Epitaxially Grown Ru Clusters–Nickel Nitride Heterostructure Advances Water Electrolysis Kinetics in Alkaline and Seawater Media
Abstract
The epitaxial heterostructure can be rationally designed based on the in situ growth of two compatible phases with lattice similarity, in which the modulated electronic states and tuned adsorption behaviors are conducive to the enhancement of electrocatalytic activity. Herein, theoretical simulations first disclose the charge transfer trend and reinforced inherent electron conduction around the epitaxial heterointerface between Ru clusters and Ni3N substrate (cRu-Ni3N), thus leading to the optimized adsorption behaviors and reduced activation energy barriers. Subsequently, the defect-rich nanosheets with the epitaxially grown cRu-Ni3N heterointerface are successfully constructed. Impressively, by virtue of the superiority of intrinsic activity and reaction kinetics, such unique epitaxial heterostructure exhibits remarkable bifunctional catalytic activity toward electrocatalytic OER (226 mV @ 20 mA cm−2) and HER (32 mV @ 10 mA cm−2) in alkaline media. Furthermore, it also shows great application prospect in alkaline freshwater and seawater splitting, as well as solar-to-hydrogen integrated system. This work could provide beneficial enlightenment for the establishment of advanced electrocatalysts with epitaxial heterointerfaces.
1 Introduction
Hydrogen energy, as the clean, highly efficient secondary energy source, will change the global energy structures in future.[1, 2] Electrocatalytic water splitting, with frequently used freshwater or more challenging seawater media, can implement high-purity hydrogen production by virtue of the generated electricity from solar energy, wind energy, or potential energy of water, thus becoming an important link of the future hydrogen economy.[3-5] The sluggish electrode reactions bring about high operation voltage for the water electrolysis apparatus, hence requiring efficient and robust electrocatalysts to expedite them.[6, 7] Owing to the satisfactory catalytic activity, the commercial Pt/C and RuO2/IrO2 catalysts have been utilized to catalyze cathodic hydrogen evolution reaction (HER) and anodic oxygen evolution (OER), respectively.[8, 9] However, the exiguous resource, exorbitant price, inferior stability, and the desired mismatched operating environment impede their large-scale application.[10-12] Thus, low-cost bifunctional catalysts with excellent activity and durability must be rationally developed to enable the sustainable hydrogen supply.
As the cheapest platinum-group metal element, ruthenium (Ru) possesses moderate adsorption strength toward hydrogen and exhibits decent catalytic activity for HER.[8, 13] As for OER, the ruthenium oxide (RuO2) is usually perceived as the commercial catalyst, while the metallic Ru can also display splendid catalytic activity through appropriate electronic structure modulation.[14-16] Consequently, Ru has good application foreground in the field of electrocatalytic overall water splitting. The size effect, geometrical morphology, crystalline structure (face-centered cubic (fcc) or hexagonal close-packed (hcp) structure), crystal plane orientation, and strong metal-support interaction (SMSI) are the most studied topics for Ru-based electrocatalysts.[17-20] In general, metallic Ru possesses a relatively high surface energy, thus tending to form fair-sized nanoparticles.[21] The catalytic performance declines along with the metal agglomeration during practical electrochemical reactions, making the durability still be the major problem. The oxidation phenomenon (from tetravalence to octavalence) and consequent dissolution at high potentials would also result in the devitalization of Ru-based electrocatalysts.[22, 23] In fact, designing suitable substrate materials can effectively stabilize the metallic Ru, which is vital for optimizing the dispersity issue, reducing particle size, and enhancing the available active site density and the cycling durability.[24, 25] Moreover, the strong coupling effect of the heterointerfaces between Ru and substrates would induce the charge density redistribution, thereby modifying the surface adsorption and reducing the reaction energy barrier.[26]
By means of the lattice similarity of two different material phases, the extraordinary epitaxial heterostructure can be achieved.[27, 28] For example, the Ti-V-N epitaxial heterostructure can be formed owing to the lattice similarity of coexisting TiN and VN phases.[29] We notice that Ru has a terrific lattice similarity with nickel nitride (Ni3N). Based on their crystal structures, they present great lattice matching (dRu (100) ≈ dNi3N (110), dRu (002) ≈ dNi3N (002), dRu (101) ≈ dNi3N (111), dRu (102) ≈ dNi3N (112), dRu (110) ≈ dNi3N (103), and dRu (103) ≈ dNi3N (113); Ru ref. JCPDS No. 01-1253 and Ni3N ref. JCPDS No. 89-5144). Thus, the epitaxial heterostructure is expected to be rationally constructed to acquire desired strongly coupled interfaces. Furthermore, our former studies have testified that the strong interaction between noble-metal atoms and metal sites of substrate materials is favorable for diminishing the particle size of noble metal into tiny clusters and further restricting their agglomeration.[30, 31] In brief, it is feasible to construct the epitaxial heterointerface between Ru clusters and Ni3N substrate.
Herein, density functional theory (DFT) calculations first reveal the redistributed charge density and the enhanced intrinsic electronic transmission capability of the Ru cluster-nickel nitride (cRu-Ni3N) heterostructure, leading to the optimized adsorption behaviors toward various reaction intermediates and the reduced thermodynamic energy barriers for both OER and HER. Experimentally, the cRu-Ni3N porous nanosheets are assembled upon the conductive nickel foam (NF) substrate (cRu-Ni3N/NF). Impressively, tiny Ru clusters are epitaxially in situ grown on the Ni3N nanosheets during the nitridation process, bringing about the strongly coupled heterointerfaces. Benefited from the ameliorative electronic states, fast kinetics mechanism, high active site density, and decent intrinsic activity, the as-obtained cRu-Ni3N/NF exhibits excellent electrocatalytic activity and stability toward OER and HER in alkaline media, which are much superior to the commercial catalysts. Furthermore, such electrode material can be utilized in alkaline overall water splitting, seawater electrolysis, and solar-to-hydrogen generation system.
2 Results and Discussion
To investigate the electronic state and underlying mechanism of the coupled heterointerface between Ru cluster and nickel nitride, DFT calculations were first carried out. As shown in Figure S1, Supporting information, the cRu-Ni3N heterostructure was built based on the Ru cluster (cRu) and Ni3N models. The charge density difference reveals that the establishment of the cRu-Ni3N heterostructure would markedly induce the charge density redistribution (Figure 1a). A large number of electrons assemble at the heterointerface between cRu and Ni3N layer, resulting in higher electronic states near the Fermi level, as relative to independent cRu (Figure 1b). This indicates the enhanced electronic conductivity for cRu-Ni3N heterostructure, which is conducive to accelerating electron transfer during electrocatalytic process.[14, 32] The adsorption behaviors of oxygenated intermediates (Figure S2, Supporting information) or hydrogen (Figure S3, Supporting information) on catalytic sites were calculated to unveil the rate-determining steps (RDS) and theoretical overpotential. For OER, the RDS for the cRu-Ni3N heterostructure is the formation of *OOH from *O, while the contrast models (cRu and Ni3N) are subject to the last step (*OOH → O2) (Figure 1c). Besides, the theoretical overpotential of cRu-Ni3N (1.51 eV) is also lower than the cRu (1.60 eV) and Ni3N (2.06 eV). The change in rate-determining step and the more favorable OER kinetics demonstrate the optimized adsorption behaviors and theoretical catalytic activity upon the cRu-Ni3N heterostructure. Similarly, with regard to HER, the RDS of the cRu-Ni3N heterostructure becomes the hydrogen adsorption step with the ΔG*H value of 0.36 eV, which is superior to the cRu (−0.48 eV) and Ni3N (−0.57 eV) limited by the hydrogen desorption step (Figure 1d).
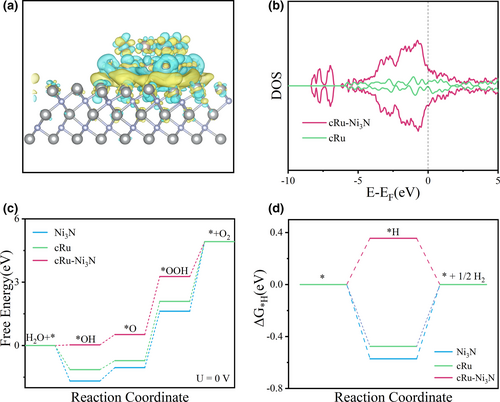
It can be concluded that once forming the cRu-Ni3N heterostructure, the charge density would be redistributed around the interfaces. Such electron-enriched heterointerfaces can effectively improve the intrinsic electron conduction. Sequentially, the adsorption of key reaction intermediates will be regulated, leading to the altered rate-determining step and reduced activation energy barrier for electrocatalytic OER and HER process.[33]
In accordance with the theoretical prediction, we rationally constructed the cRu-Ni3N heterostructure on the nickel foam substrate. As shown in the Figure 2a, the Ni(OH)2 nanosheet arrays were first grown on the NF substrate through a hydrothermal reaction. To obtain the final cRu-Ni3N/NF product, the oven-dried Ni(OH)2/NF was further subjected to the Ru3+ ion incorporating process, followed by the high-temperature nitridation at 350°C for 2 h under an ammonia atmosphere. The Ni3N/NF material was prepared in a similar way, just without the Ru incorporation.
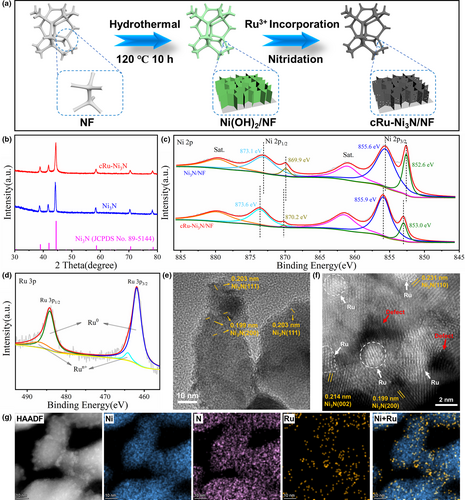
The phase composition of as-prepared materials was analyzed by X-ray diffraction (XRD). As shown in Figure 2b, the scraped powder from cRu-Ni3N/NF exhibits typical characteristic peaks of hexagonal Ni3N (JCPDS No. 89-5144). The diffraction peaks at 39.0°, 42.0°, 44.5°, 58.7°, 70.6°, and 78.5° are indexed to (110), (002), (111), (112), (300), and (113) crystal planes of Ni3N. Importantly, as compared to pure Ni3N powder, there is no characteristic peak relevant to metallic Ru in cRu-Ni3N, indicating the low content of incorporated Ru. To further analyze the elemental components and chemical states of these samples, X-ray photoelectron spectroscopy (XPS) was conducted. Firstly, as for the deconvoluted spectrum of Ni 2p state for cRu-Ni3N/NF, the doublet peaks at 855.9 and 873.6 eV are attributed to the Ni 2p3/2 and Ni 2p1/2, along with two doublet satellites (Figure 2c).[34] Besides, the shoulder peaks at 853.0 and 870.2 eV arise from the NF substrate.[35] Noteworthily, relative to pure Ni3N/NF, the positive peak shift of Ni 2p states can be observed in cRu-Ni3N/NF, heralding the beneficial electron transfer and charge density redistribution upon the as-obtained heterointerfaces, in line with the electronegativity trend and theoretical results.[27, 36] Moreover, the content of those incorporated Ru is about 4.3 at%, as detected in the full spectra (Figure S4, Supporting information), and the Ru 3p spectrum of cRu-Ni3N/NF exhibits that the dominant couple peaks at 461.9 and 484.2 eV correspond to Ru0, while the other two small peaks at 464.2 and 486.9 eV are assigned to the Run+ due to the partial surface oxidation (Figure 2d).[37] Similarly, the Ru 3d spectrum of cRu-Ni3N/NF also confirms the dominating Ru0 (280.4 and 284.4 eV), as well as a little Run+ (281.0 and 285.2 eV) (Figure S5, Supporting information).[38] The exact incorporation content of Ru was measured to be 9.3 wt% from inductively coupled plasma–optical emission spectrometry (ICP-OES). And the N 1s spectrum of cRu-Ni3N/NF can be deconvoluted into two subpeaks at 398.1 and 402.4 eV, consistent with Ni-N and N-O bonds (Figure S6, Supporting information).[36, 39, 40]
The morphology of these as-prepared materials was first observed with scanning electron microscopy (SEM). Different from the smooth surface of bare NF, plentiful Ni(OH)2 nanosheet arrays were uniformly grown on the surface of substrate after the hydrothermal process (Figures S7 and S8, Supporting information). After the nitriding process, abundant holes appeared on those nanosheets along with the phase transformation, resulting from the strong etching of ammonia (Figure S9, Supporting information). Such hole defects can effectively expose more catalytic active sites and improve the active surface area, and the porous structure is favorable for the mass transfer process in electrocatalysis.[41] Besides, the incorporation of Ru would not change the morphologic structure of Ni3N porous nanosheets (Figure S10, Supporting information). The nitridation from 2D hydroxide nanosheet could supply plentiful incorporation location for Ru clusters to achieve the fully exposed epitaxial cRu-Ni3N heterostructure.
The porous structures were further confirmed with high resolution transmission electron microscopy (HRTEM) in Figures S11 and S12, Supporting information. As shown in Figure 2e, for the TEM image of cRu-Ni3N, the legible lattice space of 0.203 and 0.199 nm could be indexed to the (111) and (200) lattice plane of Ni3N, with the absence of lattice fringes for Ru. To thoroughly disclose the existence form of incorporated Ru, the spherical aberration-corrected scanning transmission electron microscopy (ac-STEM) was utilized. Due to the difference in atomic number, the contrast of metal atoms will be significantly different. As shown in Figure 2f, the relatively dark substrate material displays multiple crystal plane orientations of Ni3N, and some bright Ru clusters with the size less than 1.6 nm are epitaxially grown along with the lattice orientation of Ni3N substrate. Such epitaxial heterostructure can be generally observed, well coincident with our expectations (Figure S13, Supporting information). The localized aggregation of Ru atoms was further confirmed by the high-angle annular dark-field (HAADF) STEM image and corresponding energy-dispersive spectroscopy (EDX) elemental mapping images, verifying the existing form of Ru clusters again (Figure 2g and Figure S14, Supporting information). Notably, the above results firmly confirm the successful construction of the epitaxial cRu-Ni3N heterostructure.
The OER performance of these as-prepared electrode materials was first investigated in alkaline media (1 M KOH, pH = 14). The typical iR-compensated polarization curves are shown in Figure 3a, revealing the activity trend of cRu-Ni3N/NF > Ni3N/NF > RuO2 on NF > bare NF. The defect-rich Ni3N/NF electrode exhibits comparable catalytic activity to commercial RuO2, while the incorporated epitaxial Ru clusters would further enhance the electrocatalytic OER performance. Specially, cRu-Ni3N/NF merely needs a low overpotential of 226 mV to achieve the current density of 20 mA cm−2, which is much better than RuO2 (293 mV), Ni3N/NF (289 mV), and NF (332 mV) (Figure 3b), and its performance at higher current densities remains ahead of the control samples. Such performance is quite competitive as compared with those reported noble-metal-incorporated nanocatalysts and self-supporting electrodes (Table S1, Supporting information).
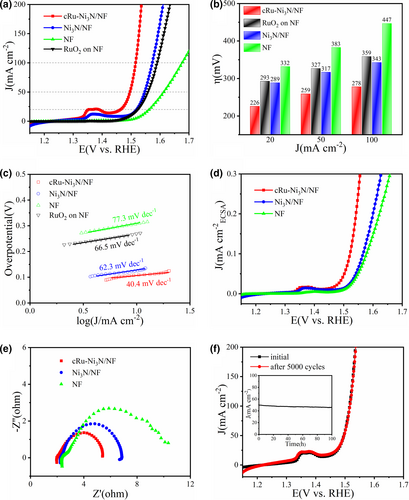
The kinetics features of these catalysts were then evaluated. First, as compared with the Ni3N/NF electrode (62.3 mV dec−1), bare NF (77.3 mV dec−1), and RuO2 catalyst on NF (66.5 mV dec−1), the cRu-Ni3N/NF electrode possesses the lowest Tafel slope of 40.4 mV dec−1, signifying the optimal electrocatalytic kinetics mechanism (Figure 3c).[4] Then, based on the cyclic voltammetry (CV) curves at a succession of scan speeds within the non-Faradaic region, the double-layer capacitance (Cdl) can be calculated (Figure S16, Supporting information). cRu-Ni3N/NF owns a large double-layer capacitance of 18.5 mF cm−2, and the corresponding electrochemical active surface area (ECSA) is reckoned to be 308.3 cm−2, which are superior to those of Ni3N/NF (12.7 mF cm−2, 211.7 cm−2) and NF (4.17 mF cm−2, 69.5 cm−2). The high ECSA of cRu-Ni3N/NF is mainly owed to the defect-rich nanosheet structure, and the epitaxial heterostructure brings about more potential active sites for electrocatalytic reactions and further enhance the ECSA value. Accordingly, the polarization curves normalized to ECSA values were drawn (Figure 3d). Distinctly, cRu-Ni3N/NF also has the most remarkable intrinsic catalytic activity toward OER, with the smallest overpotential of 312 mV at 0.2 mA cm−2ECSA. Such high intrinsic activity should be principally credited to the in situ grown epitaxial cRu-Ni3N heterostructure that lowers the reaction energy barrier. Besides, the fast reaction kinetics of cRu-Ni3N/NF was further validated by the electrochemical impedance spectroscopy (EIS). As shown in Figure 3e and Figure S17, Supporting information, cRu-Ni3N/NF possesses the lowest extracted charge transfer resistance (Rct) of 3.1 Ω among all electrode materials, suggesting the ultrafast electron transfer process upon the epitaxial cRu-Ni3N heterointerfaces. All these manifest that the construction of the epitaxial heterostructure between Ru clusters and Ni3N substrate would effectively induce the electron interaction and charge density redistribution.[27] Sequentially, the enhancement of active site density, intrinsic catalytic activity, and specific conductance testifies the modified electrochemical kinetics for OER.
The OER stability of cRu-Ni3N/NF electrode was analyzed by the CV cycling and chronoamperometry tests. Figure 3f shows that the polarization curve of cRu-Ni3N/NF remains almost unchanged after 5000 CV cycles. Besides, during the long-term operation for 100 h, the electrode material shows extremely steady current output at the OER overpotential of 260 mV. Such outstanding durability of cRu-Ni3N/NF suggests the superiority of the novel epitaxial heterostructure. After the OER stability test, the morphology and chemical states of the cRu-Ni3N/NF electrode were reanalyzed. The pristine porous nanosheets tend to reconstruct into nanowrinkles, which is also favorable for the rapid mass transfer (Figure S18, Supporting information).[4] Furthermore, the surface oxidation was confirmed by XPS and Raman spectra. As shown in Figure S19, Supporting information, the surface of Ni3N substrate is apt to generate the nickel oxyhydroxide species (as detected at 480 and 555 cm−1 in Raman spectra). The positive peak shifts of both Ru 3p and Ru 3d states, and the increasing relative amount of Run+, suggest the slight valence ascension and in situ formed oxidation layer on the epitaxial Ru clusters as well (Figures S20 and S21, Supporting information).[14, 42] The inevitable oxidized layer of oxide/oxyhydroxide serves as the accessional active site and must make a great contribution to the electrocatalytic reactions. Thus, the slight decay of the catalytic activity for cRu-Ni3N/NF during long-term operation probably mainly stems from the transformation of morphology, the mild surface oxidation, and the dissolution of active metallic moieties (Table S2, Supporting information). From this, the cRu-Ni3N/NF electrode is still very stable during the long-term continuous electrocatalysis.
Similarly, the electrocatalytic HER activity in alkaline media was then evaluated. As shown in Figure 4a, the polarization curves show that the bare NF owns terrible HER performance, while the defect-rich Ni3N nanosheets exhibit acceptable catalytic activity. Impressively, the epitaxially grown cRu-Ni3N heterostructure can afford the current density of 10 mA cm−2 with a low overpotential of 32 mV, nearly the same as the commercial Pt/C catalyst on NF (31 mV @ 10 mA cm−2) (Figure 4b). Meanwhile, the HER activity of the cRu-Ni3N/NF electrode surpasses that of Pt/C catalyst at the relatively high current densities (99 mV for cRu-Ni3N/NF and 125 mV for Pt/C @ 100 mA cm−2). Such superb activity of cRu-Ni3N/NF outperforms most reported precious metal-incorporated electrocatalysts and numerous self-supporting electrodes (Table S3, Supporting information).
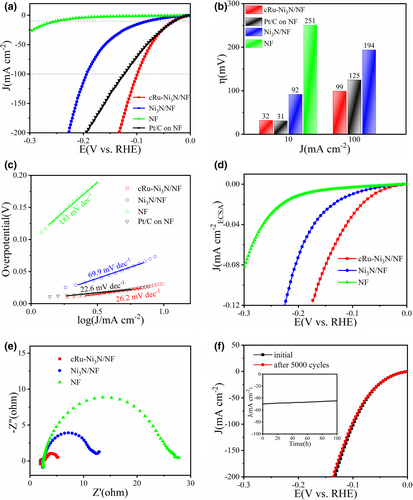
As expected, the general tendency of Tafel slopes coincides with the polarization curves at early kinetic region (Figure 4c). The cRu-Ni3N/NF exhibits the low Tafel slope of 26.2 mV dec−1, suggesting a fast HER dynamic process following the Volmer-Tafel mechanism.[43, 44] Besides, cRu-Ni3N/NF also owns the larger Cdl value than the Ni3N/NF and NF electrodes, as calculated from the sequential CV curves within the non-reactive region (Figure S22, Supporting information). It indicates the highest ECSA value and the most abundant potential active sites for cRu-Ni3N/NF. The ECSA-normalized linear sweep voltammetry (LSV) curves demonstrate the best intrinsic activity for the cRu-Ni3N/NF electrode as well (Figure 4d). Another proof of the improved HER kinetics for cRu-Ni3N/NF is the EIS curves shown in Figure 4e and Figure S23, Supporting information, testifying its glorious intrinsic conductivity and extremely rapid electron transport. Furthermore, the activity of cRu-Ni3N/NF maintains stable during the long-time i-t test at the HER overpotential of 75 mV, and scarcely any performance decay could be found from the CV cycling test (Figure 4f). All these signify that the cRu-Ni3N/NF electrode, possessing prominent activity and stability, can serve as the dual-function electrocatalyst for OER and HER under alkaline condition.
To access the application prospect of cRu-Ni3N/NF electrode materials in practical electrochemical devices, the two-electrode overall water splitting (OWS) devices with the conventional 1 M KOH electrolyte were first assembled. The voltage differences calculated from the above polarization curves (ΔV = ηOER + ηHER) exhibit that the cRu-Ni3N/NF electrode has the lower ΔV values (1.57 and 1.62 V @ 50 and 100 mA cm−2) than the commercial Pt/C and RuO2 catalyst couple (1.64 and 1.71 V @ 50 and 100 mA cm−2), implying the better utilization potentiality of cRu-Ni3N/NF for both electrodes of OWS devices (Figure 5a). Just as expected, the cRu-Ni3N/NF electrodes show excellent OWS performance, which only need the operational voltages of 1.61 and 1.71 V to afford the current densities of 50 and 100 mA cm−2, respectively (Figure 5b). Such performance is superior to the Pt/C || RuO2 couple, and many reported bifunctional electrocatalysts (Figure S24 and Table S4, Supporting information). Besides, the steady current output at 1.57 V and negligible overpotential decline testify the excellent long-term stability for cRu-Ni3N/NF electrode in alkaline OWS cell (Figure 5c). To estimate the faradic efficiency of water splitting on cRu-Ni3N/NF electrodes, the generated hydrogen and oxygen gases were collected through drainage method at separated sides of H-style electrolytic cell (Video S1, Supporting information). The volume ratio of H2 to O2 is about 1.96:1; thus, the faradic efficiency is estimated to be ≈100% (Figures S25 and S26, Supporting information). All these indicate that the cRu-Ni3N/NF electrode is a very promising substitute for electrocatalytic hydrogen generation.
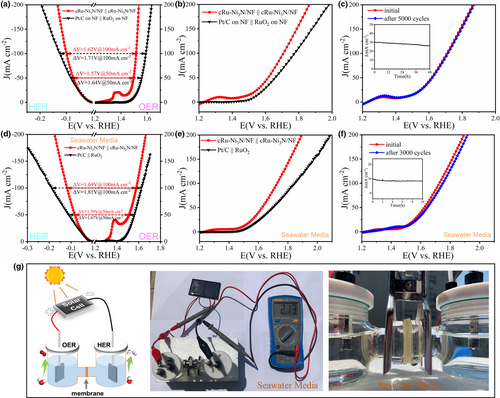
In addition, to alleviate the dependence on freshwater resource, we further measured the electrochemical behaviors of cRu-Ni3N/NF electrode in alkaline seawater media. First, we tested the semi-electrolytic cell performance of cRu-Ni3N/NF and commercial catalysts in the 1 M KOH seawater solution (Figure 5d). With respect to OER, cRu-Ni3N/NF exhibits preferable catalytic activity to commercial RuO2 catalyst. Concretely, to deliver the current density of 50 mA cm−2, the cRu-Ni3N/NF electrode merely requires the overpotential of 280 mV, which is 50 mV lower than that of RuO2 catalyst. Likewise, it also owns the lower overpotential (36 mV @ 10 mA cm−2) than state-of-the-art Pt/C catalyst (39 mV @ 10 mA cm−2) for HER. Correspondingly, it has lower voltage differences than Pt/C and RuO2 couple at different current densities. Importantly, the extremely low overpotential augment in alkaline seawater media, as compared with the activity parameters in freshwater media, proves the few side reactions as well as the higher selectivity and efficiency on the cRu-Ni3N/NF electrode for seawater electrolysis. The low Tafel slope of the cRu-Ni3N/NF electrode indicates its fast reaction kinetics, and the small performance attenuation portends its excellent long-term stability, no matter toward OER or HER in alkaline seawater media (Figure S27, Supporting information). As for the OWS performance in seawater media, the cRu-Ni3N/NF || cRu-Ni3N/NF couple displays excellent catalytic performance (1.62 and 1.73 V @ 50 and 100 mA cm−2), as compared with the Pt/C || RuO2 couple (1.70 and 1.84 V @ 50 and 100 mA cm−2) (Figure 5e and Figure S28, Supporting information). The evolved gas volumes of both electrodes were measured to be 1.94:1, very close to the ratio of H2/O2, and the anodic gas product was further confirmed to be dominated oxygen rather than chlorine through the chemical method and differential electrochemical mass spectrometry (DEMS) analysis (Figures S29–S31, Supporting information). In addition, the possible formation of hypochlorite can be eliminated with the titration of 0.1 M K2S solution (Figure S32, Supporting information). All these suggest the high selectivity toward OER in alkaline seawater media within the measured voltage range (less than 1.7 V).[45] Besides, such bifunctional electrode couple exhibits splendid durability, as unveiled from CV cycling, and i-t response test at 1.5 V (Figure 5f). These results show that the cRu-Ni3N/NF electrode can be employed in the direct seawater electrolysis free from freshwater dependence.
As mentioned above, the water electrolysis is expected to combine with photovoltaic industry for the sake of cost reduction. An integrated system was designed by directly connecting the commercial solar panel to water electrolysis devices. The schematic diagram of this solar-to-hydrogen system is shown in Figure 5g. Motivated by the sun illumination, the solar panel could offer a considerable voltage output of 2.13 V, which can drive the rapid electrocatalytic water splitting. Visually, the violent gas escape can be observed on both sides of H-type cell no matter in freshwater or seawater media, suggesting the decent hydrogen production rate upon the cRu-Ni3N/NF electrode (Videos S2 and S3, Supporting information). To eliminate the influence of anodic side reaction in seawater media, the actual applied voltage must be lowered to around 1.7 V by customizing the output voltage of solar panel, or tandem connecting external resistance. Such an integrated solar-to-hydrogen system can enable the highly efficient electrocatalytic hydrogen production, dispensing with the electric power from the existing power grid. Undoubtedly, it would provide large-scale high-purity hydrogen feedstock for hydrogen economy times, meanwhile getting rid of the dependence of long-range electrical supply.
3 Conclusion
In summary, considering the lattice similarity between ruthenium (Ru) and nickel nitride (Ni3N) as well as the size confinement effect upon the strongly coupled interface, the epitaxial heterostructures of Ru clusters and Ni3N substrate (cRu-Ni3N) were systematically investigated. DFT calculations first unveil the charge density redistribution upon the cRu-Ni3N heterointerface and the concomitant enhanced intrinsic electronic conductivity, resulting in the regulated adsorption behaviors and reduced reaction energy barriers for both electrocatalytic OER and HER. Then, the epitaxially in situ grown cRu-Ni3N heterostructure was successfully constructed, whose structural features were directly verified through advanced electron microscopes. Such electrode material exhibits excellent catalytic activity, rapid reaction kinetics, and robust durability for both OER and HER in alkaline media. Consequently, we demonstrate the application performance of this electrode material in diverse electrochemical apparatus, including overall water splitting devices with alkaline freshwater and seawater media, and the equipment combined with photovoltaic power. Thus, this work profoundly investigates the reaction mechanism and application potential of the epitaxial cRu-Ni3N heterostructure for electrocatalytic freshwater and seawater splitting systems, providing guidance for the development of novel electrode materials with epitaxial in situ grown interfaces.
4 Experimental Section
Material Syntheses
For the synthesis of cRu-Ni3N/NF, the surface of nickel foam (NF, 3 cm × 4 cm) was first cleaned by sonication in 0.5 m H2SO4, acetone and ethanol for half an hour, respectively. The pre-treated NF was placed in a 60 mL aqueous solution containing 10 mmol Ni(NO3)2·6H2O and 20 mmol HMT, which was beforehand transferred in a Teflon-lined stainless steel autoclave. The Ni(OH)2/NF can be obtained by hydrothermal reaction at 120 °C for 10 h, followed by rinsing with water and vacuum drying at 60 °C for 8 h. After that, the Ni(OH)2/NF was immersed in a RuCl3·nH2O ethanol solution (15 mg mL−1) for 30 min, and then mildly rinsed with ultrapure water. Finally, the dried Ru-Ni(OH)2/NF was nitrogenized at 350 °C for 2 h with the flowing ammonia gas (60 sccm) to obtain the cRu-Ni3N/NF. As a contrast, the Ni3N/NF was prepared with the similar strategy, just without the Ru incorporation.
Electrochemical Measurements
The electrochemical tests were conducted with typical three-electrode system connected to the electrochemical workstation (CHI750E). The as-obtained self-supporting materials with a geometric area of 0.25 cm2, graphite rod and Hg/HgO electrode were chosen as the working electrode, counter electrode and reference electrode, respectively. The contrastive commercial Pt/C and RuO2 working electrodes were prepared by coating the catalyst inks onto the clean NF. The catalyst inks were prepared by dispersing 5 mg powder into the mixture of 880 μL isopropanol, 100 μL H2O and 20 μL 5 wt% Nafion. The catalyst loading was 4 mg cm−2, similar with that of main sample. The electrolytes were 1 m KOH freshwater solution and 1 m KOH seawater solution. To prepare the alkaline seawater media, the collected seawater was first filtered to remove the insoluble impurities. Then the potassium hydroxide was added into the seawater to obtain the 1 m KOH solution. After stirring for half an hour, the solution was filtered again to remove the precipitated substances. The alkaline electrolyte mainly contains cationic K+, Na+, anionic OH−, Cl−, , as well as other trace ions.
For the OER test, the linear sweep voltammetry was recorded with the scan rate of 1 mV s−1. And the LSV curves of HER employed the scan rate of 5 mV s−1. All the polarization curves were iR-corrected. And the electrode potential was converted versus reversible hydrogen electrode (RHE): E(vs. RHE) = E(vs. Hg/HgO) + 0.926 V. The electrochemical impedance was conducted with the frequency of 0.01 to 100 000 Hz. Electrochemical specific surface area (ECSA) was obtained based on the formula: ECSA = Cdl/Cs, where the Cdl signifies the double-layer capacitance and the Cs means the specific capacitance of an ideal 1 cm2 flat surface. Here we adopt the general value of 60 μF cm−2 for Cs. The Cdl was calculated based on the cyclic voltammetry curves within the non-Faradaic region, with the scan rates from 20 to 120 mV s−1. Then, the Cdl value can be obtained by the formula: Cdl = ΔJ/2v, where the ΔJ represents the current density difference and the v is the scan rate. The stability was evaluated by comparing the LSV curves before and after the CV cycling test, and the current decline with time at a constant potential was surveyed.
As for the two-electrode water splitting performance, the polarization curve without iR compensation was recorded with the as-synthesized bifunctional electrode material as both anode and cathode. The Pt/C || RuO2 couple (4 mg cm−2) was also measured as a contrast. The stability was assessed through CV cycling test and chronoamperometry. The generated H2 and O2 were separated in a typical H-style cell, and then were collected and quantitatively evaluated by drainage method. In order to assess the practical industrial application prospect, the solar-to-hydrogen devices were assembled by connecting the commercial solar panel with the H-style cell.
Acknowledgments
J.Z. and R.L. contributed equally to this work. This work was financially sponsored by the National Natural Science Foundation of China (Grant No. 22075223, 22179104), the State Key Laboratory of Advanced Technology for Materials Synthesis and Processing (Wuhan University of Technology) (2021-ZD-4), and the Fundamental Research Funds for the Central Universities (No. 2020-YB-012). We wish to thank Xiaoqing Liu and Tingting Luo (Materials Analysis Center of Wuhan University of Technology) for TEM measurement support.
Conflict of interest
The authors declare no conflict of interest.




