Constructing Crystalline g-C3N4/g-C3N4−xSx Isotype Heterostructure for Efficient Photocatalytic and Piezocatalytic Performances
Abstract
Graphitic carbon nitride (g-C3N4) is viewed as a promising visible-light photocatalyst for industrialization due to its low processing temperature and high chemical stability. However, serious charge recombination caused by incomplete polymerization during direct calcination of nitrogen-rich precursors significantly limits its photocatalytic performances. To boost charge separation, herein, we propose a rational strategy by constructing a crystalline g-C3N4/g-C3N4−xSx isotype heterostructure through the molten salt method. Theoretical calculation reveals that apparent charge-transfer channels are formed between g-C3N4 and S-doped g-C3N4 layers in the heterostructure. Owing to high crystallinity for decreasing charge recombination and isotype heterostructure for efficient charge transfer, the as-prepared g-C3N4/g-C3N4−xSx showed remarkable photocatalytic performances with the hydrogen production rate elevated by up to 12.3 times of its singular components. Another novelty of this work is we investigated for the first time the piezocatalytic activity of crystalline g-C3N4 by characterizing its performance for H2O2 generation and KMnO4 reduction. Strikingly, its superior piezocatalytic performance over components can be further improved by NaBH4 treatment, which is uncovered to enhance the asymmetric structure of crystalline g-C3N4 by introducing extra cyano groups and removing partial NHx species in its tri-s-triazine layer structure. This work opens up new strategies for the design of highly efficient polymeric photocatalysts and highlights the piezocatalytic studies of g-C3N4.
1 Introduction
Photocatalysis that is considered as a high-potential clean and sustainable process to achieve solar energy conversion for renewable use could be a potential solution for the increasing global warming and fuel shortage. To make effective use of solar light and meet the industrial demands, developing highly efficient visible-light responsive photocatalysts with excellent stability and low cost is currently one of the fundamental and challenging issues to be solved for photocatalysis.[1-5] Graphitic carbon nitride (g-C3N4) has attracted much research attention as a promising “holy grail” photocatalyst due to the prominent merits of visible-light absorption, proper band positions for abundant redox reactions, low cost and variable modulation strategies, etc.[6-11] However, the extensively studied g-C3N4 obtained by direct polymerization of nitrogen containing precursors (such as melamine,[12] dicyandiamide,[13] urea,[14] thiourea,[15] etc.) generally has low crystallinity (semi- or non-crystal) due to the incomplete deamination/polymerization of precursors from the poor condensation kinetics.[16, 17] These g-C3N4 materials show melon structure consisting of cohered strands of tri-s-triazine units with NH/NH2 groups,[18] and the hydrogen bonds in the intralayer result in high electron localization and thus poor transport of charge carriers.[19-21] Suffering from difficult migration as well as serious recombination of charge carriers in the small ordered structure with high density defects, the visible-light utilization efficiency of these g-C3N4 materials is, therefore, greatly restricted. To address these issues, exploring effective strategies to promote charge-carrier transport and separation in g-C3N4 is highly desirable.
Developing high-crystalline g-C3N4 with condensed structure can effectively accelerate charge-transfer kinetics within the material and thus improve its photocatalytic performance. The previous study shows the reprocessing of melon in molten salt can lead to the formation of crystalline g-C3N4 with tri-s-triazine units and condensed conjugation, which exhibited better charge mobility and more promising photocatalytic activity than its melon based counterparts.[16, 22] On the other hand, heterostructure construction,[23-25] which has been widely reported to decrease charge-carrier recombination in singular components through promoting the transfer of charge carriers at the interface by the built-in electric field, is also viewed as an effective approach to improving the utilization of photo-generated charge carriers. Compared with heterostructures composed of different components, the isotype based heterostructure is advantageous for acquiring the higher driving force for charge-carrier separation because of their similar physicochemistry and intrinsic properties that can effectively decrease the interfacial barrier.[26] Recently, Shen reports an encouraging self-Z-Scheme 2D/2D g-C3N4 heterostructure composed of pristine and boron-doped g-C3N4 with melon based structure, which showed excellent photocatalytic performance due to its strong interfacial interaction and elaborate band structure modulation.[26, 27] Based on the two aspects aforementioned, the combination of crystallinity enhancement and isotype heterostructure construction may provide some potential solutions for the low charge-carrier separation efficiency of g-C3N4. Xiang recently reported the fabrication of crystalline heptazine-/triazine-based C3N4 heterostructure and characterized their photocatalytic reactivity for hydrogen generation.[28] Notwithstanding these encouraging investigations, this field is still in its infancy stage and developing the simple processing routes with in-depth understanding for heterostructure built-up discipline remains challenging.
Piezo-catalysis that is a process using the piezoelectric polarization to separate charge carriers for catalysis by induced deformation from mechanical vibration is becoming an emerging field for sustainable energy and environment use.[29, 30] Since this concept proposed about 10 years ago,[31] most of the previous piezo-catalysis researches is focused on the inorganic piezoelectric materials (such as ZnO and BaTiO3, etc.), while the organic piezoelectric material for catalysis is rarely reported.[32, 33] Recently, g-C3N4 has been found to present piezoelectric properties from the asymmetry of the triangle holes by theoretic simulations and experimental observation.[34] The subsequent analysis reports the melon based g-C3N4 from direct polymerization of melamine has the capability to realize piezocatalytic reactions under ultrasonication.[35] Similar to the photocatalysis process, the characteristics of g-C3N4 closely related to the transportation and separation of charge carriers are supposed to affect its piezocatalytic performances. The investigations on the piezocatalytic activity of g-C3N4 are thus highly anticipated, which may extensively enlarge the use of carbon nitride based materials for energy and environmental applications.
In this paper, we report the feasible construction of crystalline g-C3N4/S doped g-C3N4 (g-C3N4−xSx) isotype heterostructure through the direct reprocessing of mixed C3N4 materials obtained from urea and thiourea in molten salt. Confirmed by density functional theory (DFT) analysis, the component g-C3N4 crystalline nanoflakes with different modulated band structures form high-quality heterostructures, which significantly improved the separation of photogenerated charge carriers and decreased the transfer barriers. As a consequence of the structure improvements, this novel g-C3N4/g-C3N4−xSx isotype heterostructure shows obviously enhanced photocatalytic performances under visible-light irradiation compared with its low-crystalline and singular counterparts. The piezocatalytic behaviors of high-crystalline C3N4 based materials are also revealed for the first time, which showed excellent reactivity for H2O2 generation and KMnO4 reduction. More importantly, in addition to the further improvement of photocatalytic performance, NaBH4 treatment was confirmed to significantly enhance its piezocatalytic reactivity by changing the asymmetry of the atomic structure.
2 Results and Discussion
2.1 Structure and Morphology Analysis
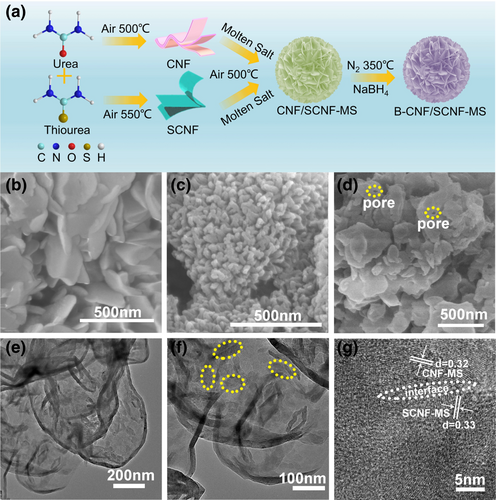
The sample synthesis process is shown in Figure 1a. Herein, g-C3N4 and g-C3N4−xSx were obtained by thermal polymerization of urea and thiourea at air, respectively, which introduces the oxidative etching of hydrogen-bonded chains of the polymeric subunits in C3N4 and thus leads to the formation of nanoflakes (Figures S1 and S2, Supporting Information) rather than the large bulk that is commonly observed. The samples are thus denoted as CNF (carbon nitride flakes) and SCNF (S-doped carbon nitride flakes). Among various C3N4 based precursors, thiourea owning the similar structure to urea but with S species can increase polymerization degree while the formed C3N4 can expose more active sites and promote catalytic activity of the reactants.[36] The high-crystalline g-C3N4/g-C3N4−xSx isotype heterostructure (denoted as CNF/SCNF-MS) was then built by molten salt treatment of CNF and SCNF mixture. Herein, molten salt serves as dual functional roles, namely ensuring the formation of high-crystalline C3N4 materials by re-organizing their polymeric structure and elevating the crystallinity, as well as promoting the interfacial chemical bonding between two phases for smooth charge transfer. Compared with the mechanical mixture or simple annealing that always lead to poor interfacial connection, molten salt treatment can form the intimate junctions with high-strength bonding.[28] Furthermore, CNF/SCNF-MS was treated by NaBH4 and the formed product was denoted as B-CNF/SCNF-MS. Both CNF and SCNF presented nanoflake morphologies but in different dimensions (Figures S1 and S2, Supporting Information). The subsequent molten salt treatment (CNF-MS and SCNF-MS) did not significantly influence the morphology but with the formation of a more compact structure (Figure 1b,c) due to the weakened O2 etching by molten salt coverage. Similar to CNF/SCNF that combines the characteristics of CNF and SCNF (Figure S3, Supporting Information), CNF/SCNF-MS are composed of nanoflakes from the components CNF-MS and SCNF-MS while the integration of two components are uniform (Figure 1d). The nanoflake based structure of samples is beneficial for enlarging their piezocatalytic effect and strengthening the mechanical energy harvesting over the bulk C3N4.[37] TEM images under different magnifications (Figure 1e,f) confirm the bridging of CNF-MS and SCNF-MS nanoflakes in different dimensions with SCNF-MS circled in the figure. The lattice spacing of d values from different phases of the heterostructure in high-resolution TEM image (HR-TEM, Figure 1g) was calculated to be 0.32 and 0.33 nm, which match well with the data from XRD as shown in the latter section, respectively. Further treatment with NaBH4 leads to the slight formation of some fragmentary flakes though the overall morphology did not change (Figure S4, Supporting Information).
The phase compositions of all samples were investigated by XRD. As widely reported, the extensively studied g-C3N4 has two characteristic peaks in the XRD patterns at around 13° and 27°, representing the periodic intralayer motif and the interlayer stacking, respectively.[15] Those two characteristic peaks without other extra peaks are clearly observed in the pattern of samples without molten salt treatment (namely CNF, SCNF and CNF/SCNF, Figure S5, Supporting Information). Interestingly, another new peak at 8.1° that is related to the reorganization of in-plane structure is detected for all molten salt treated samples (Figure 2a), demonstrating the high crystallinity of those samples. The appearance of this new peak is also reported in other crystalline structured g-C3N4 in literatures.[16, 38] Crystallinity enhancement for samples treated with molten salt can also be confirmed by comparing their full width at half-maximum (FWHM) of the interlayer stacking peaks (Figure S6 and Table S1, Supporting Information). Correspondingly, FWHM values of CNF/SCNF and CNF/SCNF-MS are situated between their components, further confirming the successful formation of heterostructures. Meanwhile, the interlayer characteristic peak with highest intensity is positively shifted from 27.22° (CNF) to 27.80° (CNF-MS), and 27.47° (SCNF) to 27.79° (SCNF-MS), indicating the stacking distance between layers was reduced for samples after molten salt treatment. Compared with those of CNF-MS and SCNF-MS, the interlayer spacing is also narrowed for their heterostructure CNF/SCNF-MS with a positively shifted peak to 27.86°. In this case, NaBH4 treatment leads to the intensity decrease of the peak at 8.1° (Figure 2b), indicating the slight destruction of in-plane periodicity. Correspondingly, the crystallinity of B-CNF/SCNF-MS was also confirmed to be slightly decreased by FWHM data (Table S1, Supporting Information) compared with that of CNF/SCNF-MS.
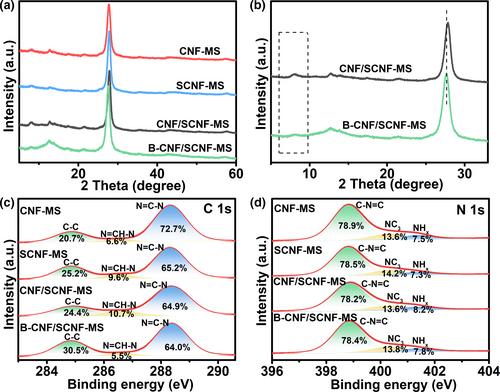
XPS survey spectra along with high-resolution elemental profiles of different samples were recorded to further reveal chemical states and atomic environment of samples, as shown in Figure 2c,d and Figures S7–S10, Supporting Information. Consistent with the XRD phase analysis result shown above, C, N, O are all observed in the XPS survey spectra of samples (Figures S7a, S8a, S9a, S10a, Supporting Information). Meanwhile, some trace amounts of S were detected in SCNF-MS, CNF/SCNF-MS and B-CNF/SCNF-MS, confirming the realization of sulfur doping (g-C3N4−xSx) in these three samples. In detail, the C 1s spectra for CNF-MS, SCNF-MS, CNF/SCNF-MS and B-CNF/SCNF-MS (Figure 2c) all show the characteristic peaks at 284.8, 286.6 and 288.3 eV (Table S2, Supporting Information), which correspond to the bonding states of C-C, N=CH-N and N=C-N, respectively.[39] The peaks in N 1s spectra (Figure 2d) at 398.8, 400.4, and 401.4eV can be assigned to sp2 bonded nitrogen in the triazine rings (C-N=C), tertiary nitrogen bonded with carbon (N-C3) and N-H species (NHx), respectively.[38, 40] Note that the peak percentages of both N=CH-N in C 1s and NHx in N 1s spectra for B-CNF/SCNF-MS decrease compared with those for CNF/SCNF-MS, indicating the loss of NHx for the sample after NaBH4 treatment. This is consistent with the XRD result that the in-plane periodicity is destroyed by NaBH4 use. Furthermore, the percentage of sp2 bonded nitrogen (C-N=C) over that of other N species, which are generally considered as one of positive factors to enhance the photocatalytic activity of g-C3N4,[41] increases for B-CNF/SCNF-MS. For O 1s high-resolution spectra, the O element content was reduced from 8.93% to 4.8% for the sample before and after NaBH4 treatment (Table S3, Supporting Information). The O 1s spectra can be deconvoluted into two peaks at 533 to 531.6 eV (Figures S9, S10, Supporting Information) that correlate to O-N intermediates and surface hydroxyl groups (O-H), respectively, while their percentage ratio decrease from 2.4:1 (CNF/SCNF-MS, Figures S9, Supporting Information) to 1.7:1 (B-CNF/SCNF-MS, Figure S10, Supporting Information) indicates the reductive effect of NaBH4.[42]
FTIR was conducted to further reveal the surface groups of samples and all samples show the typical vibration modes of graphite carbon nitride (Figure S11, Supporting Information). Specifically, the broad band from 1200 to 1650 cm−1 is attributed to the stretching modes of aromatic C-N and C=N bonds while the characteristic peaks at 810 cm−1 correspond to typical stretching and bending vibrations of triazine ring.[43, 44] In addition, the absorption band between 3000 and 3500 cm−1 are generally attributed to the stretching vibration modes of the uncondensed amine group (N-H).[45] The characteristic peak at 2180 cm−1 stems from the vibration of cyano group. It is worth mentioning that the peak area ratio of cyano group to the triazine ring apparently increases after molten salt treatment while the subsequent NaBH4 treatment can further increase this ratio to the maximum value among all samples (Table S4, Supporting Information). Combining with the XPS results, it is assumed that NaBH4 treatment has removed partial NHx groups and initiated the formation of cyano groups, which may contribute to the shift of periodic interlayer diffraction peak toward lower angle in XRD. The surface area of CNF/SCNF-MS and B-CNF/SCNF-MS is analyzed by N2 adsorption isotherms (Figure S12, Supporting Information) and the type IV characteristics of both curves confirms the mesoporosity of samples.[46] CNF/SCNF-MS and B-CNF/SCNF-MS show the specific surface area (SSA) value of 56.651 and 58.690 m2/g, which are higher than that of most reported C3N4 results from thermal condensation (commonly in the range of 5 and 30 m2/g).[15, 47] The BJH data (Table S5, Supporting Information) demonstrates mesopores are the primary pore structure in the heterostructure, which contribute to 43% SSA while micropores under 1.5 nm can also be found. The hierarchical porous structure is favorable for promoting the transfer of mass/charge carriers between the photocatalyst/reactant, and shortening the mass diffusion length.[48] Note NaBH4 treatment did not impact the SSA value and pore structure of CNF/SCNF-MS.
2.2 Electronic Band Structure Investigation
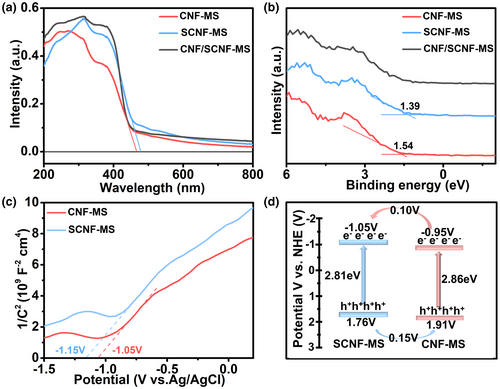
The electronic band structure was investigated to explore the possible charge-carrier transfer pathway in the isotype g-C3N4/g-C3N4−xSx heterostructure. As shown in Figure 3a, the UV-visible-light absorption edges of SCNF-MS and CNF-MS were around 477 nm and 465 nm, respectively, while the corresponding band gap (Eg) values obtained from the Kubelk-Munk function calculation are approximately 2.86 eV for CNF-MS and 2.81 eV for SCNF-MS (Figure S13, Supporting Information). For the heterojunction of CNF/SCNF-MS, its light absorption spectrum lies between the component CNF-MS and SCNF-MS that meets the expectation. This is similar to the case for CNF/SCNF and the corresponding band gap of CNF and SCNF are 2.88 and 2.83 eV (Figure S14a, Supporting Information). It can be clearly observed the molten salt treatment can extend the visible-light absorption of g-C3N4 (Figure S14b, Supporting Information). Meanwhile, NaBH4 treatment can further lead to the obvious red shift of light absorption edge from 450 nm to 466 nm (Figure S14c, Supporting Information), possibly due to the generation of extra cyano groups[49] as confirmed by FTIR result. An additional light absorption shoulder from 450 to 600 nm is also observed for B-CNF/SCNF-MS due to the formation of nitrogen vacancies.[50, 51] The aforementioned result also implies the molten salt and NaBH4 treatment is beneficial for improving the total amount of photogenerated carriers, and thus enhancing the photocatalytic performance. Retrieving back to the electronic structure characterization, the valence band spectra of molten salt treated samples (Figure 3b) indicate that the VB edge of CNF-MS (1.54 eV) is 0.15 eV lower than that of SCNF-MS (1.39 eV). The obtained Mott-Schottky diagrams (Figure 3c) of CNF-MS and SCNF-MS both show positive slopes, indicating their n-type semiconductor characteristics.[52] The flat band potential (Efb) are determined at −1.15 and −1.05 V for SCNF-MS and CNF-MS versus Ag/AgCl based on the Mott-Schottky formula, which are further converted into −0.95 and −0.85V versus normal hydrogen electrode (NHE) according to the equation of ENHE = EAg/AgCl + 0.197 V.[53] For many n-type semiconductors, Efb is closely related to conduction band (CB) and deemed to be more positive about 0.1 V than its conduction band potentials (ECB). Therefore, the ECB of SCNF-MS and CNF-MS are calculated to be about −1.05 and −0.95 V (vs. NHE). According to these results, the calculated electronic band structure is illustrated in Figure 3d. It can be clearly observed that Type II heterostructure is formed between the two components, which has been widely proposed to be advantageous for improving the photocatalytic performance.[54, 55] In our case, upon visible-light irradiation, the photo-excited electrons on the surface of SCNF-MS can transfer to CNF-MS while the photo-excited holes transfer from CNF-MS to SCNF-MS, facilitating the efficient charge separation and promoting better utilization of charge carriers.
2.3 Photocatalytic Performance Analysis
To evaluate the photocatalytic visible-light activities of the samples, both RhB degradation and hydrogen production tests were conducted with the results shown in Figure 4. The photocatalytic degradation rates of RhB for all samples are improved after molten salt treatment (Figure 4a and Table S6, Supporting Information). In detail, the reactivities of CNF-MS and SCNF-MS are 1.41 and 1.08 times those of CNF and SCNF, respectively, while this enhancement is enlarged for their heterostructure with a value of 2.09 times for CNF/SCNF-MS over its untreated counterpart, implying the high crystallinity assisted optimization of g-C3N4/g-C3N4−xSx heterostructure is effective in photocatalysis. Correspondingly, the degradation rate of CNF/SCNF-MS is about 0.04229 min−1 (Table S6, Supporting Information), which is about 2.59 times and 1.58 times those of SCNF-MS and CNF-MS (Figure 4a), respectively, confirming the effectiveness of the heterostructure construction for photocatalytic activity elevation. The highest photocatalytic reactivity for dye degradation is achieved by B-CNF/SCNF-MS, which is 1.73 times that of CNF/SCNF-MS. In addition, the component ratio of the g-C3N4/g-C3N4−xSx heterostructures as well as proportions of NaBH4 to raw material is also optimized with the detail presented in Tables S7 and S8, Supporting Information, respectively.
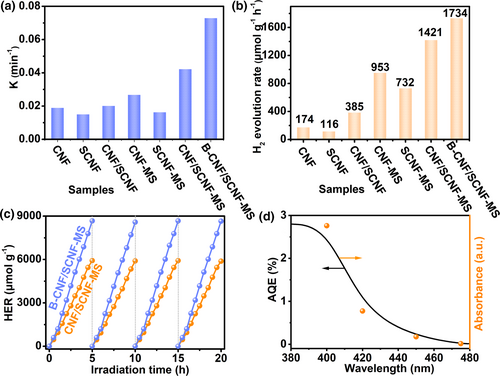
The photocatalytic hydrogen production performances of samples are evaluated with 3 wt% Pt loaded as co-catalyst (Figure 4b). CNF-MS and SCNF-MS have hydrogen evolution rates 5.48 times and 6.31 times those of CNF and SCNF, respectively. Meanwhile, CNF/SCNF-MS shows excellent hydrogen production performance of 1421 μmol h−1g−1, which is about 1.94 times and 1.49 times those of SCNF-MS and CNF-MS. Consistent with dye degradation, these results further reveal the high crystallinity induced by molten salt treatment and the heterostructure construction have positive influence on the photocatalytic activities. B-CNF/SCNF-MS displayed a hydrogen generation rate of 1734 μmol h−1g−1, which is 1.22 times that of CNF/SCNF-MS. The stability of the as-prepared photocatalysts was also evaluated. Take CNF/SCNF-MS and B-CNF/SCNF-MS as examples, the photocatalytic hydrogen production rates were quite stable with the results of four repeated circulation operations presented in Figure 4c. The apparent quantum yield (AQE) of CNF/SCNF-MS was also calculated (Figure 4d and Table S9, Supporting Information) with a value of 2.760% under λ = 400 nm and the AQE change trend with wavelength is consistent with its light absorption characteristics, which indicates the close correlation of hydrogen generation to the photo-generated electrons in the heterostructure.[28]
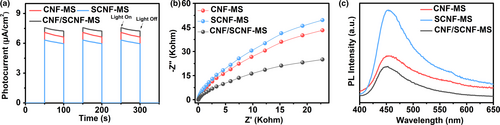
Furthermore, photoelectrochemical characterizations were also conducted to characterize the samples for charge separation efficiency under working conditions and their photocurrent density trends (Figure 5a and Figure S15, Supporting Information) are consistent with those of photocatalytic RhB degradation and hydrogen evolution activities. The enhanced photocurrent density of CNF/SCNF-MS (7.58 μA/cm2) compared with CNF-MS (7.17 μA/cm2) and SCNF-MS (6.37 μA/cm2) demonstrates excellent charge-transfer kinetics in the heterostructure.[56] The heterostructure built-up between g-C3N4 and g-C3N4−xSx can promote charge-carrier transfer between components and this statement can be further supported by the EIS Nyquist plots presented in Figure 5b. Apparently, the arc radius of CNF/SCNF-MS is smaller than that of CNF-MS and SCNF-MS, indicating the lower charge-transfer resistance and the more efficient separation of photo-generated charge carriers in the heterostructure for photocatalytic reactions.[57-59] Meanwhile, the lower charge-transfer resistance of CNF-MS and SCNF-MS compared with CNF and SCNF (Figure S16, Supporting Information) proves that the higher crystallinity benefits charge-carrier transfer process. Moreover, post treatment by NaBH4 can further promote the charge transfer and separation of samples attributed to the introduced cyano groups that can serve as strong electron withdrawing sites.[60, 61] Photoluminescence (PL) analysis was performed to acquire additional information about the charge transfer and recombination. As shown in Figure 5c, the PL emission peak intensity of CNF/SCNF-MS is weakened by 26% and 64% compared with those of CNF-MS and SCNF-MS, respectively, both of which show lower peak density than their untreated counterparts (Figure S17, Supporting Information). These results indicate the recombination of photo-generated electrons and holes is effectively inhibited through crystallinity improvement and heterostructure construction.[62] Furthermore, the addition of cyano groups introduced by NaBH4 treatment that can serve as the charge capture center further retard charge recombination, as the PL intensity of B-CNF/SCNF-MS is even smaller than that of CNF/SCNF-MS.
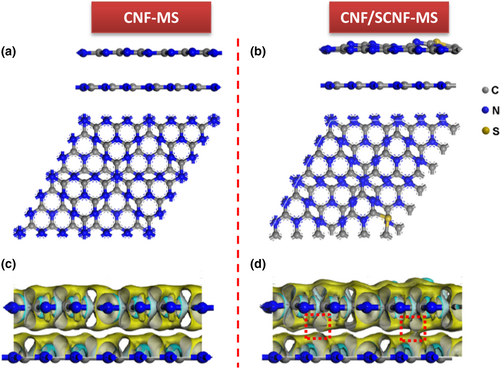
To better understand the charge-transfer mechanism in the heterostructure, DFT calculations were performed to compare the simplified structure model of CNF-MS that is consisted of two pristine g-C3N4 layers (Figure 6a) and the heterostructure CNF/SCNF-MS with the focus on the interface that is presented with one layer of S-doped g-C3N4 and one layer of pristine g-C3N4 (Figure 6b), respectively. Due to the introduction of S, the bond lengths of S-C elongate (Figure S18, Supporting Information) compared with that of the original N-C in the tri-s-triazine ring (Figure S19, Supporting Information) while the buckling effect that part of the N and S atoms deviate from the same horizontal surface can be observed. The Mulliken charge analysis showed that the S atom in the CNF/SCNF-MS possessed a positive charge (around +0.42), which means a loss of the electrons. The corresponding calculated differential charge density of the two structures (with the side-view map presented in Figure 6c,d) demonstrates that the apparent charge-transfer channels are formed (indicated by the enlarged contour of the electron density difference) between g-C3N4 and S-doped g-C3N4 for the possible charge transfer between those two layers in CNF/SCNF-MS, which is not observed in that of CNF-MS. Combining these results, it can be supposed that the electron transfer will proceed from S-doped g-C3N4 layer to the pristine g-C3N4 layer through the “electron bridge” in the CNF/SCNF-MS.
DFT calculations can also be used to understand reaction mechanisms.[63] We carried out the calculation of hydrogen evolution reaction (HER) process on CNF-MS and CNF/SCNF-MS. The ΔG values at N site of CNF-MS and at S, N1, N2 sites for CNF/SCNF-MS were calculated, respectively. As shown in Figure S20, Supporting Information, the ΔG value at N site in CNF-MS is −1.03 eV, while the ΔG value at S site in the CNF/SCNF-MS is as large as 1.11 eV. However, the ΔG value at various N sites in the S-doped g-C3N4 can decrease to less than −0.40 eV, which means that the CNF/SCNF-MS performs better catalytic activity since the ΔG values become much closer to the zero in the CNF/SCNF-MS.
2.4 Piezocatalytic Performance Analysis
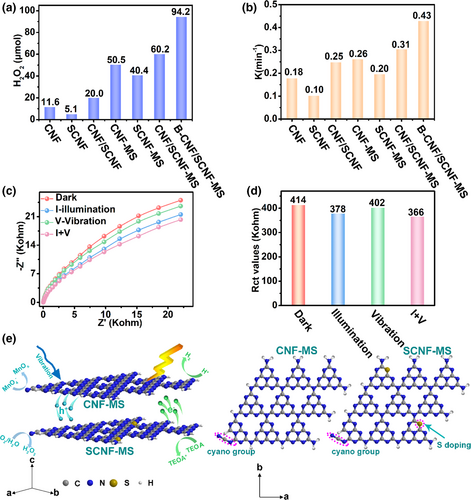
The piezoelectric effect of g-C3N4 has been recently reported both theoretically and experimentally,[35] and its abundant catalytic sites as well as diverse modulation strategies endow it great potential as efficient piezoelectric catalyst. Herein, we also evaluated the piezocatalytic effect of the as-prepared g-C3N4 based samples by analyzing their reactivities for H2O2 generation from water/O2 and KMnO4 reduction under dark with ultrasonic vibration condition, respectively, which are viewed as two approved methods to measure the piezocatalytic effect of catalysts.[35] As shown in Figure 7a, the piezocatalytic yield of H2O2 from CNF-MS and SCNF-MS is 4.35 times and 7.92 times those of CNF and SCNF, respectively, indicating crystalline C3N4 samples presented better piezocatalytic performance. Meanwhile, their heterostructure CNF/SCNF-MS reached the H2O2 generation yield of 60.2 μmol, which is about 1.19 times and 1.49 times those of CNF-MS and SCNF-MS, respectively. B-CNF/SCNF-MS displayed the highest piezocatalytic performance among all samples. Furthermore, the piezocatalytic KMnO4 degradation also confirmed the same trend with that of H2O2 generation (Figure 7b), both of which are similar to their photocatalytic performance. Although photocatalytic and piezocatalytic reactions have different excitation sources, both their subsequent processes require high-quality charge-carrier transportation and surface reactions. This may be the reason why the same trend was obtained in the as-prepared samples for both processes. The performances of some similar photocatalysts reported previously were also listed in Table S10, Supporting Information. The results of XRD (Figure S21, Supporting Information), SEM (Figure S22, Supporting Information) and XPS (Figure S23, Supporting Information) characterizations for CNF/SCNF-MS before and after piezocatalytic reactions show similar morphologies, phases and atomic binding environment, indicating the piezoelectric catalytic reaction did not change the physical and chemical properties of the CNF/SCNF-MS.
To further verify the origination of the H2O2 generation and KMnO4 reduction reactions, the corresponding control experiments were designed with the result shown in Figures S24 and S25, Supporting Information. Without the addition of catalysts, the absorbance of KMnO4 did not decrease over time (Figure S24, Supporting Information), which eliminates the possibility of self-reduction of KMnO4 under ultrasonic treatment. Moreover, in the absence of ultrasound (with only stirring), the concentration of KMnO4 did not decrease and the H2O2 generation was not detected even in the presence of CNF/SCNF-MS (Figure S25, Supporting Information), demonstrating the importance of piezoelectric properties emerged in g-C3N4 for the KMnO4 degradation and H2O2 generation.
The piezoelectric properties of g-C3N4 originate from its asymmetrical structure (triangular-shaped non-centrosymmetric holes), and is directly related to its piezocatalytic performance.[35] Herein, low crystallinity can lead to the in-continuous in-plane atomic structure and weakening of this asymmetrical structure in g-C3N4 plane, and thus the decreased piezocatalytic performance. Meanwhile, from the aspect of charge transfer within the material itself, high crystallinity can ensure the smooth transfer kinetics while the suitable band alignment between two components can efficiently retard charge recombination, both of which can guarantee more charge carriers (either generated by light irradiation for photocatalysis or ultrasonication for piezo-catalysis) to participate in the surface redox reactions. Electrochemical impedance spectroscopy (EIS) analysis of CNF/SCNF-MS under different conditions is also conducted to understand the reactivity contributions from different sources (Figure 7c) with the corresponding charge-transfer resistance (Rct) values provided in Figure 7d. The charge transfer is apparently promoted under both illumination and ultrasound conditions (with lower Rct values than that of sample under dark), demonstrating both the photo- and piezo-catalysis processes can lead to the more efficient accessible charges for the surface reactions while the photocatalysis in this C3N4 related configuration can promote the charge transfer faster than that of piezo-catalysis (Rct under illumination: 378 Kohm VS Rct under vibration: 402 Kohm). More importantly, the photocatalysis and piezocatalysis effect can collaborate for coaction to further improve the charge-transfer process in the catalyst and accelerate the electron exchange between charges and electroactive species in the solution, as confirmed by the smallest Rct value of sample under illumination/vibration (I + V) condition. This observation is consistent with the most recently report that we noticed during the writing of this work.[37] According to the above results, the schematic illustration of heterostructure constructed in photocatalytic/piezoelectric reactions in given in Figure 7e. The NaBH4 treatment that can introduce extra cyano groups and remove NHx species (as confirmed by XRD, XPS and FTIR results) of heterostructure without apparent SSA change increases the asymmetry of the atomic structure, thus resulting in the increase of its piezocatalytic activity. Note the presence of cyano groups breaks the integrity of g-C3N4 structure, and this highlights its piezocatalytic performance enhancement (more than photocatalytic performance enhancement). The roles of piezocatalytic and photocatalytic processes were also integrated to extend the use of this heterostructure for potential mechano-solar-(electro)chemical system construction and further reactivity enhancement for dye degradation (by 16%) was observed for the sample under light and ultrasonic vibration (Figure S26, Supporting Information).
3 Conclusion
To sum up, we have successfully constructed a high-crystalline g-C3N4/g-C3N4−xSx isotype heterostructure by molten salt method for photocatalytic water splitting and piezocatalytic reactions. Attributed to the high crystallinity and well-aligned band structure from isotype heterostructure, efficient charge-carrier transfer was achieved in the bulk and through the interface between isotype g-C3N4 components that is confirmed by both DFT calculations and experimental analysis, leading to the significant improvement of photocatalytic and piezocatalytic processes. More importantly, the post NaBH4 treatment introduces extra cyano groups and removes NHx species, resulting in the enhancement of asymmetry structure and thus further increase of piezocatalytic reactivities. The enhanced light absorption induced by NaBH4 treatment also elevates the photocatalytic activity of the heterostructure. This work provides new insights for the design of high-performance isotype heterostructure for solar energy conversion and highlights the potential piezoelectric applications of g-C3N4.
4 Experimental Section
The experimental detail can be found in the Supporting Information.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51902045, 51904059), Fundamental Research Funds for the Central Universities (N182503030, N182505036, N2002005), Liao Ning Revitalization Talents Program (XLYC1807123) and Young Elite Scientist Sponsorship Program by CAST (YESS) 2019–2021QNRC.
Conflict of Interest
The authors declare no conflict of interest.




