including high ionic conductivity and reversible redox activity), POMs have shown great potential in becoming a new generation of solid-state electrolytes. In this review, an overview is investigated about how POMs have evolved as ion-conducting materials from basic research to novel solid-state electrolytes in energy devices. First, some expressive POM-based ion-conducting materials in recent years are introduced and classified, mainly inspecting their structural and functional relationship. After that, it is further focused on the application of these ion-conducting electrolytes in the fields of proton exchange membranes, supercapacitors, and ion batteries. In addition, some properties of POMs (such as inherent dimension, capable of forming stable hydrogen bonds, and reversible bonding to water molecules) enable these functional POM-based electrolytes to be employed in innovative applications such as ion selection, humidity sensing, and smart materials. Finally, some fundamental recommendations are given on the current opportunities and challenges of POM-based ion-conducting electrolytes.
1 Introduction
With the development of printed electronics and wearable electronics, light-weight, small-sized, and flexible energy systems face huge opportunities.[1, 2] Solid-state electrolytes combine the advantages of small size and high density, while avoiding the leakage problem of traditional liquid electrolytes in the common clean energy equipment.[3-7] Yet, the low ion conductivity and slow dynamics of solid-state electrolytes have been considered as the disadvantages to restrict their practical application.[8] Many solid-state conducting materials (such as covalent organic frameworks,[9-11] metal–organic frameworks,[9, 12-15] porous aromatic frameworks,[16] Nafion,[17, 18] and various oxides[19]) with high ionic conductivity have been developed to overcome this problem.[20]
Polyoxometalates (POMs) have fascinating and variety of structures formed by connecting multiple pre-transition metal oxygen clusters (mainly containing V, Nb, Ta, Mo, W…) through shared oxygen atoms.[21, 22] Ion conduction can occur in most POMs due to the hydrophilic properties and the rich terminal oxygen on their surface. Besides, some redox-active POMs allow the storage of electrons along with the ion transfer, broadening the application of POMs in energy conversion and storage.[23-25] This makes POMs exhibiting plentiful physical and chemical properties different from the mononuclear oxides. The design and synthesis of novel functional POMs are still being the engine to maintain the high level of research and development of POMs chemistry, which greatly broadens their application fields such as ion conduction, capacitor, catalysis, photoelectromagnetic functional materials, and biomedical chemistry.[26-30] Among them, the ion conduction (especially proton conduction) allows POMs to be good candidates for the next generation of excellent solid-state conducting materials.[31, 32]
Proton conduction, as a common ion conduction, is widely used in proton exchange membrane fuel cells and sensors. Hydrated POMs have the highest proton conductivity among inorganic solid proton conductors at room temperature (such as 27 mS cm−1 for H4SiW12O40•28H2O),[33] which is ascribed to the abundant water clusters on the surface.[34, 35] Such a high hydrophilic pathway leads to a "quasi-liquid" state and rapid proton transport in hydrated POMs.[36, 37] Protons are transported through the breaking and reorganization of hydrogen bonds (Grotthuss mechanism) or with the assistant of proton carrier (Vehicle mechanism, such as H3O+, H5O2+, and NH4+).[38] Additionally, aprotic POMs have also shown the possibility of becoming ion-conducting materials (such as Li+, Na+, K+), in which the ordered structure of POMs constructs free volume and channel to help ion storage and transportation, as well as enhances the mechanical stability of the composite electrolyte.[39] While serving as the ion source, these POMs contain abundant terminal oxygens on surface, which are regarded as Lewis base and used as the adsorption and transporting site of alkali metal ions,[40] thus enriching the ion conduction network in solid-state electrolytes. However, there are few reports about POMs as the principal electrolyte, especially as the only ion source.
Various physical and chemical properties, such as good phase compatibility, easy processability, and structural controllability, allow POMs to display more applicable space in energy-related devices. For example, POMs with inherent dimensional characteristics enable themselves as space limiters to achieve specific ion selection in solid-state electrolyte.[41, 42] POMs display reversible proton conductivity as the humidity changes,[43, 44] for which the reversible bonding to water molecules makes POMs as humidity sensing materials.[45] In addition, POMs are easy to form stable hydrogen bond with various hydrophilic materials, which enables the development and application of POM-based solid electrolytes as smart materials.[46-48] These structure–property interaction and controllability have enabled the development and application of POM-based solid-state electrolytes in various clean energy devices and various functional devices due to their tunable versatility. However, as mentioned in many reports, there are still some scientific problems in POM-based ion-conducting electrolytes, such as their unclear structure–function relationship, high dependence on humidity, and leakage from composite materials under high relative humidity. Therefore, it is necessary to make a phased summary of these problems that hinder the progress of POM-based solid electrolytes.
In this review, we first reviewed the design and synthesis of function-oriented POM-based conducting materials and polymer electrolytes in recent years. Inspired by these principles, some opinions are summarized on the subsequent synthesis of POMs in ion-conducting materials. Furthermore, we classified, analyzed, and summarized the reports of the energy equipment (represented by proton exchange membrane fuel cells, solid-state supercapacitors, and ion batteries) with POM-based solid ion-conducting electrolytes. Then, we analyzed several representative functionalized POM-based solid electrolytes applied in other cutting-edge fields (ion selectivity, smart materials, humidity sensing) in detail. At last, we have given some recommendation for the programs of POM-based solid-state electrolytes in the future, being intended to provide some practical inspiration for future research.
2 Ion Conduction in POM-Based Solid-State Electrolytes
Polyoxometalates with a relatively large size and low charge usually own a minimal relative surface charge, reducing the binding strength between anions and nearby counter-cations.[49] This makes POMs a substitute for various ion-conducting materials. POMs become excellent candidates in the field of ion conduction due to their large solubility in a variety of solvents and easy dispersion in a variety of polymers.
2.1 Crystalline POM Materials
Proton is the lightest and smallest cation, requiring the least energy in transport.[50] Proton conduction is based on two mechanisms, namely the Grotthuss and the Vehicle mechanisms.[51] In the Grotthuss mechanism, the conduction of protons depends on the hydrogen bond network of water molecules, accompanied by hydrogen bond breakage and recombination. In this way, protons "jump" along the conduction path through the protonation and deprotonation of water molecules. The Vehicle mechanism transports protons through the self-diffusion of the proton carrier. Therefore, the Grotthuss mechanism process generally requires a smaller activation energy (0.1–0.4 eV), while the Vehicle mechanism involves the transmission of larger ionic species, leading to a greater activation energy (>0.4 eV).
Solid-state POMs are suitable as proton carriers for their strong Bronsted acidity.[52] The adsorption and dissociation of protons on the POM surface are easily achieved because of the delocalization of negative charges on the surface of polyanions and the M=O double bonds.[27] Further, POMs possess completely hydrophilic oxygen-rich cluster structures and hydrogen bonds are easily formed near the rich abundant oxygen sites.[53] This dynamic process of hydrogen-bonding formation and dissociation is also highly reversible, which is advantageous for proton transport.[54, 55] At the same time, POMs can be assembled to form an ordered structure induced by some cations and polymer frameworks,[56, 57] forming an ordered proton conduction pathway and continuous hydrogen-bonding network, which greatly promotes proton transmission and reduces the activation energy.[58, 59] Under the association of these additives, the water clusters and hydrogen bonds become more stable even at low humidity. This enables POM-based proton-conducting materials to display a suitable proton-conducting performance in a wide range of humidity. In this chapter, we will review the synthesis of new POMs with high proton conductivity in recent years.
Hollow open polyhedral cages, such as the classic Mo132,[60] Mo368,[61] and Mo72Fe30,[62] have attracted much attention in ion/proton conduction due to their high symmetry and high nuclearity.[63, 64] In 2018, Lan et al. synthesized different hollow structures in Mo132 and found that the imidazole-loaded Mo132 single-crystal sample contained more proton sources and offered a high proton conductivity of 4.98 × 10−2 S cm−1.[65] In 2020, Lan continued to report a case of a giant polymolybdate cage with 240 nuclei, composing tripod-shaped [Mo6O22(SO3)]n−/[Mo6O21(SO4)]n− building blocks with three connected vertices and 30 cubane-type [Mo4O16]n− edge building blocks (Figure 1a).[66] This is the highest nuclear cavity reported to date in a dodecahedral cage with a large inner cavity (maximum diameter of 1.8 nm) and 12 open pentagonal windows. Interestingly, this cage exhibited excellent stability in solution after the formation of tetrabutylammonium salt. In addition, a bulk sample of this compound has an ultra-high proton transfer rate of 1.03 × 10−1 S cm-1 at 80°C and 98% RH (RH: relative humidity), the highest one among polyoxometalate-based crystalline proton conductors. This sets a precedent for further exploration of more solution properties for the application of such compounds.
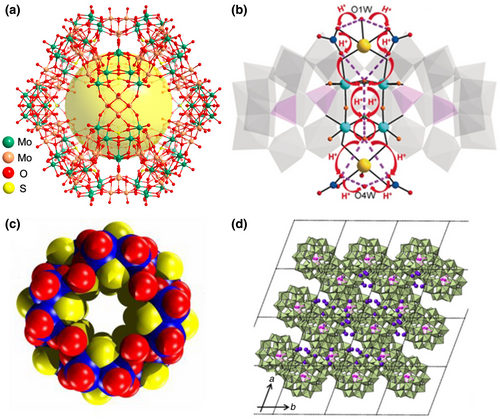
The host–guest engineering design used to encapsulate countless molecular guests has recently dominated the proton-conducting chemistry,[69, 70] in which small molecules H3PO4,[71, 72] H2SO4,[73] imidazole,[74-76] and phytic acid,[77] are frequently used. Khashab et al. embedded nano-scale 16-AlIII-32-oxo cluster units (Figure 1b) into P8W48 archetype polyanions by means of inorganic host–guest self-assembly, {[Na(NO3)(H2O)]4[Al16(OH)24(H2O)8(P8W48O184)]}16- and its GaIII analogue, [Ga16(OH)32(P8W48O184)]24−,[78] representing the discrete components containing the largest number of Al/Ga ions found in POM chemistry. It showed an excellent proton conductivity of 4.5 × 10−2 S cm−1 (85°C, 70% RH), which was the highest proton conductivity of the POM-based single-crystal proton conductor reported at that time. The presence of Lewis acid–Lewis base pairs and the nitrate-facilitated hydrogen-bonding network was the cause of high proton conductivity in {[Na(NO3)(H2O)]4[Al16(OH)24(H2O)8(P8W48O184)]}16−. The synergistic effect of the abundant Lewis acid–base pairs and the acid nitrate function in the crystal is largely conducive to proton conduction. This host–guest self-assembly method provides a novel synthetic method for multi-level inorganic crystalline materials.
In traditional POM systems, tetrahedral ions, such as PO43−, SO42−, and AsO43−, are typically used as structural linkers.[79, 80] In 2004, incorporation of pyramidal geometry anions such as SeO32−, IO3−, TeO32− was put forward by Cronin et al. to lead the self-assembly process toward the formation of complex architecture.[81] Relying on this principle, a lot of POM-based proton conductor materials with ordered hydrophilic pathways were designed and successfully synthesized.[82-84] Typically, [Mo2O2S2]2+ cation and selenite anion were used to synthesize the first case of thiometalate proton-conducting cluster (Figure 1c).[85] The {Mo16} ring showed a super-proton conductivity of over 10−2 S cm−1 under high relative humidity at 55°C. Such high proton conductivity is attributed to the abundant O sites on the surface of the {Mo16} unit, and the high-water absorption under high relative humidity. This indicates that the proton conductivity of selenite-based oxothiometalate species makes it a promising alternative material for fuel cell applications.
Selecting different counter-cations to balance the charge of POM anions can weaken the binding force on protons, enhancing the proton conductivity.[86] In 2019, Uchida reported a Preyssler-type polyoxometalate ([Bi(H2O)P5W30O110]12-) composites (Figure 1d), K8H4[Bi(H2O)P5W30O110]•0.03PAA5000•19H2O,[87] which was crystallized from aqueous solutions together with potassium ions and poly(allylamine) (PAA) as a good proton conductor. They showed that proton migration was promoted by reducing the electrostatic interaction between POM and protons. PAA was selected because the amine group helped to increase the number of protonation sites and expand the hydrogen bond network. When PAA was positively charged by protonation, the electrostatic interaction with POMs would improve structural stability. All compounds synthesized from polyallylamine with different molecular weight showed a proton conductivity of over 10−2 S cm−1 at moderate humidity and under low temperature conditions. Highly proton conductive but hygroscopic POMs and amine polymers can achieve a stable existence by electrostatic interaction in the solid state, which launches a constructive study for the application of POM-based proton-conducting materials under low humidity or even non-humidity conditions.
2.2 Non-Crystalline POM Materials
Most newly synthesized POMs have shown excellent proton conductivity, but both their high solubility in water and instability at higher temperatures have hindered their practical application. So, some safer strategies have an obligation to prepare POM-based solid-state electrolytes in a larger application platform. At the same time, most of the reported conducting performance can only be achieved under high humidity, which limits their practical applications under harsh conditions. In this part, we have summarized several effective ways to address these problems, including the construction of covalent bonds, through the electrostatic attraction, stronger hydrogen bonds, and the introduction of moisture-retaining polymer in solid-state electrolytes.[88, 89]
The anion replacement method between the cationic framework and the POM anion is implemented to prepare the POM-based proton-conducting materials.[90] This methodology endowed the composite with strong Coulomb interaction and increased quantity of the transport medium.[91] In 2016, the first proton-conducting covalent organic framework–POMs (EB-COF: PW12) was designed and synthesized by combining a cationic covalent organic framework (EB-COF:X, X = F, Cl, Br, I) and PW12O403- (Figure 2a).[92] This is the first time that a stable cationic crystal framework allows the production of a series of COFs charged through an ion exchange process. The exchange of extra framework ions can well adjust the porosity and nanometer-sized pore size of COFs. More importantly, the ordered channels can provide a lot of free space for the transport of protons and the positive charge of the skeleton accelerates the dissociation of protons on the surface of POMs, greatly improving the proton conductivity. Among the reported porous organic materials, EB-COF: PW12 shows the best proton conductivity at room temperature.
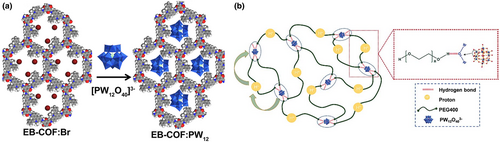
Polyoxometalates–polymer electrolytes tend to show high proton conductivity because POMs easily form a stable hydrogen bond network with some hydrophilic polymers. In 2001, Wu et al. reported H4GeW12O40-PVA polymer electrolytes with a GeW12 content of 80%, which has the high conductivity of 2.11 × 10−2 S cm−1 at 20°C.[93] In 2006, Honma et al. reported heteropolyacid-encapsulated self-assembled materials (H4PW12O40 and polystyrene sulfonic acid) with a conductivity of 1 × 10−2 S cm−1 at 180°C when the H4PW12O40 content was 10%.[94] In these reports, the migration of protons mainly relies on the hydrogen bond network between the polymer and POMs. In 2019, Yin et al. reported that a high concentration of PW12 was dissolved in PEG400 polymer to obtain a semi-solid nanocomposite, PEG400-PW12, which showed a promising proton conductivity under ambient conditions.[95] PW12 clusters form dynamic cross-linked hydrogen bonds with PEG400 through their bridging oxygen ligands. In addition, protons can temporarily bind to O atoms on PEG and be transported along the polymer chain (Figure 2b), which is the novelty of PEG400-PW12 among previously reports. Therefore, protons can successfully jump between hydrogen bonds, and at the same time, the movement of the PEG segment is also crucial for the transport of protons from one PW12 to its neighboring PW12. Thanks to these advantageous conditions, the conductivity of PEG400-70%PW12 reached 1.01 × 10−2 S cm−1 at 80°C without humidity. This work further shows that POM–polymer solid electrolytes prepared using polymers with good interface compatibility and wettability are expected to optimize and adjust the dependence of the proton conduction of POMs on high humidity.
Polyoxometalates-based proton conductor materials often face the problem of leakage under high humidity during practical application. The introduction of counter-cations can effectively overcome this issue through the electrostatic force of positive and negative charges.[96-98] For example, Liu et al. reported a way of using graphene oxide (GO) aminated by ethylenediamine (EN) to absorb H3PW12O40 (PW12) molecules,[99] achieving high proton conductivity above 10−2 S cm−1, while the materials showed low PW12 dissolution. In 2018, our group reported a new type of composite proton-conducting material composed of positively charged niobium hydroxide and PW12 molecules.[100] These studies have shown that the high solubility problem of POMs has been solved to some extent after being complexed with other materials.
Due to their uprising performance in the field of proton conduction and good phase compatibility, POMs can be combined with a variety of polymer materials to prepare various POM-based proton-conducting materials in some energy-related devices. The authors believe that the synthesis and preparation of POM proton-conducting materials need to meet the following requirements in future research and even applications: First, it has an open multi-dimensional structure, providing a lot of space for proton transport; second, an orderly proton transport pathway and a continuous hydrogen bond network; third, abundant acid–base sites that can be used for proton jumping, reducing the electrostatic interaction between POM anions and protons; and fourth, using highly moisturizing polymer species in the POM-based composite materials to enhance the applicability under different working conditions.
3 Energy-Related Devices
3.1 Proton Exchange Membranes
With the use of a large amount of fossil energy, serious environmental pollution is accompanied, and thus, it is urgent to develop efficient clean energy technologies.[101] In the past decades, the proton exchange membrane fuel cells (PEMFCs) have attracted much attention due to their high energy conversion rate and environmental-friendliness.[102-104] The proton exchange membranes (PEMs), as a key component of the electrolyte in transmitting protons and separating the cathode and anode, ensure the successful assembly and efficient operation of the proton exchange membrane fuel cell.[105, 106] POMs exhibit excellent proton-conducting behaviors, which brings the potential application as solid electrolytes for PEMFCs.[107] However, the inherent high water solubility of POMs (gradually leaking from the membrane) is recognized as “the first threshold” to obstruct the adoption of POM-based PEM,[108, 109] which results in a gradually decreasing proton conductivity. Therefore, new methods are needed to improve the stability of POM composite PEM without sacrificing the proton conductivity.[110, 111] To date, several strategies that improve the stability of POMs in PEM have been expanded, such as synthesizing some waterproof POMs and developing some multiple POM composite materials with high proton conductivity.
The formation of covalent bonds with POMs (C-O-W/Mo, C≡N-W, Nb-O-B) can directionally assemble orderly 3D nanochannels that can store large amounts of free water,[112, 113] which is an effective strategy to prevent the leaching of POMs. Yin et al. reported a highly water-retentive PEM composed of ferrocyano-coordinated poly(4-vinylpyridine) (CP4VP), H3PW12O40 (PWA), and polysulfone (PSf) under a strong magnetic field (Figure 3a).[114, 115] In particular, a microporous water-insoluble but highly hydrophilic and proton conductive Prussian blue analogue (PBA) framework with the new type of Fe-C≡N-W bonding was formed by H3PW12O40 and CP4VP, in which a ~5.4 Å hydrophilic micropore existed. This 5.4 Å channel was between the diameter of water molecule (2.8 Å) and the distance from the edge of the first hydration shell to the proton conductor (6–6.5 Å).[17, 116-118] This approved distance enabled larger amounts of non-freezable water in PBA framework, giving a better water retention at low relative humidity and elevated temperatures in PEM. Through the formation of water-insoluble PBA, the leaching of H3PW12O40 in the membrane was rejected. Such a strong H3PW12O40 retention in the membrane represents a major advancement in the manufacturing strategy beyond the typical PEM system based on POM–polymer interactions.
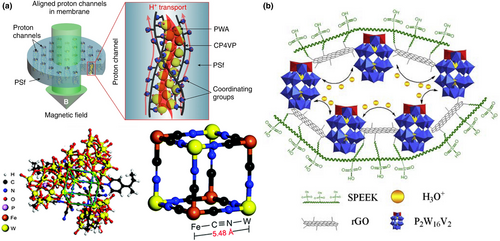
Achieving mutual cross-linking between multiple components can enhance the water absorption of the proton exchange membrane and improve the water retention capacity under low humidity. The cross-linked multi-dimensional network helps to resolve the problem that POMs are easily soluble in water, and enhances the mechanical stability of the POM matrix proton exchange membrane.[119] Wu et al. reported a new type of organic–inorganic composite membrane rGO-P2W16V2@SPEEK (Figure 3b) (SPEEK is sulfonated poly(ether ether ketone)),[120] in which reduced graphene oxide (rGO) was used as a cross-linking agent to connect the clusters and the organic polymer skeleton. This composite was made into an 86 μm thick film and showed an excellent proton conductivity of 7.90 × 10−2 S cm−1 at 50°C. This was the first report on the application of Dawson-type HPA for PEM. This work expanded the application of polyoxometalate-based materials in the field of fuel cells.
3.2 Supercapacitors and Ion Batteries
In recent years, the demand for flexible and recyclable electronic products has increased, and there is an urgent need to develop diverse energy devices. Supercapacitors and ion batteries using solid-state electrolytes as separators and ionic conductors have caught the attention for their high energy density, energy efficiency, and safety.[121]
At room temperature, polyoxometalates with high ionic (especially proton) conductivity allow POM-based polymer electrolytes to replace traditional H2SO4 or H3PO4 in supercapacitors.[122] POMs have very high solubility in the polymer matrix, which is beneficial to increase the concentration of protons and the number of proton carriers in the polymer electrolyte. Typically, an appropriate amount of POM salt (most utilizing Keggin-type POMs) is dissolved in a selected polymer solution and then coated on a specific plane (or on the electrodes) to form a quasi-solid electrolyte or solid electrolyte, in which ion conduction features a more complicated mechanism than its pure solid counterpart due to a possible difference in the hydrogen-bonding networks.[3]
Polyoxometalates with high conductivity and redox activity are designed as solid-state electrolytes in pseudo-capacitors, in which two common POMs are silicotungstic acid (SiWA, H4SiW12O40) and phosphotungstic acid (PWA, H3PW12O40) as the ionic-conducting filler.[123, 124] In 2009, Lian et al. reported a case of SiWA-PVA (PVA is polyvinyl alcohol) electrolyte with a high proton conductivity of 0.01 S cm-1 at room temperature,[125] successfully applied in RuO2 pseudo-capacitors. The electrochemistry device with the PWA-PVA polymer electrolyte was successfully demonstrated in the RuO2/graphite asymmetric pseudo-capacitor, with a 0.2 mm thick and a 1.5 V operating voltage. From its CV curves, two different capacitive regions are obviously identified (Figure 4a).[126] The first region (at 0–0.7 V) was primarily a double-layer electrode with a capacitance of 2 mF cm−2, and the second region (at 0.7–1.55 V) shows a pseudo-capacitance increasing to about 22 mF cm−2. This proves POMs can function two roles: enabling enough proton conductivity and offering pseudo-capacitance due to the redox reaction of POMs with the active electrode.[127, 128]
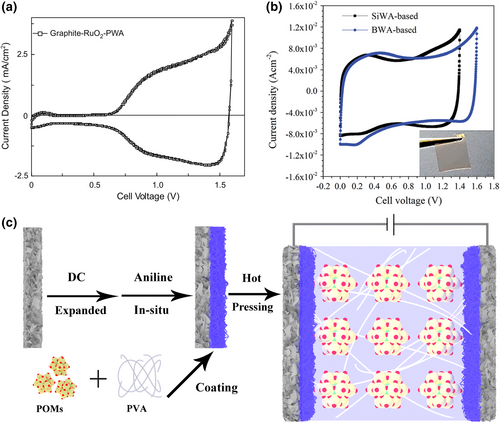
In order to further optimize proton conductivity, SiWA and PWA are combined into a composite electrolyte (SiWA-PWA-PVA polymer electrolyte), showing higher conductivity than its individual components at the same concentration.[129] The solid RuO2 pseudo-capacitor with the SiWA-PWA-PVA polymer electrolyte provided a capacitance of 50 mF cm−2 at 500 mV s−1. Considering the low water retention of POMs under low humidity conditions, a small amount of H3PO4 was introduced to form a ternary compound (SiWA-H3PO4-PVA electrolyte) for replacing the rigid and even brittle POM–polymer binary compound.[130] In addition, solid H3PO4 can be used as a plasticizer and an additional proton donor to promote the retention of water and the thermal stability of the polymer electrolyte.[131]
In 2015 and 2016, Lian et al. continuously developed a BWA (H5BW12O40)-based polymer electrolyte with BWA as the proton donor in supercapacitors, which showed a wider operating voltage than that of SiWA-based capacitors.[132, 133] The electrode potential tracking method was used in liquid BWA and SiWA electrolytes to determine the factors that affect the voltage of supercapacitor, revealing the respective roles of electrodes and electrolytes. This was caused by the redox activity of the Keggin anion. Both have a positive potential of 1.2 V due to oxygen evolution, but the negative potential limit of SiWA was −0.25 V and a negative potential limit of BWA was −0.45 V, producing a 1.65 V potential window, about 200 mV wider than SiWA. The electrochemical potential window can be increased by using B heteroatom in place of Si in POMs because that substitution leads to a greater reduction overpotential. Finally, the solid capacitors using BWA-based polymer electrolyte achieved a voltage of 1.6 V, which was 0.2 V wider than the battery voltage of SiWA-based devices (Figure 4b).
Molybdenum-based polyoxometalates exhibit high redox activity and are commonly used in battery electrode materials and electrocatalysis. Redox behavior of POMs allows multiple electrons to be stored, accompanied by the reversible combination of protons, which is called a proton–electron coupling process.[134, 135] Based on the excellent proton conductivity and redox activity of POMs, our group reported a compound POM–polymer electrolyte applied to a polyaniline (PANI) symmetrical pseudo-capacitor in 2020 (Figure 4c).[136] The assembled capacitor exhibited an area capacitance of 7.69 F cm−2 with an energy density of 0.533 mWh cm−2 (266.6 Wh kg−1) at 0.5 mA cm−2. In order to reveal the matching relationship between the types of POMs and the electrode active materials, the electrochemical performance of PANI electrodes coated with PW12 or PMo12 was tested using a three-electrode system in 1 M H2SO4 solution. The redox potential of PMo12 matched better with PANI than that of PW12, which brought about a greater increase in the capacitance of the PANI electrode. Finally, PW12 with high proton conductivity and PMo12 with high redox activity are both incorporated into the solid polymer electrolyte, showing higher capacitance performance than the individual components. An appropriate match between the electrode and electrolyte voltage can further improve the capacitance performance of the electrode, providing an unprecedented research strategy for developing a pseudo-capacitor with high energy density, which is also applicable to other energy storage devices.
At present, the application of proton-conducting POMs to supercapacitors has shown encouraging performance. Among them, high proton conductivity and suitable redox activity allow POMs to have a potential stage in pseudo-capacitors. However, there are still some researching problems that remain to be solved in this field: POMs will lose proton conductivity due to the loss of crystal water in the solid state, and the specific electrochemical behavior and stability of POMs are unclear in the solid state (whether it is the same as in solution). For the first one, although there are some researches currently seeking effective strategies to solve it, more exploration and research are still required to truly realize the use under actual conditions. The second one is still in its infancy, and suitable POMs still need to be synthesized.
In addition to the above application of POM electrolyte in supercapacitors, POM-based solid-state electrolytes also show satisfactory potential in ion batteries. POMs with different counter-cations (NH4+, Li+, K+, Na+, K+, Cs+…) can be selectively synthesized by replacing synthetic raw materials, which makes POMs promising for various ion conduction media.[137, 138] The solid-state Li+, K+, Na+ ion batteries can offer high energy density, and some batteries using POMs and their derivatives as solid-state electrolytes have also been reported. In 2019, Dong et al. used Li7V15O60(CO3) (LVC) with a hollow structure and rich lithium content to design a polyoxometalate-based polymer electrolyte (PPE) for lithium-ion batteries, with a high ionic conductivity of 9.13 × 10−5 S cm−1 (Figure 5a).[39] The LiFePO4|PPE-15 wt%|Li cells showed excellent rate performance and high specific discharge capacity (Figure 5b). The introduction of rigid framework LVC not only enhances the mechanical strength and thermal stability of the composite electrolyte, forming a good solid network to inhibit the development of lithium dendrites, but also reduces the crystallinity of PEO and increases the free space for Li+ ion conduction. At the same time, the presence of LVC can increase the solubility of movable Li+ ion. This shows the potential of the POM-based Li-ion polymer electrolyte in high-safety solid-state lithium-ion batteries.
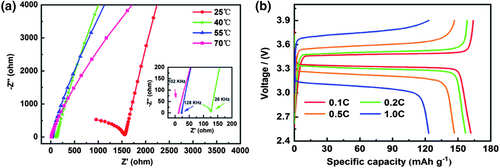
In 2020, Lan et al. added polyoxovanadate to sodium-4-vinylbenzenesulfonate solution to prepare polyoxovanadate–polymer hybrid nanowires (POVs/PSS) by in situ thermal polymerization at 80°C, and fabricated a POVs/PSS solid-state electrolyte after alkali ion exchange, showing a high ionic conductivity (3.30 × 10−3 S cm−1, 2.00 × 10−3 S cm−1, and 4.55 × 10−3 S cm−1 for Li+, Na+, and K+) in Figure 6a.[40] Also, the assembled LiFePO4|Li battery provided a specific capacity of 148 mAh g−1 at 100 mA g−1 at 25°C (104.8 mAh g−1 at 20 mA g−1 for K-ion battery). These admirable properties benefited from the abundant terminal oxygen structure on the POM surface, as a Lewis base to adsorb and transport alkali ion. In addition, the 1D fibrous structure of POVs/PSS nanowires provided effective and continuous ion conduction pathways to reduce interface barriers, giving a significant increase in ion conductivity.
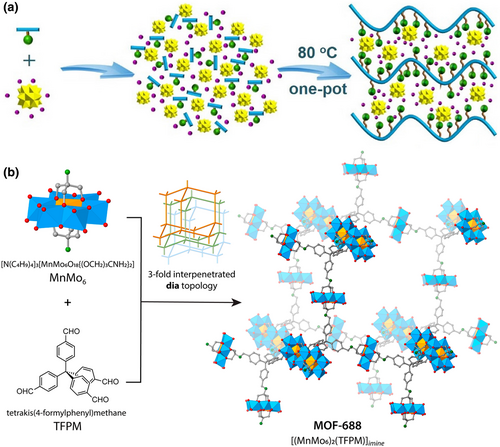
Metal–organic frameworks of organic vertices and polyoxometalate linkers (abbreviated as POMOF) often have open ion transport pathways, in which POM anions and ligands are linked by covalent bonds to obtain an unusually stable structure.[139] In 2019, Yaghi et al. used Li+ ion exchange to realize the possibility of POMOF (MOF-688, Figure 6b) as a solid electrolyte for Li-ion batteries. Ditopic amino-functionalized Anderson-type polyoxometalate [N(C4H9)4]3[MnMo6O18{(OCH2)3CNH2}2] and 4-connected tetrahedral tetrakis(4-formylphenyl)methane (TFPM) were linked through imine condensation for synthesizing MOF-688.[140] After exchanging with Li-salts, MOF-688 showed a high ionic conductivity (3.4 × 10−4 S cm−1 at 20°C), a high lithium-ion transference number (0.87), and low interfacial resistance (353 Ω) against metallic lithium. At room temperature, the lithium metal battery constructed using MOF-688 as the solid-state electrolyte was successfully cycled at a practical current density of ~0.2 C. This strategy immobilizing POM anions in framework materials demonstrates the possibility of using POM materials directly in Li-ion solid electrolytes.
In general, there are not many articles using POMs as solid electrolytes in ion batteries, especially about POMs as the only ion source. The controversial challenge is concentrated on the concentration of activated and movable ions.
4 Functionalized Electrolytes with POMs
As mentioned above, doping POMs would be favorable for the construction of high-performance ion-conducting electrolytes. This strategy provided a fantastic platform merging the advantages of every component. Recently more and more researches have demonstrated that POM-functionalized electrolyte membranes are endowed with many unexpected properties, for example, ion selectivity, humidity sensing, and self-healing, which greatly expanded the application of POMs in multifunctional electronic and energy devices.
4.1 Ion Selectivity
Ion selectivity is of great significance in both biological and energy storage processes. Recently, it has been considered that inorganic materials could form ion selecting channels like protein in nature while exhibiting enhanced stability relative to protein due to their rigid structures. Among them, POMs were the most promising candidates to allow selective transmembrane transport of cation in aqueous environments.[141, 142]
Among these POM macroions, [Mo132: {(Mo)Mo5(linker)30O21(H2O)6}12 {Mo2O4(ligand)}30] are of unique structural features. The architecture presents 20 {Mo9O9} crown-ether-type pores, which can be closed by hydrogen bonding, for example, guanidinium-type cations, while smaller cations such as alkali ones can directly enter the capsule cavity. Interestingly, the {Mo9O9} pores/gates can also show flexibility for the transportation of larger molecules.[143]
On this basis, in 2015, Mahon et al. reported one ion channel of the POM–macroion hybrids: [DODA40(NH4)2{(MoVI)MoVI5O21(H2O)6}12{MoV2O4 (CH3COO)}].[144] Alkali ion transports will take place across bilayer membranes with the hydrophobic acetate-type hybrid (Figure 7a). The ion transport activity of the hybrid follows the sequence Li+ > K+ > Na+ over a large concentration range. In the {Mo132}-type capsules, the diameter of the {Mo9O9} pores demands partial dehydration of Na+ and K+ when entering the capsule, thus resulting in the lower Na+ conduction due to the higher hydration-free energy. Similarly, a fullerene-shaped molecular cluster: {Li48+mK12(OH)m[UO2(O2)(OH)]60(H2O)n} (U60) also shows selective permeability to different alkali ions.[145] This cluster has subnanometer pores caused by the water-ligand-rich surface. The pores block the Rb+ and Cs+ ions and allow the Na+ and K+ ions to enter the cluster (Ka values of these ions with U60 are 1642, 1712, 1944, 2242). When combining with Na+ and K+, the U60 surface pores were more dominant than their solvation forces; therefore, the hydration layer and counterions would be severely damaged during the entrance of the U60 cages, and thus, the system would achieve an overall lower free energy by gaining more entropy.
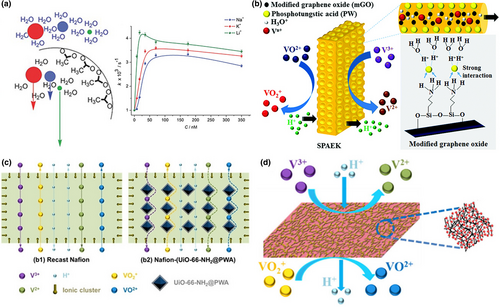
Besides the intrinsic ion selection property, POMs can also improve the ion selectivity of functional electrolytes, especially in the vanadium redox flow battery (VRB) system.[41, 146] The VRB was considered as a promising candidate for large-scale energy storage systems due to the high capacity, quick response, and environment-friendly properties.[147] When the VRB is discharging, the electrochemical reduction and oxidation of vanadium ions will be carried out, while VO2+ was reduced to VO2+ in positive electrolyte and V2+ was oxidized to V3+. But if the cross-mixing of these vanadium ions occurs, non-electrochemical redox reaction will happen; as a result, active species of the VRB will be reduced. So, both the proton conductivity and ion selectivity of the PEM greatly affect efficiency and durability of the VRB system. Unfortunately, the ion selectivity of the commercial Nafion is not good enough to avoid the ion crossover. To address this issue, various strategies have been explored to enhance the ion selectivity of PEM by incorporating inorganic fillers into the polymer matrix. Among them, designing and engineering of POM-based composite PEM was an effective strategy due to that POMs’ high proton conductivity and strong interaction with the PEM could block its polar structure. Sulfonated poly(arylene ether ketone) (SPAEK) membrane shows better ion selectivity than Nafion but still need further modification to meet the requirements for practical applications. In 2017, Aziz et al. reported highly ion-selective membrane consisting of SPAEK and phosphotungstic acid coupled with graphene oxide (PW-mGO) (Figure 7b).[148] The strong interaction between the POMs and the modified graphene blocked the porous structure of SPAEK, thereby partially reducing the vanadium-ion permeability via a tortuous pathway effect. Meanwhile, the combination of the strong acidity of PW and the proton transport pathway provided by GO has resulted in significantly boosting the proton conductivity. Owing to the high stability, proton conductivity (71 mS cm−1), and superior ion selectivity (2.5 × 106 S min cm−3), the membrane has been considered as a promising candidate in replacing the commercial Nafion membrane.
Metal–organic frameworks (MOFs) have also attracted great attention to act as fillers for avoiding ion crossover due to their distinctive features containing adjustable pore structures, tunable void cages, and controllable surface chemistry. In 2019, Yang et al. developed a strategy to fill the PW coupled with UiO-66-NH2 through electrostatic interaction in Nafion, as a result of significant modification on both proton conductivity and ion selectivity (Figure 7c).[149] The Nafion-(UiO-66-NH2@PWA)-3 wt% membranes with optimal doping ratio show high proton conductivity (0.092 S cm−1) and a preferable ion selectivity (2.66 × 105 S min cm−3). As a result, the VRB constructed with the modified Nafion exhibits superior battery performance.
Many nanohybrid filters based on POMs have been reported. However, when introducing inorganic fillers into the PEM, the doping of inorganic composite often leads to agglomeration due to their incompatibility with the polymer matrix. As a result, only low concentration of fillers can be doped into PEMs, which limits the effectiveness of fillers. To address this issue, Yang et al. designed a hydrogen-bonding composite filter with Nano Kevlar Fibers (NKFs) and PW.[150, 151] This membrane was prepared by incorporating the composite into the Nafion matrix via a solution-casting method. Highly favorable compatibility of NKF with the Nafion resulted in the high content of 30 wt% while the membrane retained compatibility (Figure 7d). With such a high content of NKF and PW, proton/vanadium selectivity of the resulting membrane achieved 2.48 × 105 S min cm−3, and retained high proton conductivity at room temperature.
4.2 Humidity Sensor
Conducting polymers such as polyaniline (PANi) or polypyrrole (Ppy) have attracted an extensive theoretical interest and showed potential in humidity sensing technology, but these pure systems cannot avoid the drawback of poor linearity and relatively long response time. Modification of these materials with functional components are in urgent need. POMs have a strong sensitivity to humidity for the easily forming of hydrogen bonds with water. Many current results suggested that proton conductivity of POM-based ion crystals or framework materials was significantly relevant to humidity, which was primary feature of humidity sensor. Owing to the high hydrophilicity and compatibility with conducting polymer, POMs can be used to construct high-performance humidity sensing electrolytes.[152]
In 2010, P. Chithra Lekha et al. reported a humidity sensor based on PW12-doped PANi (P12TPA) with increased conductivity and humidity sensitivity (Figure 8a).[153] Under all humidity conditions, the doping of PW12 significantly improved the conductivity. It was clear that P12TPA showed two-stage sensing responses. In the high RH area, the conductivity of P12TPA showed a higher response to changes in humidity than that of PANi. It was because an uninterrupted trajectory for proton migration formed more hydrogen bond networks among POMs, PANi and water molecule at high humidity, which followed a typical Grotthuss mechanism. As a result, the humidity sensitivity of the composite was significantly improved.
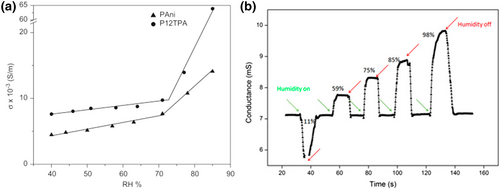
In 2018, Miao et al. reported the fabrication of hybrid polymer film of polypyrrole/H3PMo12O40 (POMs/Ppy) with nanometer grade thicknesses by co-electrodeposition of free pyrrole monomers with POMs.[45] In Figure 8b, the conductivity of POM/Ppy materials increased with increasing relative humidity, which was the typical response properties of the humidity. Owing to the synergistic effect between the doped proton acid and the oxidation doping structure, the sensing response and recovery time were 1.9 s and 1.1 s, respectively, in a 59 nm thick sample with a sensing range of 11%–98% relative humidity.
4.3 Self-Healing
For the more demanding practical device applications, insightful understanding for the strong interaction between POMs and the host material in the hybrid electrolyte system is crucial for achieving the unique property, for example, self-healing feature.
In 2014, Herrmann et al. reported the first example of POM-IL (polyoxometalate-based ionic liquid)-based acid-resistant corrosion protection coating. The POM-IL was synthesized by transition metal-functionalized lacunary Keggin clusters [α-SiW11O39TM(H2O)]n-(TM=CuII, FeIII) and quaternary alkylammonium cations (CnH2n+1)4N+.[47] Besides the acid-resistant corrosion protection, the self-healing property of the POM-IL coating was also discovered. The POM-IL coating could self-repair upon mechanical damage (e.g., scratching) and re-seal the hydrophobic coating and recover their protective properties. As illustrated in Figure 9a, upon scratching, the coating was temporarily removed; while the viscous POM-IL subsequently self-repaired and resealed the scratch, giving the fully repaired surface after near 60 s. The scratching could not permanently damage the coating, and the self-repair process fully recovered the acid protection property.
In 2017, Xu et al. reported a strategy to fabricate water-insoluble material through the aqueous assembly of basic amino acid monomers and silicotungstic acid (SiW).[48] The self-assembly process was composed of abundant hydrogen bonds, which was recognized as the key point in self-healing property. As expecting, the self-healing property of the material was also investigated. The adhesive was first broken by applying a 200% strain through a strain amplitude sweep, which caused the modulus values to diminish drastically. The healing behavior of the adhesive was then monitored over time by continuing to oscillate the adhesive at low (1%) strain amplitude. It was observed that the adhesive recovered its original modulus values within 5 min.
With the increasing demands for developing wearable technologies, self-healing material-based devices including POM-based devices were paid much attention. The integration of self-healing ability into luminescent switches has attracted increasing interest in designing smart optical devices. In 2014, Wei et al. reported the facile preparation of hybrid luminescent hydrogels by assembling triblock copolymers [poly(2-(2-guanidinoethoxy) ethylmethacrylate)-b-poly(ethyleneoxide)-b-poly(2-(2-guanidinoeth oxy)ethyl methacrylate)] (PGn-b-PEO230-b-PGn) with Na9EuW10O36 (EuW10) through electrostatic interactions in aqueous solution.[154] The hydrogel exhibited excellent luminescence and self-healable properties. Specifically, the column-shaped self-standing hydrogel was cut into three parts, and the three parts can be combined together with light pressing in only 3 min. The fast recovery observed in the hybrid hydrogels may be attributed to the disassociation and reformation of the coacervate core arising from quickly responsive and nondirectional nature of electrostatic attraction. In 2018, Yang et al. fabricated the responsive hydrogels containing with [Eu(SiW10MoO39)2]13− (Eu-POMs) and poly(2-acrylamido-2-methyl-1-propanesulfonic acid (PAMPSA),[155] which exhibited self-healing performance without the use of any kind of healing agent. In the damaged situation, the state of hydrogel is between solid and fluid near this critical point, suggesting that the hydrogel behaves as a liquid and solid alternately with a quick recovery with negligible reduction in the two regimes.
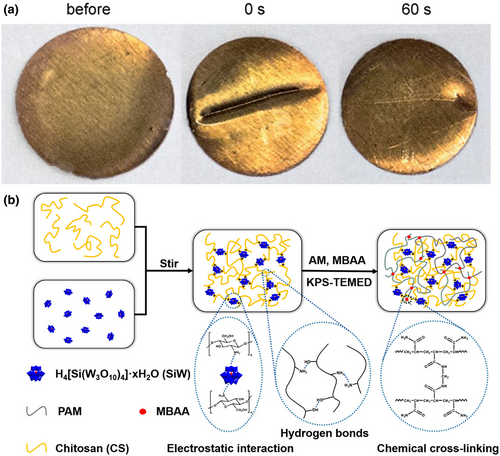
In 2020, Wei et al. developed a simple strategy to obtain chitosan-polyoxometalate-based self-healing conductive hydrogel, which can act as a wearable strain sensor. Chitosan (CS), silicotungstic acid (SiW), and cross-linker were used to fabricate the CS/SiW-PAM [PAM is poly(acrylamide)] hydrogel (Figure 9b).[156] After contacting for 60 s, the self-healing region could withstand stretching without any damage. Further experiment suggested that self-healing behavior was entirely reversible and repeatable during four cyclic measurements. The self-healing properties originated from the connection network fabricated by strong electrostatic interaction and hydrogen bond during the damage process, which would be favorable for reconstruction of the hydrogel after the stress was slowed down.
As mentioned above, the breaking and reorganization of the hydrogen bond network is a prerequisite for self-healing properties and proton conduction performance. In our previous report,[132] the POM gel was first cut into two pieces and then self-healed under 85% relative humidity, providing a same performance for the capacitor as the intact one. It shows that both self-healing and proton conduction are compatible. However, it must be clear that there are few reports on the self-healing properties of POM-based electrolytes with ions other than H+. Therefore, this is a very interesting idea: In POM-based electrolytes (Li+ and K+…), can there be repair properties such as H+ conduction? This will expand the application of POM chemistry on a larger stage. Therefore, we list POM-based self-healing electrolytes as a prospect to promote the development of self-healing devices based on POM-based electrolytes.
5 Summary and Outlook
- Proton conductivity. The proton conductivity of POM-based solid-conducting electrolytes is still a hot topic in POM chemistry, and we hope that more breakthrough work and innovations will be reported in the future. The design and synthesis of POM-based solid-conducting electrolytes with high proton conductivity is critical to the successful assembly of energy devices. The balance between reaction kinetics and ion mobility is essential to achieve optimal conductivity.
- Water retention capacity and sensitivity to the environment. Although POMs have high conductivity at room temperature, it is depended on hydration conditions. When POM-based solid-conducting electrolytes are exposed to low humidity conditions, the bounded water molecules of POMs are easily lost naturally, which may cause a reduced conductivity. Therefore, different hygroscopic materials or plasticizers should be introduced to overcome and improve environmental stability.
- Chemical stability and equipment durability. The strong acidity and high redox activity of POMs limit the use of certain catalysts and metal current collectors in energy equipment. This puts forward new requirements for the selection of inert materials and assembly technology.
Acknowledgements
D.C. and K.L. contributed equally to this work. The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 21871042, 21471028, No. 21671036, No. 21673098, No. 21975211), and the support from the Innovative Research Group Project of NSFC (22021001), the National Key Research and Development Program (2021YFA1502300), Changbai Mountain Scholarship, Natural Science Foundation of Jilin Province (No. 20200201083JC), Natural Science Foundation of Department of Education of Jilin Province (No. JJKH20201169KJ), the Fundamental Research Funds for the Central Universities (20720190035), and Nanqiang Young Top-notch Talent Fellowship in Xiamen University. The project was supported by Open Research Fund of State Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences.
Conflict of Interest
The authors declare no conflict of interest.




