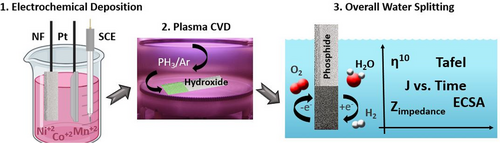
Unveiling the Optimal Interfacial Synergy of Plasma-Modulated Trimetallic Mn-Ni-Co Phosphides: Tailoring Deposition Ratio for Complementary Water Splitting
Abstract
Designing highly active, durable, and nonprecious metal-based bifunctional electrocatalysts for overall water electrolysis is of urgent scientific importance to realize the sustainable hydrogen production, which remains a grand challenge. Herein, an innovative approach is demonstrated to synthesize flower-like 3D homogenous trimetallic Mn, Ni, Co phosphide catalysts directly on nickel foam via electrodeposition followed by plasma phosphidation. The electrochemical activity of the catalysts with varying Mn:Ni:Co ratios is assessed to identify the optimal composition, demonstrating that the equimolar trimetallic phosphide yields an outstanding HER catalytic performance with a current density of 10 mA cm−2 at an ultra-low overpotential of ~14 mV, outperforming the best reported electrocatalysts. This is asserted by the DFT calculations, revealing strong interaction of the metals and the P atom, resulting in enhanced water activation and optimized GH* values for the HER process. Moreover, this optimal composition appreciably catalyzes the OER by exposing more intrinsic active species in-situ formed on the catalyst surface during the OER. Therefore, the Mn1-Ni1-Co1-P-(O)/NF catalyst exhibits a decreased overpotential of ~289 mV at 10 mA cm−2. More importantly, the electrocatalyst sustains perfect durability up to 48 h at a current density of 10 mA cm−2 and continued 5000 cycling stability for both HER and OER. Meanwhile, the assembled MNC-P/NF||MNC-P/NF full water electrolyzer system attains an extremely low cell voltage of 1.48 V at 10 mA cm−2. Significantly, the robust stability of the overall system results in a remarkable current retention of ~96% after a continuous 50-h run. Therefore, this study provides a facile design and a scalable construction of superb bifunctional ternary MNC-phosphide electrocatalysts for efficient electrochemical energy production systems.
1 Introduction
The unprecedented dilemma of the ever-growing shortage of energy resources and resultant ecological concerns, such as massive carbon-emitting fuels and acid rains, put forward an urgent search for sustainable, uncontaminated, and secured energy sources.[1, 2] As an ultimate green energy carrier, hydrogen fuel with efficient, renewable, everlasting, and totally recyclable features has been recommended as a potential candidate to overcome the unusually up-growing fossil fuels depletion.[3-7] Water electrolysis, involving both hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), is widely recognized as a prosperous, most convenient, and effective technology for industrialized H2 fuel production, especially when the electricity is supplied from renewable energy sources.[8-10] However, various associated thermodynamic and kinetic hindrances of inherent upfill HER and OER reactions are the bottlenecks in water electrolysis technology. Higher electrode overpotential is therefore indispensable to initiate the overall water splitting, reducing the overall efficiency.[11, 12] Thereby, the rational design of nonprecious, durable, and active electrocatalysts is mandatory for expediting the sluggish kinetics of both half reactions and thus accelerating the electrocatalytic reaction rates. These remain significant challenges.[6, 13-15] Traditionally, precious-metal-based catalysts (e.g., Pt/C, IrO2, and RuO2) have been recognized as ideal catalysts that could display preeminent electrocatalytic behavior for both HER and OER processes. From the commercialization perspective, the extortionate cost and intense scarcity of such catalysts, along with their limited stability in alkaline medium, could seriously restrain their widespread utilization in electrochemical water electrolyzers.[6, 12, 16-18] Despite the fact that OER and HER electrocatalysts could function well in different electrolytes, implementing similar electrocatalysts in one type of electrolyte is highly preferable for water electrolysis to sustain high reaction efficiency and to overcome the unresolved sluggish kinetics of such processes.[19, 20] Particularly, the overall efficacy of such applications could be prompted in an alkaline medium compared with the acidic counterpart.[20]
As the main objective of water electrolysis systems is to generate pure H2 and O2 gases using more simplified designs that could lessen the energy barrier of the overall process and accelerate the reaction, the development of cost-effective, non-noble, and active electrocatalysts is a crucial necessity.[9, 11, 19, 21] Thus, many studies have extensively investigated the electrochemical behavior of most transition metal-based sulfides, selenides, phosphides, hydroxides, etc. as promising and highly active alternatives to the precious electrocatalysts.[11, 19, 21-23] Specifically, transition metal-containing phosphides have been recently explored as effective and stable bifunctional electrocatalysts that can solve the complexity of achieving high catalytic activity in water electrolyzers.[12, 15, 16, 22, 24-26] Among them, the electrochemical performance of several highly active nonprecious 3D mono- or bimetallic composites of Ni, Co, Fe, and Mo-based phosphides with various morphologies has been recently explored, such as MoP2 nanosheets,[27] CoP nanotubes,[28] Mn-NiP2 nanosheets,[26] flowers-like NiFe,[29] flowers-like NiCoP,[23] CoFeP nanocubes,[30] MoP-Ni2P nanoflowers,[31] FeP microspheres,[32] etc. This is not surprising, as the multielectron orbitals of the P atom have an ensemble effect: P atom strongly bonds with the hydrogen atom, weakening the connection between the transition metal and the corresponding H atom (M-H), thus greatly stimulating the desorption rate of the H2 molecules.[33, 34] Furthermore, it is well-recognized that proton acceptor phosphorous-containing electrocatalysts increase the electronic conductivity of various transition metal-based catalysts for both HER and OER half-reactions. They also impede the corrosion behavior of those TM catalysts, further augmenting their long-term stability, for better performing water electrolyzers.[6, 24, 25] Another important factor is the compositional diversity of those reported electrocatalysts that might explain their exotic electrochemical activity for overall water splitting.[35]
Nevertheless, the electrochemical activity of those reported TM-based-phosphide electrocatalysts is not well-adopted as that of the efficient benchmark noble-electrocatalysts because of stability issues and the limited intrinsic catalytic activity for OER and HER catalysis. As for the bifunctional activity of overall water electrolysis, monometallic cobalt phosphides have shown a significant intrinsic catalytic performance for industrial HER processes along with their good stability in various electrolytes. On the contrary, this scenario usually changes for the OER process with lags in its activity because of some morphological and compositional transformations as well as the inability to well-elucidate the real active sites.[16, 35, 36] On the other hand, while nickel is widely recognized for electrocatalysis applications, especially HER, because of its corrosion resistance and high applicability, the intrinsic activity of nickel phosphides is still limited by the surface atoms type that highly affect the kinetic energy barrier of the HER process.
To this end, the synergetic modulation of both atoms could be an effective pathway to boost the catalytic behavior of the monometallic phosphides. Thus, binary NiCo phosphides-based compounds were known to offer ameliorative characteristics with respect to their high conductivity, enhanced structural and chemical stability, and good electrochemical performance compared with single metal electrodes.[23, 37] Those findings have inspired more researchers to utilize the synergism between more components for tuning the catalytic activity. The proper correlation between the multi-metallic components is of primordial importance to realize high catalytic activity for the water oxidation process. Eventhough, little work has been so far conducted to explore the overall electrochemical performance of quaternary phosphides. Commonly, to enhance the electrochemical performance, two main strategies have been developed for this purpose. Either inserting proper extrinsic metals into the lattice, thus benefiting from the synergistic effect and selectively inducing more active sites, or utilizing well-conductive substrates with high surface area such as carbon paper and nickel foam (NF) supporters.[38-40]
Inspired by the above-mentioned considerations, a series of complementary quaternary MnNiCoP nanoflowers-like electrocatalysts immobilized on NF was inimitably set out with various ratios, using a facile electrodeposition pathway followed by PH3 plasma treatment as bifunctional electrocatalyst for superior water electrolysis. On the one hand, it seems that the incorporation of manganese into the lattice of nickel and cobalt could be a pivotal pathway to modulate the electronic structure of the trimetallic system, thus achieving remarkable electrochemical performance for catalyzing the overall water splitting.[41-43] This enhanced conductivity was further confirmed by the XPS analysis of the fabricated MnNiCo-P nanostructures, which revealed the presence of active species with rich redox chemistry in various oxidation states. In addition, the use of the plasma-enhanced chemical vapor deposition (PECVD) system is an industrially scalable and safe technique, which ensures the homogeneous distribution of the phosphine precursor in the reaction medium.[44] On the other hand, the direct electrodeposition on NF substrate avoided the necessity of using polymer binders, thus maximizing the number of active sites and contributing, to some extent, to the OER activity.[37, 43, 45] Furthermore, the convenient electrodeposition approach could wisely control the electrode thickness and allow for better homogeneity than the other tedious synthesis pathways.
In this study, we have extensively explored the influence of varying the relative ratios of the trimetallic components along with the direct phosphidation of the hybrid catalysts on the real catalytic activity and the reaction kinetics for both HER and OER in alkaline electrolyte. The typical synthesis approach is demonstrated in Figure 1. It is clearly realized that the optimal interaction of the equimolar metal components in the electrodeposition bath as well as the conductivity improvement via phosphorous doping specifically facilitates the charge transfer kinetics and boosts the overall efficiency for water electrolysis. In this regard, the hybrid porous Mn1Ni1Co1-P/NF electrode imparted superior HER and OER performances with η10 of 14 and 289 mV vs. RHE in 1.0 m KOH, with a real MnNiCo- (oxide/oxyhydroxide) species during the OER process. Achieving the most benefit, we probed the bifunctional activity of the synthesized prime catalyst in a full water electrolyzer setup in alkaline KOH solution, revealing superb electrochemical activity with an ultralow η10 of 1.48 V vs. RHE and outstanding chronoamperometric stability up to 50 h with insignificant 4% current density losses.
These experimental findings provided us with a fresh impetus to implement density functional theory (DFT) calculations to rationalize our observations by exploring the effect of P-ions incorporation on the electronic structure of the coupled trimetallic MnNiCo-OH as well as the surface adsorption energies of the intermediates as a good descriptor during HER and OER processes. The rationalized combination of the experimental findings and theoretical predictions is so far indicating the intriguing characteristics of the well-designed porous MnNiCo-P structure as a robust, high-performance, and cost-effective bifunctional electrocatalyst that could maximize the HER and OER catalysis. This study demonstrates the establishment of good electrocatalysts via promoting the optimal metallic synergism and tuning the reaction reactivity for the water electrolysis process and other practical applications.
2 Results and Discussion
2.1 Theoretical Basis
To realize the pertinent surface chemistry, the catalytic performance was probed via DFT calculations to unravel how the characteristic phosphorous ions and the electronic interaction between the metallic constituents influence the catalytic activity of the composite. The DFT calculations enabled the estimation of ΔGH*. The details of the calculations based on the standard Cambridge Serial Total Energy Package (CASTEP) as implemented in Materials Studio version 2017 are presented in the supporting information.[46] The Sabatier principle assumes that, for optimum activity, H binding should not be too strong or too weak. Having it approximately thermo-neutral minimizes the energy barrier and hence improves the rate of reaction.[34] We did geometry optimization to reach minimum energy configurations of H adsorbed at different sites on the (0001) surface of MnNiCoP to study their activity beyond hydrogen evolution reaction (HER). This crystallographic plane is related to the surface of the catalyst used experimentally. As demonstrated in Figure 2a, MnNiCoP exhibits typical metallic nature with zero bandgap, which is favorable for efficient electron transport in electrocatalysis. The Total DOS and the corresponding projected DOS are depicted in Figure S1 and Figure 2b, respectively. Based on Figure 2b, the unoccupied 3d orbitals (unfilled region) promote strong orbital with the HOMO orbital of the H2O molecule. Therefore, smaller DOS was observed, which preferred the valence and conduction bands coupling, resulting in excess interband electrons transfer that further promote the HER and the OER kinetics.
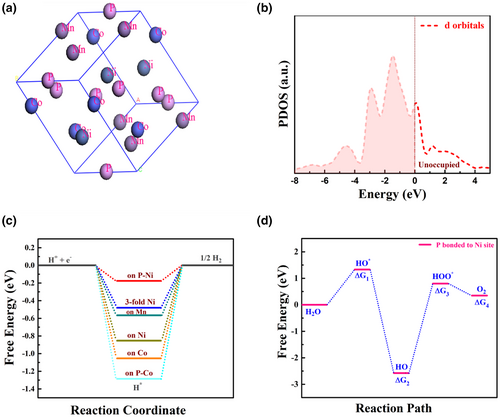
Considering the HER mechanism, HER generally involves three steps: starting with H+ + e−, followed by an intermediate state, and ending with ½ H2 product.[47] To determine the active sites, we calculated Gibbs free energy for six representative different possible sites as shown in Figure 2c, to further assess the electrocatalytic activity of the reaction in depth. Note that the top of P bonded to Ni site of MnNiCoP is the optimum site for hydrogen adsorption with ∆GH* of −0.17 eV, which is almost thermoneutral, even comparable to that of the Pt (111) surface, thereby revealing superior HER catalytic behavior.[23, 34] Moreover, the second preferential site for the H-adsorption is 3-fold with Ni atoms, in agreement with previous studies.[34] Meanwhile, the third favorable site for H-adsorption is the top of Mn site of the MnNiCoP electrocatalyst. Such catalytic activity of the MnNiCoP can be attributed to the excess favorable sites available on its surface and the contest between Mn and 3-fold Ni adsorption sites that are beneficial for boosting the HER activity. Accordingly, the alkaline HER mechanism on the MnNiCoP catalyst was schematically investigated as depicted in Figure S2. The whole process is summarized as follows: HER begins with the adsorption of H2O molecules followed by OH bond-breaking via Volmer reaction (discharge reaction). Then, the adsorbed hydrogen combines with another adsorbed H2O molecule forming H-H bond after which the desorption of H2 molecule occurs through either the Heyrovsky reaction (ion + atom reaction) or the Tafel reaction.[25, 34] Indeed, all of these features are key factors for the observed high HER activity, as will be later discussed.
From further insights into the origin of the overall water electrolysis performance, we decipher the optimum factors for a higher OER catalytic activity, which is also of crucial importance. Indeed, the OER pathway has sluggish kinetics, making it the bottleneck in catalysis compared with the HER counterpart. To monitor the catalytic activity beyond the OER process, we did geometry optimization and thermodynamic calculations for OH, O, and OOH adsorbed intermediates at the top of the P atom sites (which is the favorable site similar to that reported for NiCoP).[48] The calculated Gibbs free energies of the investigated intermediates at the equilibrium potential of 1.23 V are displayed in Figure 2d. Note that among all catalytic sites of the studied MnNiCoP electrocatalyst, the best performance was provoked for the metal-Ni site with the rate-determining step being the third step (O* to OOH*). Notably, the four electron-proton reaction steps concurrently involve the desorption of the oxygen molecules from the catalyst surface, which could estimate the adsorption affinity of the O2 molecules on the catalyst surface for a better understanding of the reaction mechanism. All these predictions reveal strong desirable water adsorption on the surface of the electroactive electrode and further indicate the strong bonding between the metallic components and their best synergism with P atom for peculiar catalytic performance. The detailed calculations of the H-adsorption energies, as well as binding energies of OER intermediates, are listed in the SI file, See Figures S3 and S4.
2.2 Morphology, Structure, and Composition
The ternary metallic manganese nickel cobalt phosphide (MNCP) was prepared via a two-step synthesis approach. First, the precursor metallic hydroxide was electrodeposited on flexible nickel foam substrate (MNC-OH/NF) using cyclic voltammetry by sweeping in the potential range of −0.13 V to 1.27 V vs. RHE. The potential of −0.13 V is more negative than the reduction potentials of Mn (−0.075 V), Co (0.325 V), and Ni ions (0.27V) vs. RHE, which should ensure the deposition of all three cations on the nickel foam.[14] Moreover, the cyclic voltammetry technique has the advantage of depositing homogenous films with controlled thickness upon varying the number of sweeping cycles.[42] After electrodeposition, the MNC-OH/NF gets phosphidized at a low temperature of 250°C via PH3 plasma treatment without phosphatizing the substrate.[23] After the phosphidation process, the electrode converted from greenish-yellow to black, revealing the synthesis of phosphides (Figure S5).
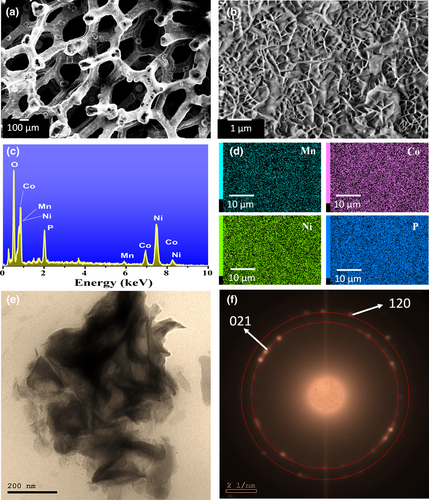
Figure 3a,b and Figure S6 show typical FESEM images of the fabricated catalyst, revealing three-dimensional flower-like hierarchy composed of interconnected and highly porous nanosheets.[23, 49] The EDX spectrum (Figure 3c) confirms the presence of Ni, Co, Mn, P, and O with uniform distribution as indicated via the EDX elemental mapping (Figure 3d). The interconnected nanosheet structure was also confirmed via TEM imaging (Figure 3e). Moreover, the selected area electron diffraction (SAED) analysis revealed the polycrystalline nature of the fabricated catalyst (Figure 3f). Three different sets of planes can be identified with lattice spacing of 0.142, 0.178, and 0.204 nm, which can be assigned to the (112), (120), and (021) planes of the hexagonal NiCoP crystal structure. For further elucidation of the crystallinity of the catalyst, the XRD spectra of catalyst on Ni foam were recorded as depicted in Figure S7 and compared with MNC-OH and bare Ni foam bulk samples. Before phosphidation, diffraction peaks can be observed at ~44.5°, 51.9°, and 76° that can properly be assigned to the Ni foam substrate with the corresponding reflections of (111), (200), and (220), respectively (ICDD: 04-001-0091). No further peaks were detected, revealing the amorphousness of the deposited film before plasma treatment. Upon a closer inspection of the XRD spectra of the plasma-treated electrode, additional diffraction peaks can be distinguished at ~43.7°, 44.9°, 51.1°, and 76.7°, in good agreement with the hexagonal NiCoP (ICDD: 04-001-0091). These diffraction peaks can be assigned to the crystal phases of (111), (021), (120), and (122), respectively, in accordance with the TEM findings. Notably, the XRD patterns of the prepared samples (Figure S7) showed a gradual shift in the peak positions relative to the standard reference card, which might be originated from the incorporation of the manganese atoms within the lattice structure. Consequently, these results strongly confirm the formation of hexagonal MNC-P intentionally. To further confirm the successful formation of the MNCP nanoflowers-like films upon surface plasma treatment, the Fourier transform infrared (FTIR) spectra of the MNC-OH and MNC-P films on Ni foam were compared as depicted in Figure S8. Apparently, a more intense broad peak at ~3640 cm−1 was clearly observed, which can be assigned to the hydroxyl group stretching mode in nickel-containing hydroxyl composites.[50] On the other hand, upon phosphidation, the intensity of the surface-OH peak was greatly minimized, thus affecting the electrocatalytic properties, as will be discussed later. These findings further propose the possibility of the chemical transformation of the as-deposited MNC-OH films via the incorporation of phosphide species during the PH3-plasma process.
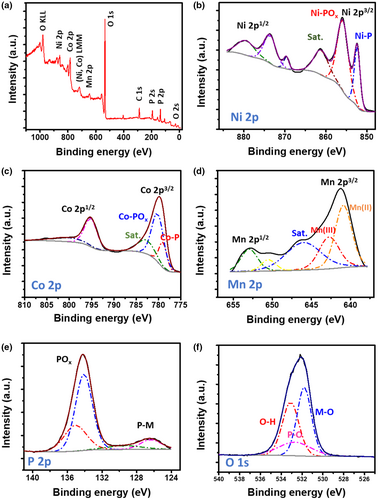
Regarding the detailed analysis of the surface chemistry of the synthesized MNCP catalyst, X-ray photoelectron spectroscopy (XPS) measurements were performed, Figure 4. The survey spectrum (Figure 4a) shows the presence of Ni, Co, Mn, P, and O. In the high-resolution XPS spectrum of the Ni 2p (Figure 4b), two peaks from (2p3/2) and (2p1/2) appear at 852.4 eV and 869.6 eV, respectively, which are more positively shifted from that of metallic nickel, corresponding to the partially charged Niγ+ (0 < γ < 2), indicating the formation of nickel phosphide bond (Ni-P).[24, 51] Oxidized nickel phosphate (Ni-POx) also possesses two peaks at 873.6 eV (2p1/2) and 855.9 eV (2p3/2), which can be ascribed to the superficial oxidation because of air exposure.[24, 52] The Co 2p spectrum (Figure 4c) shows two spin-orbit doublets at 795.2 eV (Co 2p3/2) and 779.0 eV (Co 2p1/2), along with two shake-up satellites at 800.5 and 782.8 eV, ascribable to the cobalt oxidation state in cobalt phosphide (Co-P), while the peak at 780.2 eV is characteristic of oxidized Co (Co-POx).[53-55] As for the Mn 2p spectrum (Figure 4d), two deconvoluted peaks appear at the binding energies of 640.9 and 642.7 eV (2p3/2), which can be assigned to Mn2+ and Mn3+ species, with a satellite appearing at 646.0 eV characteristic of Mn2.[56, 57] Regarding the characteristic P 2p spectrum shown in Figure 4e, the phosphide (P-M) and phosphate (POx) chemical states can be detected at 134.2 eV and 126.5 eV, respectively.[24, 53] Compared with pure phosphorus, the phosphide peak is shifted to lower binding energy, indicating electron density transfer from the present metals to the phosphorus.[23, 34] This negatively charged phosphorus can thus behave as a base that traps the protons with positive charges during electrocatalysis. Finally, the O1s spectrum (Figure 4f) can be fitted into three peaks at 531.8, 532.7, and 533.1 eV, which can be ascribed to metal-oxygen bond, broadened peak because of P penetration into the lattice, and O-H bond, respectively.[52] It can therefore be further inferred from the XPS data that manganese nickel cobalt phosphide has been successfully synthesized.
2.3 Electrocatalytic Hydrogen Evolution Reaction (HER)
The HER activities of ternary metallic MnNiCo-P/NF, MnNiCo-OH/NF, and bare NF electrodes were effectively evaluated using a standard three-electrode system in 1.0 m KOH solution and benchmarked against the 20 wt% commercial Pt/C catalyst under the same experimental conditions. The deposited electrocatalysts with a geometric area of 1 cm × 1 cm were directly used as a working electrode, Pt sheet as a counter electrode, and Ag/AgCl as the reference electrode, see Figure S9. The binder-free nature of the synthesized electrocatalysts should boost extra active sites, minimize the series resistance, limit proton diffusion, and sturdily improve their long-term stability.[42, 58] To further investigate the influence of catalyst composition on the electrocatalytic behavior and explore the optimal electrocatalyst composition, we not only studied the influence of plasma treatment on the synergism of MnNiCo precursors but we also systemically evaluated the impact of the structural variations and different deposition ratios (MnNiCo-P/NF 1:1:1, MnNiCo-P/NF 5:2:2, and MnNiCo-P/NF 4:4:1) on the catalytic HER performance. Prior to the activation step for the HER, scans in a positive potential, from low potentials to high potentials, were necessary during the recording of the cyclic voltammograms (CVs) to prevent any possible interferences that may result from the reduction peak of NiOx to Ni and affect the accurate determination of the overpotential (see Table S1 for the electrochemical protocol).[51, 59] Figure 5a records the IR-corrected LSV polarization curves of the prepared electrocatalysts at a scan rate of 5 mV s−1 where their overpotentials at specific current density (10 mA cm−2) were compared. Impressively, the MNC-P/NF electrode with Mn-Ni-Co deposition ratio of 1:1:1 demonstrated the optimal HER performance among the five investigated catalysts, with a tremendously satisfactory cathodic geometric current density coupled with an extremely negligible overpotential of 14 mV@–10 mA cm−2, ~115 mV@–100 mA cm−2, and ~162 mV@–200 mA cm−2, Figure 5a. This remarkable performance is surprisingly very close to that of the 20 wt% Pt/C commercial benchmark (~0 mV@–10 mA), see Figure S10 for comparison, and overwhelmingly surpassed that of recently reported HER metallic phosphide catalysts as listed in Table S2. The exceptional catalytic performance of the electrocatalyst with the equimolar composition (MnNiCo-P 1:1:1) can be attributed to the remarkable enhancement in the electrocatalytic behavior achieved by the formation of newly active species with enhanced electrocatalytic rates upon the incorporation of phosphorous ions via plasma treatment. Besides, coupling Mn, Ni, and Co components contributed to improving their synergetic interaction compared with their existence individually. As such, the excellent performance of the fabricated MNC-P/NF 1:1:1 heterostructure can be related to the unusual synergism between the incorporated P ions and the coupled transition metals deposited on highly conducting substrate, thus resulting in facilitating charge transfer and enhancing the adsorption of intermediates during the electrochemical reaction.[34, 60] On one hand, the incorporation of nickel and cobalt should improve the electrical conductivity and ensure superior redox characteristics of the novel phosphide heterostructure, achieving a remarkable HER performance.[18] Furthermore, manganese addition to Ni and Co could adjust the electronic density of nickel d-orbitals, further upgrading the H2 adsorption-desorption rate and intrinsically improving the electrocatalytic performance for the HER.[26, 61, 62] Accounting on the electrocatalytic synergism of the synthesized catalysts, the equimolar metallic interaction has led to a significant improvement in their bond strength, thus boosting the intermetallic stability of the fabricated electrocatalyst for the HER.[63, 64] It is important to note that the enhanced activity can also be ascribed to the thin nanoflowers-like structure that could provide excess active sites and good contact with the electrolyte.[26, 65] Contrarily, overpotentials of 57, 98, 114, and 224 mV are required for the MnNiCo-P/NF 5:2:2, MnNiCo-P/NF 4:4:1, MnNiCo-OH/NF, and the bare NF to deliver the same current density of −10 mA cm−2, respectively. Notably, blank NF displayed negligible current density in the range of 0 to −250 mV vs. RHE, demonstrating that the conductive NF substrate did not show any significant contribution to the catalytic behavior of the fabricated catalysts associated with the absence of its major electroactive catalytic centers, thus confirming the superior catalytic performance of the synthesized MnNiCo-P.[60, 66]
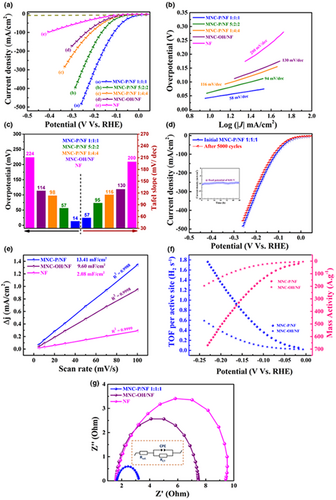
It is generally recognized that the HER mechanism in alkaline medium follows three main processes: the first involves proton discharge at the cathode (Volmer reaction), followed by either an electron-desorption pathway (Heyrovsky reaction) or Tafel slope that assume the direct formation of hydrogen molecule from two adsorbed H* species (See SI for equations and calculation methods).[34, 60] To further provide profound investigations on the fundamental HER kinetics, Tafel plots of the corresponding polarization curves are considered a valuable key to assess the inherent catalytic properties of the fabricated electrocatalysts by evaluating the rate-determining step of the electron-transfer reactions. Figure 5b analyzes the Tafel plots of all investigated catalysts from their small polarization area, further evidencing the high performance of the plasma-treated electrocatalysts. Note that the equimolar MNC-P/NF 1:1:1 electrode demonstrated the smallest Tafel slope of about 58 mV dec−1 among the five investigated electrocatalysts, which is comparable to that of the 20 wt% Pt/C benchmark and much lower than that of the MNC-P/NF 5:2:2 (94 mV dec−1), MNC-P 1:4:4 (116 mV dec−1), MNC-OH (130 mV dec−1), and blank NF (200 mV dec−1) counterparts. In accordance with Tafel slopes, such low values of the phosphide-containing electrodes suggest that the HER pathway is probably proceeded by the Volmer-Heyrovsky mechanism, with the desorption step being the rate-determining step. On the other hand, the higher Tafel slope of the MNC-hydroxide electrocatalyst and bare Ni-foam counterpart may indicate a different electrocatalytic process, with the Volmer step being the main mechanism controller, resulting in sluggish kinetics on the electrode surface.[34, 67] It can reasonably be inferred that the noticeable variation in the Tafel slope and the HER performance of the phosphide electrocatalysts fluctuate from varying the relative metallic content, with the optimum catalytic efficiency aroused from equal ratios of Mn-Ni-Co precursors (1:1:1). Further increasing the deposition ratios, the catalytic synergism of the deposited trimetallic components becomes obstructed, resulting in minimizing the charge mobility in Mn5-Ni2-Co2 and Mn4-Ni1-Co1 phosphides and lowering their HER performance. That can be attributed to the possibility of the agglomeration of some precursors particles within the composite matrix, leading to a catalytic HER retardation.[63] Such composition-dependent behavior reveals the vital role of the optimized Mn-Ni-Co deposition ratios on electrocatalytic performance. Overall, the values of the overpotentials and respective Tafel slopes (kinetic metrics) at 10 mA cm−2 of the variously deposited electrocatalysts are comparatively illustrated in Figure 5c. Particularly, these findings highlight the outstanding HER activity of our catalyst in the alkaline media, which is comparable and even superior to other valuable representative catalysts reported in the literature, Table S2.
The stability of the catalyst is another indispensable parameter for assessing the HER catalytic activity of the valuable electrocatalysts and examining their effectiveness for industrial use. In this context, accelerated stability test via successive CV sweeps in the potential range of −0.2 to 0.2 V vs. RHE at 100 mV s−1 was conducted to elucidate the electrocatalyst durability for the HER. Figure 5d compares the LSV of MNC-P 1:1:1 in 1.0 m KOH before and after 5000 CV cycles. The polarization curve of the MNC-P 1:1:1 after 5000 cycles almost overlapped with the first cycle, with a barely current density variation and a slight attenuation in the overpotential value, revealing outstanding stability for catalyzing the hydrogen evolution reaction. Further assessing the electrocatalyst durability, chronoamperometry measurement (i-t) in KOH alkaline solution at ~-0.01 V vs. RHE was monitored up to a continuous 48 h run (see inset in Figure 5d). The electrocatalytic hydrogen evolution activity was sustainable and kept a steady current density, retaining ~92% of the initial current after 48 h, revealing the long-term catalytic stability of the deposited MnNiCo-P/NF 1:1:1 electrode for the HER. Importantly, post structural SEM characterization was conducted for the MNC-P/NF 1:1:1 after prolonged HER durability measurement to investigate any possible structural variations (Figure S11). The SEM imaging of the post investigated HER electrocatalyst revealed that it maintained almost its pristine morphology with no obvious structural deterioration, revealing structural sturdiness by the intact morphology of the synthesized electrocatalyst during the HER.
2.4 Electrocatalytic Intrinsic Activity
To further explore the origin of the enhanced robustness of the fabricated electrocatalysts for the HER, the electrochemical active surface area (ESCA)[23, 34, 68] was evaluated to monitor the double-layer capacitance (Cdl) of the MNC-P/NF, MNC-OH/NF and bare NF at the solid/liquid interface, Figure 5e. The capacitive current was calculated based on the reported cyclic voltammetry technique using various CV scans in the absence of the faradic processes.[23, 34, 68] Linear fitting of the maximum current at various scan rates of 5 to 100 mV s−1 was necessary for the accurate determination of the Cdl (Figure S12). As demonstrated in Figure 5e, the MNC-P/NF 1:1:1 electrocatalyst showed the highest Cdl of ~13.341 mF cm−2, which is ~7.6-folds higher than that of the bare NF (2.08 mF cm−2) and considerably 1.4 times enhanced compared with that of the MNC-OH/NF counterpart (9.6 mF cm−2). Accordingly, ECSA was estimated for the fabricated electrocatalysts as detailed in the SI. Clearly, the equimolar MNC-P/NF 1:1:1 electrode showed the highest electrocatalytic intrinsic activity with high surface roughness, improved conductivity, and more accessible active sites for the HER, Tables S3 and S4. Notably, normalizing the current density to the ECSA at a specific onset potential is really critical for a better comparison of the intrinsic electrocatalytic activity.[65, 69, 70] In this context, the normalized current density of the equimolar plasma-treated electrode was compared with that of the MNC-OH/NF counterpart as demonstrated in Figure S13, evidencing higher current output originating from the MNC-P/NF 1:1:1 electrocatalyst. Moreover, the turnover frequency (TOF) is an important parameter for tracking any changes in the electrocatalytic activity and to get more insights into the inherent catalytic properties of the electrocatalysts by assessing the number of H2 evolved/active site.[6, 71, 72] As depicted in Figure 5f, the TOF values were computed for the MNC-P/NF 1:1:1 electrocatalyst and compared with that of the MNC-OH/NF counterpart at different overpotentials, supposing that all active centers for the transition metals and phosphorus atoms are included in the HER process (corresponding calculation details depicted in the SI). Amazingly, the average TOF value of the MNC-P/NF was found to be 0.35 s−1 and 0.80 s−1 at overpotentials of −100 and −150 mV, respectively. This value evidently surpassed that of its hydroxide electrocatalyst counterpart (0.06 s−1 at η-100), implying a 3-fold enhancement that might reveal a more active interface between the deposited components and the phosphorus atom, thus confirming an excess number of active sites. The active sites density was estimated directly from the CV plot at 20 mV s−1 scan rate, Figure S14. Despite the uncertainty of the TOF calculations as it is relatively difficult to determine the exact number of active centers precisely, it is still an applicable way to correlate the catalytic activity, particularly when comparing electrocatalysts under the same experimental conditions.[73] Meanwhile, the mass activity (MA) is considered a quantitative parameter and a more reliable criterion for assessing the electrocatalytic performance of electrocatalysts.[74, 75] The MA values can be obtained by normalizing the current density of the reported electrocatalysts to the total mass loading of the synthesized catalyst (detailed calculations are depicted in the SI).[74] As demonstrated in Figure 5f, the MNC-P/NF 1:1:1 electrocatalyst possessed an extremely high MA up to 136 A/gmetal load at an overpotential of −100 mV, which is remarkably 5.9 times higher than that of the MNC-OH/NF (~23 A/gmetal load) counterpart. It is worth mentioning that higher MA can be accomplished by minimizing the mass loading to further minimize the bulk resistance and allow for better electrical ionic conduction by the electrolyte ions to the electrocatalyst surface.[75, 76] Accordingly, the higher mass activity attained by the MNC-P/NF 1:1:1 catalyst can reveal higher current output at lower mass loading, and thus a higher catalytic activity. Generally speaking, the higher catalytic activity in the multicomponent electrocatalysts, in contrast to single components, can strongly be ascribed to the better interaction at the interface, revealing the benefit of the interface effects in accessing more active centers of the catalyst.[23, 25]
As the electronic conductivity directly impacts the electrocatalytic HER performance, electrochemical impedance spectroscopy (EIS) was investigated to effectively determine the charge transfer resistance (RCT) of the deposited electrocatalysts and ensure full characterization of the HER process at the electrode/electrolyte interface. Figure 5g compares the EIS Nyquist plot of MnNiCo-P/NF 1:1:1 to those of MNC-OH/NF and blank NF counterparts at a fixed potential of −200 mV vs. RHE. The semicircles were properly fitted using equivalent Randle’s circuit (inset Figure 5g) to determine RCT of the electrocatalysts. Obviously, the MNC-P/NF 1:1:1 electrocatalyst exhibited the least RCT value (1.59 Ω) with the smallest semicircle diameter in contrast to the metal hydroxide (5.84 Ω) and blank NF (8.00 Ω) counterparts, in agreement with the ECSA measurements. On the other hand, the solution resistance (RSOL) was almost kept unchanged (1.65 Ω) for all studied samples during the HER impedance measurements. It is worth noting that the lowest RCT reported for the MNC-P/NF electrocatalyst can predominantly be ascribed to the phosphidation treatment of the deposited electrocatalyst that improves the interfacial electrode surface area and boosts the electronic conductivity. Moreover, the enhanced interfacial contact between the metallic components and NF substrate should highly improve the electrical conductivity within the nanostructured electrocatalyst, thus leading to lower RCT at the electrode/electrolyte boundary.[77] That could reveal an ultrafast Faradic process and effective electron transfer process for better HER kinetics.[25, 78]
2.5 Electrocatalytic Oxygen Evolution Reaction (OER)
The electrochemical OER activity of the designed electrocatalysts was also investigated using a 3-electrode setup in N2 saturated 1.0 m KOH solution. Polarization curves of the MNC-phosphide/NF with three different relative metallic ratios were reported and compared with those of MNC-OH along with the bare Ni foam counterpart. Before electrochemical testing, all working electrodes underwent repetitive CV scans to achieve a relatively stable state. As displayed in Figure 6a, the IR-corrected LSV curves of the MNC-P/NF 1:1:1, MNC-P/NF 5:2:2, and MNC-P/NF 1:4:4 were compared with those of MNC-OH/NF and blank NF at a scan rate of 5 mV s−1. Note that MNC-P/NF 1:1:1 exhibits by far the earliest OER onset potential (ῃOER) of about 289 mV to attain a current density of 10 mA cm−2 along with the considerably improved current density. In contrast, the untreated MNC-hydroxide electrode supported on NF showed an OER overpotential of ~345 mV to deliver the same current of 10 mA cm−2, much higher than those of the plasma-treated MNC-P/NF electrodes, indicating that the phosphidation of MNC-OH/NF greatly enhanced the OER performance. This ῃOER of the MNC-P/NF 1:1:1 is ~2 times less compared with that of the bare NF counterpart (530 mV). On the other hand, the onset potential of the MNC-P/NF 1:1:1 electrocatalyst (289 mV) at 10 mA cm−2 is even much lower than those of MNC-P/NF 5:2:2 and MNC-P/NF 1:4:4 with overpotentials of 346 mV and 390 mV, respectively, revealing the influence of varying the relative amounts of the different electrodeposited metal ions on the electrocatalytic activity toward OER. These values are favorably comparable and outperform some of the recently reported electrocatalysts, as listed in Table S5. Importantly, an anodic shoulder around 1.3–1.4 V vs. RHE was noticed for all the deposited catalysts, which can be ascribed to the presence of Ni and Co ions, consistent with other reported Ni or Co-containing catalysts.[23, 45] Interestingly, this anodic shoulder is shifted to a lower potential of approximately 1.3 V vs. RHE for the equimolar MNC-P/NF electrocatalyst, implying more modified electronic structure for this optimal metallic ratio in the electrodeposition solution. This, in turn, could contribute to lowering the thermodynamic barrier for the oxygen evolution process.[79] It is worth noting that the outstanding catalytic activity of the MNC-P/NF 1:1:1 electrode can be ascribed to the superior synergistic effect among equimolar Mn, Ni, and Co in the phosphide-based trimetallic structure with adjusted relative amounts, thus greatly tuning the electronic structure of the designed electrode, as well as lowering the required energy barrier for the electrolysis process.[34, 45]
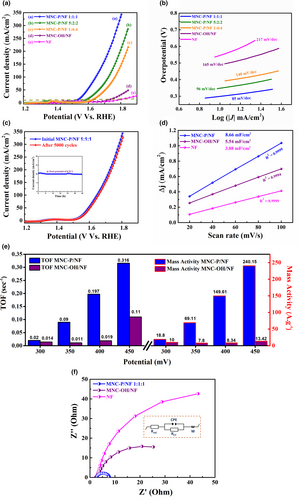
To further appraise the OER kinetics of the deposited electrocatalysts, Tafel analysis was examined for all reported catalysts and extracted from their corresponding LSV plots, Figure 6b. Tafel slopes of the MNC-P/NF 1:1:1, MNC-P/NF 5:2:2, MNC-P/NF 1:4:4, MNC-OH/NF, and bare NF were found to be 85, 95,114, 165, and 217 mV dec−1, respectively. Among the previously explored catalysts, the smallest Tafel slope reached minima for the MNC-P/NF 1:1:1 electrocatalyst, indicating fast charge transfer for the OER mechanism associated with the uniform coverage of the deposited precursors with an optimal concentration within the lattice structure, in line with the lattice modification by the plasma-phosphidation treatment. The fact that the OER enhancements of the electrocatalysts follow the same sequence of the HER process highlights the effect of the composition engineering on the electrocatalytic activities.[13, 34, 80] We believe that the incorporation of high-valence state manganese with Ni and Co- components could significantly affect the OER reaction mechanism by promoting the adsorption of such metal cations to the hydroxyl (OH-) groups, thus minimizing the overpotential and lowering the Tafel slope for rapid OER kinetics. This might be linked to the lower energy barrier needed for the proton-electron transfer process as well as the ease of O-O bond linking via the optimum synergism of Mn, Ni, and Co active centers in the presence of phosphide ions.[13, 79, 81] In turn, this could support the idea that our hybrid electrocatalyst is one of the most efficient bifunctional electrocatalysts reported so far for the oxygen evolution process.
Post-XPS analysis was further examined for the Mn1 -Ni1 -Co1 -phosphide electrocatalyst after the OER process to detect any possible variations in the chemical composition and the dominant metallic valences on the electrode surface during the OER process. It is extremely important to examine the metastable catalytic state of the reported electrocatalysts, which is a prerequisite during the OER process.[13, 23, 45] On the post-OER electrolysis, an obvious change in the chemical composition of the catalyst can be noted, as depicted in Figure S15. The low valent Mn, Ni, and Co metal peaks, attributed to the metal-P bonds in the deposited sample, completely vanish, while the P signal becomes very weak and below the detection limit of XPS. Instead, new peaks emerge at 780.7 and 857.1 eV and can be assigned to oxidized cobalt and nickel,[82] respectively. Meanwhile, the O 1s peak is shifted to lower binding energy compared with the pristine sample, revealing the formation of low valent metal oxides.[82] This P-deficient surface may reveal the complete oxidation of the metal-phosphides to oxides or oxy-hydroxide species, thus providing more desirable adsorption/desorption energies for the OER intermediates (OH* and OOH*).[22, 23, 83] Accordingly, these features demonstrate that the subsequent phosphide-derived MnNiCo-oxide or MnNiCo-oxyhydroxide heterostructures can significantly promote the OER activity and durability by facilitating the charge transfer from the metallic Mn-Ni-Co-P to the surface layer, coinciding with other reported studies for OER over the TM-based phosphides.[23, 37, 83] We also assessed the long-standing stability as another valuable factor to address the electrocatalytic applicability of the synthesized electrodes toward OER. The prolonged cycling stability evidenced by closely overlapped LSV scans after 5000 CV cycles demonstrates that MNC-P/NF 1:1:1 exhibited outstanding durability for OER, Figure 6c. This was reinforced by the chronoamperometric measurements conducted at ~0.28 V vs. RHE until 48 h with a minimum fluctuation in the current density, revealing highly efficient catalytic performance (see the inset in Figure 6c).
To elucidate the origin of the intrinsic electrocatalytic activity of the synthesized electrocatalysts, the Cdl was further estimated at various scan rates of 20–100 mV s−1. As demonstrated in Figure 6d, the MNC-P/NF electrode exhibits the highest Cdl of 8.66 mF cm−2 that is almost 3 times larger than that of blank NF electrode (3.08 mF cm−2) and even much improved compared with the MNC-OH/NF counterpart (5.84 mF cm−2). This may reveal a greater number of preferably active sites for the plasma-treated electrode associated with a higher ESCA, thus enhancing the adsorption of H2O molecules, see Table S6.
Moreover, to confirm the inherent catalytic activity of the functionalized electrocatalysts during the OER process, a graphical representation of the TOF and mass activity values was assessed and compared in Figure 6e, assuming the activity of all of the metal species of the catalysts. The TOF and MA values were quantified at various overpotentials of 300, 350, 400, and 450 mV, further demonstrating the trend in the electrochemical OER activity of the synthesized catalysts. Particularly, the TOF values of the MNC-P/NF 1:1:1 at an overpotential of 350 is ~0.09 s−1, significantly larger than that of the MNC-OH/NF counterpart (0.011 s−1) and even better than other relative Co or Ni phosphide-based OER electrodes reported in the literature, e.g., CoMnP NP (0.004 s−1),[23] NixCo3-xO4 nanowires (0.007 s−1)[84] at the same η of 350 mV, revealing the potential of the equimolar MNC-P/NF as one of the most efficient OER candidates. Furthermore, the MA values of both hydroxide and phosphide electrodeposited MnNiCo precursors were studied at the same overpotentials to elucidate the effect of plasma treatment on the pronounced catalytic activity. As displayed in Figure 6e, the MNC-P/NF electrode showed an enhanced mass activity of ~69.11 A gM−1, which is significantly 8.9 times higher than that of the MNC-OH/NF counterpart (7.8 A gM−1); see SI for more details. This improved enhancement agrees with TOF and ESCA trends, thus verifying the optimal catalytic properties of the trimetallic MNC phosphide with the augmented OER rate.
Aside from the higher OER intrinsic activity and the outstanding stability of the plasma-treated electrocatalyst, electrochemical impedance spectroscopy (EIS) is a complementary approach to further evaluate the OER kinetics. Figure 6f depicts the Nyquist plots of the fabricated electrocatalysts compared with that of bare NF electrode at a potential of 1.53 V vs. RHE. To extract the needed physical parameters from the Nyquist plots, a consistent equivalent circuit is used for the accurate fitting, as indicated in the inset of Figure 6f. Noticeably, all three electrodes almost account for a close unchanged RSOL of about 2.26 Ω. On the other hand, the RCT values were extracted to infer the OER kinetics on the electrode/electrolyte interface. As expected, the faradic RCT response was drastically increased from 5.54 Ω for the electrodeposited MNC-P/NF electrode to ~21.09 Ω and 76 Ω for the MNC-OH/NF and bare NF counterparts, respectively. Importantly, a much smaller semicircle diameter of the plasma-treated electrode compared with the other catalysts can be a good indication of its excellent activity and faster kinetics for the OER process. In turn, this could persuasively reflect the role of phosphidation on the catalyst conductivity and the creation of additional active sites.[12, 15, 29, 81]
2.6 Electrocatalytic Performance for Overall Water Splitting
Inspired by the aforementioned superior electrocatalytic activity exhibited by the MNC-P/NF 1:1:1 catalyst toward both the HER and OER, a full single water alkaline-electrolyzer system was constructed for overall water splitting using the bifunctional MNC-P/NF 1:1:1 as both the anode and cathode in alkaline KOH solution. As seen in Figure 7a, the full cell polarization curves of the assembled electrode system MNC-P/NF 1:1:1|| MNC-P/NF 1:1:1 at the scan rate of 1 mV s−1 in 1 m KOH delivered ultralow cell voltages of only 1.48 V to attain a current density of 10 mA cm−2. Notably, the gas bubbles could be virtually observed from both the anode and the cathode (inset of Figure 7a). Remarkably, this performance outperforms the combination of the two benchmark Pt/C || RuO2 system, which showed a current density of 10 mA cm−2 but at a higher cell voltage of ~1.50 V, consistent with other reports under the same experimental conditions.[85] Impressively, contrasting these findings to recently reported bifunctional phosphide electrocatalysts such as chalcogenides, and transition metal-based catalysts, it is clear that this performance stands out among the best-rated bifunctional water splitting electrolyzers.[13, 23, 66, 86-88] For comparison, Figure 7b and Table S7 demonstrate the cell voltages of a plethora of previously reported bifunctional electrocatalysts.

Note that the noticeable oxidation peak of our electrocatalysts that usually appears in the LSV before the OER potential (Figure 6a) is minimized and broadened when electrodes were utilized as both the anode and the cathode in the water electrolyzer system (Figure 7a). This phenomenon is very common for most bifunctional water splitting electrocatalysts.[13, 45, 58]
Meanwhile, to rigorously evaluate the robustness of the assembled electrodes to the overall water splitting performance, a long-term durability test of the MNC-P/NF 1:1:1 || MNC-P/NF 1:1:1 electrolyzer was explored via continuous chronoamperometry operation at the onset potential of 1.48 V in 1.0 m KOH at room temperature, see Figure 7c. After nonstop 50 h, the MNC-P/NF 1:1:1|| MNC-P/NF 1:1:1 system showed outstanding stability and a steady-state current density with only 4% decay after 50 h. Moreover, the LSV after 50 h long-term durability test (i-t), Figure 7d, showed a very stable and intact LSV curve with almost no variation in the cell voltage and a negligible minimization in the current density, revealing the superior stability of our constructed water electrolyzer. Accordingly, the combination of the MNC-P/NF 1:1:1 || MNC-P/NF 1:1:1 electrode couple is a good choice for designing a remarkable water electrolyzer with outstanding stability. It can be seen from post-structural SEM images (Figure S16) that the morphology of the combined electrodes did not show any significant variation after the long-term electrolysis, revealing the perfect durability of our constructed system. Likewise, the post-electrolysis EDX analysis confirms the presence of all metals with high oxygen content, indicating the formation of phosphide-derived oxidized species, consistently with the post-measurements XPS analysis (Figure S17).
2.7 Origin of the Superior Electrocatalytic Activity of the Designed Bifunctional Electrocatalyst
The excellent electrocatalytic behavior of our constructed electrolyzer can be ascribed to: 1) the phosphidation treatment of the electrodeposited catalysts that adds excess active centers in favor of high electrocatalytic performance, consistently with the lower energy barriers for the H-atom adsorption on the P atom surface, as revealed from the DFT calculations; 2) constructing 3D-heterostructure electrocatalyst with the nanoflowers-like morphology enhances the surface area and allows higher accessibility to the active interfaces, which eases the electrolyte diffusion and promotes the catalytic reaction; 3) despite the similar chemical nature of our proposed catalysts, rational integration of equimolar ratios of the individual metallic components during the synthesis process enhances the electronic conductivity and ensures fast electron transfer; and 4) the choice of the highly conductive nickel foam as an ideal supporter not only provides high mechanical adhesion and good electrical conductivity of the electrocatalysts but also eliminates the need for a binder, thus enhancing the electrocatalytic performance. Overall, our findings manifest the potential of the ternary metallic Mn, Ni, and Co components (with an equimolar ratio in the electrodeposition solution) supported on NF and treated under PH3 plasma as a bifunctional electrocatalyst with plentiful active sites. The major benefit of the metallic interaction and the phosphidation treatment was reflected in the overall catalytic activity of the water electrolyzer and its long-term durability, which could be further utilized for industrial water splitting applications.
3 Conclusion
In summary, we have presented the facile synthesis of a series of nickel foam-supported trimetallic phosphide nanoflowers-like electrocatalysts using electrodeposition of Mn-Ni-Co precursors followed by low-temperature selective PH3 plasma treatment. Based on both DFT calculations and experimental findings, the equimolar trimetallic Mn1Ni1Co1-P/NF has proven to be a highly active bifunctional electrocatalyst for water electrolysis with a remarkable stability in alkaline solution. The results revealed the benefit of the optimal metallic synergism and the phosphidation treatment. The resultant porous MnNiCo-P/NF heterostructure showed superior electrochemical activity for both HER and OER evidenced by the outstanding durability and considerably ultralow overpotentials of 14 and 289 mV, respectively at 10 mA cm−2, outperforming the most active nonprecious bifunctional electrocatalysts. Note that the structural analyses after the catalytic OER process revealed that the MnNiCo-P derived (oxy)hydroxide actually served as the real active species, thus boosting the OER performance. Even more importantly, the excellent activity of the plasma-treated electrocatalyst, together with its facile synthesis, have helped to construct a full water electrolyzer in 1 m KOH that only required an extremely low cell voltage of 1.48 V to generate 10 mA cm−2. Furthermore, the designed electrolyzer endowed prominent durability with an insignificant decay up to 50 h for efficient water electrolysis. This splendid activity can be ascribed to the uniform porous nanostructure of the catalyst with high metallic conductivity and appropriate tuned composition, along with the simultaneous metal-phosphide bonds that completely modified the electronic structure of the catalyst. We hope our study offers a desirable platform for achieving high electrochemical activity and paves avenues for the rational construction of advanced and optimized multi-metallic phosphide nanostructures for water electrolysis and other practical applications.
Acknowledgement
The financial support of this work by the American University in Cairo is highly valued.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.




