Seasonal water use strategy of canopy tree species and possible implication for their coexistence in a subtropical secondary forest
Abstract
We investigated the seasonal water use patterns of five canopy tree species, Schima superba, Liquidambar formosana Hance, Quercus griffithii Hook, Quercus acutissima Carruth, and Pinus massoniana, in a subtropical secondary forest in Guangxi province, which were pervasively planted in South China. The hypothesis is that the trees can make different temporal use of environmental resources that change seasonally and consequently contribute to their coexistence. Sap flow technique was used to measure the daily and seasonal transpiration during the period from June 2016 to May 2017. The sap flow—derived daily transpiration (EL), leaf water potential (ΔΨ), hydraulic conductance (K), as well as the responses of EL to driving factor—water vapour pressure deficit (VPD) was determined in dry and wet seasons. Measured data of stem sap flow showed that L. formosana, Q. griffithii, and P. massoniana transpired more water than S. superba and Q. acutissima during the whole monitoring period, whereas daily EL of P. massoniana rapidly decreased under dry season. We attributed the different water use patterns to the significant difference in ΔΨ, K, and wood density among the five species. Moreover, linear relationships between the daily transpiration and VPD might suggest diverse water use strategies. For the species of S. superba, Q. acutissima, and P. massoniana, less water consumption and relatively flat change of transpiration with VPD were observed, suggesting a relatively tighter stomatal control. The slope values for L. formosana and Q. griffithii, which were much higher even under dry season, might own a weak stomatal control. These different functional and hydraulic traits, together with the associated water use strategies, might facilitate the coexistence of these five canopy tree species, which would assist in predicting the impact of future environmental changes on plant biodiversity and ecosystem services in the investigated region.
1 INTRODUCTION
Although forest plantations cover 5% of the forested lands at a global scale, they provide 15% of the world's wood production (Carnus et al., 2006; Grossiord, Granier, Gessler, Pollastrini, & Bonal, 2013). To fulfil the increasing demand for industrial wood products over the last decades, large areas originally occupied by natural forests have been replaced by industrial tree plantations throughout the world. However, such forest plantations are highly controversial because they might lead to the decrease of species diversity and water storage in catchments due to their fast growth and intensive water consumption (Erskine, Lamb, & Bristow, 2006; Jackson et al., 2005; Paquette & Messier, 2010). In this context, mixed forests have been advocated as a potential compromise between high wood production and biodiversity conservation and seem to be an effective management practice to help forest ecosystems acclimate to future climatic changes (Grossiord et al., 2014; Kelty, 2006). Generally, there are two potential mechanisms that would explain the positive effects of mixed forests on ecosystem services. One is the complementarity of resource use between species with different functional traits, allowing species to coexist in the same habitat; the other one is the selection effects resulted from a higher probability of several highly productive species in more diverse stands (Loreau & Hector, 2001; Sterck, Markesteijn, Schieving, & Poorter, 2011).
Plant functional traits that integrate the interactions and adaptations of species with biotic and abiotic environment are linked to species fitness and ultimately influence ecosystem properties (de Bello et al., 2010; Grossiord et al., 2014). Functional traits, including physiological and hydraulic characteristics, determine the intensity and pattern of resource uptake and utilization (de Bello et al., 2010; Zinnert, Shiflett, Vick, & Young, 2013). Tree species with different functional traits might result in the contrasting species-specific responses to fluctuating environment (Markesteijn, Poorter, Bongers, Paz, & Sack, 2011; Seyoum, Fetene, Strobl, & Beck, 2014). For instance, in terms of the xylem structure, Bush et al. (2008) reported that the ring-porous trees might continuously access deep soil water and own different transpiration rates, carbon gain, and growth compared with the diffuse-porous tree species. Consequently, the ring- and diffuse-porous trees would show different water use strategy, stomatal regulations, and responses to the environmental factors (Bush et al., 2008; Ford, Hubbard, & Vose, 2011; Ouyang et al., 2017). A useful framework classifies plants as isohydric versus anisohydric species, based on stomatal regulation of leaf water potential in response to changes in atmospheric moisture demand (Bush et al., 2008). This dichotomy has been proposed as a predictive trait of the specific underlying mechanism of trees physiology (Bush et al., 2008). Similarly, as a major determinant of hydraulic capacitance, wood density also has a large influence on tree water use. Previous studies have proved that tree species with softer wood can store and utilize more water than species with hard wood (Pratt, Jacobsen, Ewers, & Davis, 2007; Scholz et al., 2007). Moreover, Köcher, Horna, and Leuschner (2013) conducted a study to explore the stem water storage in five coexisting temperate broad-leaved tree species and considered that the variation of wood density was the main determinant for the difference in the stored stem water.
Such differences in functional traits and responses might be beneficial to the successful interspecies competition for limited resources and to coexistence of many woody species. To be specific, in an ecosystem where the water and nutrient resource were limited, co-occurring species may exhibit partitioning of the resource either spatially or temporally and use water more efficiently (Sterck et al., 2011). For instance, a previous study has reported that co-occurring holm oaks and pines have different rooting depths and extract water from different soil layers (del Castillo, Comas, Voltas, & Ferrio, 2016). The results indicated Aleppo pine and holm oak shared the same hydrological niche during the wet season but shifted to distinct water sources during the summer drought period (del Castillo et al., 2016). Other works also showed evidence of facilitation processes in water limited environments, for example, hydraulic lift by deep-rooted species favouring neighbour shallow-rooted species (Prieto, Armas, & Pugnaire, 2012; Rodríguez-Robles, Arredondo, Huber-Sannwald, & Vargas, 2015). Yet it is still a matter of controversy whether positive or negative influence define species coexistence (del Castillo et al., 2016; Diaz-Sierra, Zavala, & Rietkerk, 2010; Valladares, Bastias, Godoy, Granda, & Escudero, 2015) and how resource scarcity could promote or inhibit the coexistence (Craine & Dybzinski, 2013). Observational studies in mixed forests produced inconsistent results as well. Recent studies have shown that water scarcity increases competition for water in a mixed Mediterranean forest (Grossiord et al., 2014), reducing the potential benefits of species coexistence in Iberian forests (Jucker et al., 2014). Moreover, a report has found that more soil water was consumed under mixed stands due to the deeper reaching and more intensifying root system of European beech, exacerbating the stress of water scarcity in lower Austria (Schume, Jost, & Hager, 2004). Attributed to the difference in climate, environment, and tree species, results from previous studies are mixed with regard to the behaviours and interactions of these different tree species. Therefore, deeper insights into the mechanisms, by which diversity or tree identity are influencing water turnover in forest ecosystems under both resource-unlimited and -limited conditions, are urgently needed (Lübbe, Schuldt, Coners, & Leuschner, 2016).
Environmental factors including light, vapour pressure deficit (VPD), and soil moisture, would fluctuate temporarily or spatially in a forest land and affect tree water use. According to studies above, co-occurring tree species may show different responses to such changing environmental conditions, even grow at the same site (Seyoum et al., 2014). As previously reported, South China would likely to experience the reduction of water supply via changes in precipitation patterns, as well as increases in mean annual temperature and atmospheric evaporative demand (Gao, 2015; Zhou et al., 2011). For instance, the precipitation in this region was unevenly distributed, consequently leading to more allocation of precipitation to wet seasons, and less water storage, lower soil water content, and the longer duration in dry seasons, namely, dry gets drier, and wet gets wetter (Gao, 2015). These changes could have important effects on tree growth due to abiotic constraints and indirect effects as a result of increases in resource competition. The potential soil water limiting conditions in South China may have negative consequences on species survival, ecosystem productivity, and ecosystem resistance to soil drought. In this context, it is becoming vitally important to understand the water use patterns of the coexisting tree species planted in South China, especially when the water supply is potentially becoming scarce.
A large-scale afforestation practice has been prevailing during past three decades in South China, and the forest coverage in this region has increased from 26.2% in 1979 to 50.1% in 1998 and to almost 60% in 2016. The various types of forest have been developed into stable secondary evergreen broad-leaved forests after long-term natural growth without external disturbance. These forests have played important roles in coping with climate change, maintaining regional carbon balance and improving local environment (Bureau of Statistics of Guangdong Province, 2016; Peng, Hou, & Chen, 2009). Moreover, previous studies about water use mechanisms for coexisting trees mainly focused more on arid and semiarid regions, such as the Mediterranean region or those characterized with Mediterranean climate (del Castillo et al., 2016; Forner, Aranda, Granier, & Valladares, 2014; Grossiord et al., 2014), the North-eastern China (Wu et al., 2016), whereas the studies on water use of coexisting tree species in subtropical moist areas at low latitudes are still rare. Different from the arid and semiarid regions, our experimental site is located in a region where the forests are of high species diversity, and thus, the investigation of tree water use and associated mechanism of the coexistence species in the subtropical moist area might have a distinctive and irreplaceable ecological significance. Furthermore, it is noticeable that previous studies have emphasized more on different distribution of spatial niche for the coexistence trees, such as the root depth and the biomass allocation but ignored the temporal niche dynamics (Schwinning & Kelly, 2013), which we try to address in this study. Therefore, a field study on the tree-level transpiration and related functional traits of five coexisting canopy tree species, Schima superba, Liquidambar formosana Hance, Quercus griffithii Hook, Quercus acutissima Carruth, and Pinus massoniana, was conducted in Guangxi province, South China. The main objectives of this study are (a) to investigate the inherent differences in water use of the five dominant coexisting tree species in a subtropical forest, particularly those differences linked to divergent wood anatomy; (b) to explore the related water use mechanism of coexisting trees by examining the seasonal variations in leaf water potential, soil-to-leaf hydraulic conductance, and water use strategy; and (c) to understand how co-occurring species compete and coexist in a potential water-limited system.
2 MATERIALS AND METHODS
2.1 Site description
The study was performed in a secondary evergreen broadleaf forest at an ecological observation station located in Guilin Botanical Institute, Guangxi Province, China (25°29′N, 110°28′E). It has a typical subtropical oceanic monsoon climate, characterized by a hot–humid period from April to September (wet season), and a cold–dry period from October to March (dry season). Long-term average of annual air temperature (T) from 1981 to 2010 was 19.1°C, with July and January as the hottest (28.2°C) and coldest (8.1°C) months, respectively. Average annual precipitation (P) is 1,887.8 mm, with more than 70% concentrating on wet seasons (http://www.cma.gov.cn/2011qxfw/2011qsjgx/). The forest contains a soil of clay loam with pH of 4.30, organic content of 4.23%, and total nitrogen content of 0.20% (data were obtained from soil samples at depth of 30 cm). The forest had developed a closed canopy at the height of between 10 and 15 m above the ground.
The forest was growing on a steep north-facing slope with an inclination of 25°. Forest density in this experimental site was approximately 2,200 trees per hectare. An instrument tower (20-m tall) was set up within the plantation to provide an anchor station for the installation of various environmental monitoring sensors (described in the following section).
2.2 Micrometeorological parameters
 (1)
(1)2.3 Wood density and sapwood area
Tree wood density was determined by the method described in Gao (2015). For each species, we drilled the sapwood from six to seven trees outside the experimental site by using an increment borer (diameter: 5 mm). The drilled wood cores (1–2 per tree) were wrapped with a wet towel immediately after sampling and then placed in sealed plastic bags. The materials were quickly transported to the laboratory and weighed on an electronic balance (Shinko, Japan), with an accuracy of 0.0001 g and then were dried to a constant weight to obtain a dry weight. Finally, the wood density was calculated from the dry mass divided by fresh volume. The values are 0.59 ± 0.03 g cm−3 (S. superba), 0.53 ± 0.04 g cm−3 (L. formosana), 0.46 ± 0.03 g cm−3(Q. griffithii), 0.57 ± 0.00 g cm−3(Q. acutissima), and 0.46 ± 0.02 g cm−3(P. massoniana), respectively. As we could not core the trees installed with sap flow sensors due to the long-term operation of the experimental set-up, the sapwood area (As) was estimated from the allometric equation for each tree species (von Allmen, Sperry, & Bush, 2015). The allometric equation was established by the regression relation between the As and the tree diameter at breast height (DBH). To be specific, we chose six to seven trees of each species with different DBH that were outside and close to the experimental plot and obtained the wood core using a borer of 5 mm in diameter. The sapwood depth was directly determined by the distinction of colour difference between sapwood and heartwood. The allometric equations for the sapwood area of the five sampled tree species using the DBH were respectively established. Measured DBH and calculated As in different months during the whole experimental period are listed in Table 1. Further, leaf area index (LAI) of this plot was measured using a LAI 2000 plant canopy analyser (Li-Cor, Inc., Lincoln, NE). We randomly captured image data using the analyser at 10–15 sample points within the experimental plot and finally obtained the LAI data.
| No. | Species | Tree diameters (DBH) | Sapwood area (As) | Allometric equation | ||||
|---|---|---|---|---|---|---|---|---|
| May in 2016 (cm) | Dec in 2016 (cm) | Apr in 2017 (cm) | May in 2016 (cm2) | Dec in 2016 (cm2) | Apr in 2017 (cm2) | |||
| 1 | Schima superba | 12.7 | 13.1 | 13.3 | 103.1 | 109.8 | 113.2 | As = 0.68*(DBH)2.03 |
| 2 | S. superba | 12.2 | 12.6 | 12.7 | 95.0 | 101.4 | 103.0 | |
| 3 | S. superba | 17.6 | 18.9 | 18.9 | 199.9 | 231.1 | 243.3 | |
| 4 | Liquidambar formosana | 11.7 | 13.0 | 13.4 | 102.3 | 126.2 | 134.1 | As = 0.79*(DBH)2–1.28*(DBH) + 8.55 |
| 5 | L. formosana | 11.1 | 11.7 | 12.0 | 92.2 | 102.3 | 107.6 | |
| 6 | L. formosana | 13.5 | 14.0 | 14.3 | 136.1 | 146.4 | 152.7 | |
| 7 | Quercus griffithii | 12.4 | 13.2 | 13.7 | 117.6 | 132.3 | 141.9 | As = 0.53*(DBH)2 + 4.72*(DBH)-23.0 |
| 8 | Q. griffithii | 20.2 | 20.5 | 20.8 | 290.2 | 298.1 | 306.1 | |
| 9 | Q. griffithii | 22.8 | 24.2 | 24.9 | 333.6 | 403.8 | 425.5 | |
| 10 | Quercus acutissima | 20.1 | 20.5 | 20.9 | 234.0 | 246.1 | 258.6 | As = 1.40*(DBH)2.55 |
| 11 | Q. acutissima | 9.5 | 10.0 | 10.4 | 34.6 | 42.5 | 43.6 | |
| 12 | Q. acutissima | 11.0 | 11.6 | 11.8 | 50.3 | 57.6 | 60.2 | |
| 13 | Pinus massoniana | 33.5 | 33.7 | 34.3 | 686.5 | 695.9 | 724.6 | As = 0.84*(DBH)2.29 |
| 14 | P. massoniana | 35.9 | 37.0 | 37.4 | 804.3 | 861.9 | 883.4 | |
| 15 | P. massoniana | 26.4 | 27.5 | 28.1 | 397.9 | 436.9 | 459.0 | |
- Abbreviation: DBH, diameter at breast height.
2.4 Sap flux and whole tree transpiration
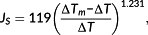 (2)
(2)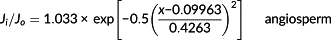 (3)
(3)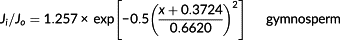 (4)
(4)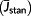 and finally obtained the actual mean sap flux density
and finally obtained the actual mean sap flux density
 . The whole-tree sap flux
. The whole-tree sap flux
 was estimated by multiplying the mean sap flux density by the sapwood area, whereas the daily tree water use was calculated as the integral of the daily courses of the whole-tree sap flux. To remove the effect of tree size on transpiration, we adopted a normalized tree transpiration EL expressed as E/DBH proposed by Besson et al. (2014).
was estimated by multiplying the mean sap flux density by the sapwood area, whereas the daily tree water use was calculated as the integral of the daily courses of the whole-tree sap flux. To remove the effect of tree size on transpiration, we adopted a normalized tree transpiration EL expressed as E/DBH proposed by Besson et al. (2014).2.5 Leaf water potentials and whole-tree hydraulic conductance
Leaf water potentials (Ψleaf) were measured at both predawn and midday on 3–5 twigs with intact leaves of sampled trees that were near the tower by using PMS-1000 pressure chambers (PMS Instrument, Corvallis, OR, USA) for 3 consecutive days in wet (August 2016) and dry (October 2016) seasons. Twigs with leaves were cut off and immediately transferred into a pressure chamber to determine Ψleaf. All measurements were typically completed within 15 min. Three replicates were made for each of the sampled trees.
 (5)
(5)2.6 Statistical analyses
Significance differences of the leaf water potential and hydraulic conductance among tree species were tested by ANOVA (the post hoc LSD test) in the SPSS software package (SPSS Inc. 2003). The correlation between whole-tree transpiration and the VPD was analysed using linear regression in Origin 8.0. To determine the seasonal significant differences in the linear regressions, homogeneity of the regression slopes and analysis of covariance were performed using the SPSS software package (SPSS Inc., 2003). Differences between the treatments were considered statistically significant at p < .05.
3 RESULTS
3.1 Seasonality of climate
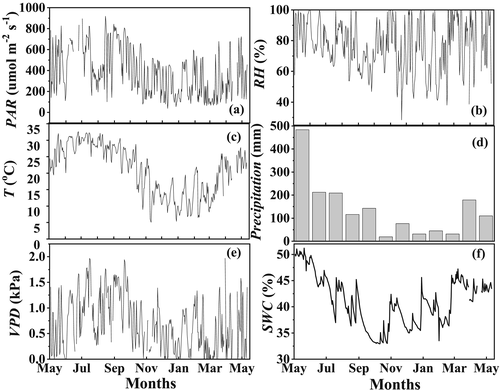
As shown in Figure 1, the measured environmental variables exhibited pronounced seasonal variations. Total precipitation during the experimental period was 1,658.4 mm and unevenly distributed. Approximately 77% of the annual total occurred between April and September (wet season). Heaviest P occurred in May, with value of 483.9 mm. Monthly mean SWC ranged from 33.6% (October) to 47.9% (May) during the whole experimental period, following the seasonal pattern of precipitation and decreasing from the wet to the dry season (Figure 1f). Maximum monthly daytime mean value of T occurred in July with value of 30.1°C, whereas the minimum monthly mean T was 12.3°C occurring in January. The range of monthly daytime mean RH was from 69.6% to 87.1%, and the minimum RH occurred in December. Large variation was also observed in monthly daytime mean photosynthetically active radiation, ranging from 256 (January) to 665 (July) μmol m−2 s−1. Monthly daytime mean VPD varied from 1.27 kPa in September to 0.25 kPa in March.
3.2 Water use of different tree species
The LAI did not significantly differ between wet and dry season, being 3.41 ± 0.33 m2 m−2 (March) and 3.95 ± 0.17 m2 m−2 (July), respectively. Daily normalized tree transpiration EL of five tree species under wet (from May to September) and dry (from October to January) season is presented in Figure 2. During the experimental period, the L. formosana, P. massoniana, and Q. griffithii trees transpired more than the other two species for most sunny days, and more pronounced interspecific difference was observed in the wet season. No significant difference in normalized EL was observed between the S. superba and Q. acutissima. Moreover, as listed in Table 2, mean daily EL values (data based on August 29–30 and October 10–12) of L. formosana and Q. griffithii were significantly higher than those of other three species (p < .05). In term of the temporal change, clear difference in EL was also shown for the five tree species. EL values of P. massoniana were clearly higher from May to August (more than 50 kg day−1 m−1) and sharply decreased during the late wet season and the subsequent dry season (less than 30 kg day−1 m−1), whereas the EL of Q. griffithii fluctuated during the early wet period but maintained stable after June (Figure 2a). The other species, however, kept relatively steady transpiration for the whole wet season. Differing from those in the wet season, the daily EL decreased significantly under dry season. Though the transpiration of L. formosana and Q. griffithii was still relatively higher than that of the other species during the early dry seasons, the EL for all the five tree species was lower than 50 kg day−1 m−1after November (Figure 2b).

| Species | predawn Ψleaf (MPa) | midday Ψleaf (MPa) | ΔΨleaf (MPa) | EL (kg day−1 m−1) | K (kg day−1 m−1 MPa−1) |
|---|---|---|---|---|---|
| August in 2016 | |||||
| Schima superba | −0.42 (0.11) ab | −1.44 (0.29) b | 1.06 (0.29) ab | 6.70 (0.42) a | 7.3 (1.35) ab |
| Liquidambar formosana | −0.35 (0.13) ab | −1.69 (0.32) ab | 1.29 (0.15) bc | 20.1 (1.99) b | 16.5 (2.78) c |
| Quercus griffithii | −0.38 (0.06) ab | −1.62 (0.25) b | 1.17 (0.22) ab | 34.0 (6.77) c | 26.2 (6.16) d |
| Quercus acutissima | −0.30 (0.02) b | −1.84 (0.12) a | 1.45 (0.19) c | 5.73 (0.32) a | 3.7 (0.94) a |
| Pinus massoniana | −0.53 (0.06) a | −1.48 (0.07) b | 0.97 (0.09) a | 9.73 (3.54) a | 10.3 (1.79) b |
| October in 2016 | |||||
| S. superba | −0.63 (0.04) b | −1.57 (0.19) b | 0.99 (017) a | 8.3 (1.92) a | 9.4 (0.39) b |
| L. formosana | −0.49 (0.09) c | −1.69 (0.32) b | 1.24 (0.09) b | 14.1 (2.57) b | 11.1 (2.16) b |
| Q. griffithii | −0.72 (0.02) ab | −1.57 (0.07) b | 0.90(0.02) a | 21.2 (3.00) c | 25.5 (3.56) d |
| Q. acutissima | −0.66 (0.04) ab | −2.28 (0.16) a | 1.54 (0.09) b | 4.21 (0.02) a | 2.9 (0.51) a |
| P. massoniana | −0.75 (0.01) a | −1.56 (0.15) b | 0.81 (0.08) a | 7.50 (3.58) a | 7.7 (0.93) b |
3.3 Leaf water potentials and hydraulic conductance
As shown in Table 2, predawn Ψleaf (a surrogate of soil water potential close to tree roots) decreased from August (ranging from −0.30 to −0.53 MPa) to October (ranging from −0.49 to −0.75 MPa) in the five species. Significant difference in predawn Ψleaf values was observed among the species for the 2 months. Similarly, we also found significant differences in the midday Ψleaf and ΔΨ among the five selected tree species; that is, lower values were observed in Q. acutissima, whereas higher values were detected in S. superba and P. massoniana. The mean hydraulic conductance (K) was also found to be significantly different among the species, with the highest K occurring in Q. griffithii and the minimum K in Q. acutissima. For all tree species except S. superba, K values were nearly equal for both months.
3.4 Tree water use in response to VPD
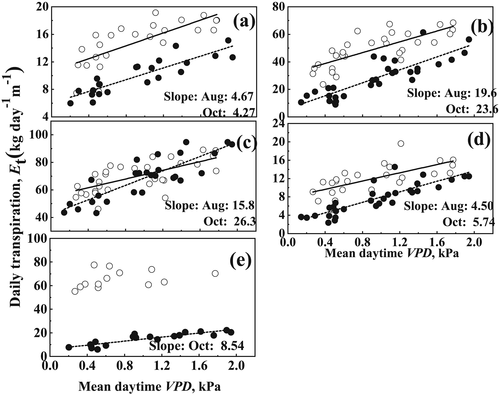
Response of daily EL to mean daytime VPD for the five species in August and October is presented in Figure 3. Except for P. massoniana in August, significant linear relationships were established between daily EL and VPD (R2 values ranged from .45 to .78, p < .05). According to the statistical analysis, there was a significant difference in the slopes of fitted linear relationships for different tree species within the same month (p < .05). For instance, different from that in L. formosana and Q. griffithii, a relative flat change in EL with increasing VPD was observed in S. superba and Q. acutissima. As shown in Figure 3, the slopes of fitted line in L. formosana and Q. griffithii ranged from 15.8 to 26.3, much higher than those in other three species (with slope values ranging from 4.27 to 8.54). Similarly, for the same tree species, daily EL also exhibited significantly different responses to the changing VPD in the different months (p < .05). For L. formosana, Q. griffithii, and Q. acutissima, a much weaker increase of EL (lower slope values) was observed with the increasing VPD in August than in October.
4 DISCUSSION
4.1 Effect of tree species on water transpiration
In this study, when water supply was sufficient (wet season), L. formosana, Q. griffithii, and P. massoniana significantly consumed more water than S. superba and Q. acutissima. As reported in previous studies, different tree species vary largely in terms of water transpiration and water use strategy, which is mainly due to hydraulic traits and biometric parameters (Seyoum et al., 2014; Zinnert et al., 2013). For example, the predawn Ψleaf has been typically regarded as an approximate indicator of soil water availability (Baldocchi, Xu, & Kiang, 2004). As listed in Table 2, values of predawn Ψleaf were higher than −1.0 Mpa in the tree species except Q. acutissima, which might indicate that the studied plants accessed water in the soil layers where little change in the soil water content occurred (White, Turner, & Galbraith, 2000). In addition, S. superba and Q. acutissima possessed a relative higher wood density (p < .05) and consequently transpired less water compared with tree species with softer wood density (Pratt et al., 2007; Köcher et al., 2013).
With less precipitation, lower SWC and T, decreases in transpiration were observed for all tree species during dry season, whereas Q. griffithii still showed much higher transpiration rates than others, especially in the early October. Possible reasons for the non-uniform response of transpiration among species are discussed below. As indicated in Table 2, K values of Q. griffithii in both wet and dry seasons were two times higher than those of the other tree species. The higher K value even under dry season might be beneficial to efficient water transpiration (Do et al., 2008; Reid, Silins, & Lieffers, 2006). In addition, the possible deep root system of Q. griffithii might enable the trees to gain access to abundant soil water in deep soil layers, allowing the maintenance of relatively high transpiration rates even under dry condition. However, the quantitative measurement of root system has not been conducted in this study and deserves further research.
Differences found in growth and water use among the studied species in response to the changing environmental factors can be also explained by differential water use strategies. Based on the different responses to fluctuating environmental conditions, plants can be classified as isohydry and anisohydry. Isohydric plants have strong stomatal control and anisohydric plants often have weak stomatal sensitivity to VPD and soil water content (Bucci, Scholz, Goldstein, Meinzer, & Arce, 2009; Franks, Drake, & Froend, 2007). We used relationships between transpiration and VPD for the five selected tree species to give insight into their water use strategy. As shown in Figure 3, less water consumption and relatively flat change of transpiration with VPD for S. superba and Q. acutissima were observed, and the slope values for L. formosana and Q. griffithii were much higher. Despite the fact that the distinction among water strategies remains unclear in some cases, with many intermediate behaviours (Franks et al., 2007), we still think that L. formosana and Q. griffithii could be ascribed to the anisohydric behaviour, whereas S. superba and Q. acutissima follow an isohydric strategy. As we stated above, Q. griffithii maintained rather high transpiration rates even during the dry seasons, whereas S. superba and Q. acutissima maintained a relatively constant water use in both dry and wet seasons. Under a potential extreme drought, the conservative strategy followed by S. superba and Q. acutissima could result in carbon starvation. In contrast, Q. griffithii may be in risk of hydraulic failure and death due to cavitation processes (Forner et al., 2014). Water transpiration of P. massoniana, as a coniferous species, sharply decreased after late September due to the lower ΔΨ and hydraulic conductance. Such seasonal variation for the coexisting species would suggest the plasticity in the use of water sources, which is similar with the result of del Castillo et al. (2016). In their research, the coexistence of oaks and pines has resulted in the limited use of shallow water by oaks in the presence of pines, which forced the oaks to shift to deep-soil water use, whereas pines had more restricted access to deep water in the presence of oaks and therefore significant lower transpiration in dry season.
4.2 Coexistence patterns of the studied five tree species
How might these differences in water use among species affect the interaction and coexistence of tree species? According to the niche theory, the functional trait differences across species govern the specialization of species for different fundamental niches, thus allowing species to coexist in the same community (Silvertown, 2004). Reports also showed the contrasting water use patterns might contribute to minimizing the competition for ephemeral soil moisture variations during prolonged drought periods (Kambatuku, Cramer, & Ward, 2013; Wu et al., 2016). In this study, responses of transpiration for the studied canopy tree species to the changing climate and their associated water use strategy were significantly different. For example, the structural and functional traits of L. formosana and Q. griffithii, for example, the higher whole-tree hydraulic conductance, the lower wood density and the larger increase of daily EL with the increased VPD, would facilitate water flow from soil to leaves to sustain the higher transpiration. These two species might be regarded as acquisitive and consequently help themselves to obtain high biomass. In contrast, those trees with high wood density, such as S. superba and Q. acutissima, were often associated with a possible higher resistance to xylem embolism (Pratt et al., 2007) and presented a reduced transpiration even under the wet season, indicating a conservative strategy (Poorter et al., 2010; Willson & Jackson, 2006). Moreover, when the potential water scarcity was about to happen, the water consumption of P. massoniana rapidly decreased. As revealed by Pretzsch (2013) and Forrester (2014), the complementary effects increased as water availability decreased. Therefore, we speculated the combination of acquisitive and conservative water use patterns might ultimately help for reasonable use of temporal distribution of water and guarantee the survival of trees under dry conditions.
Our results suggested that the five species had different hydrological niche and temporal water use patterns. For instance, the acquisitive L. formosana and Q. griffithii obtained possible higher growth rates, whereas the conservative S. superba and Q. acutissima might guarantee their survival under water scarce environment. This different niche allocation might be a complementary advantage for their coexistence. However, future climatic changes, such as changes in the amount, distribution, frequency, and intensity of precipitation, may have important consequences for the competitive relationship among coexisting species (Craine & Dybzinski, 2013; IPCC, 2013). In the studied region, the precipitation patterns have experienced alterations in recent decades (Cao et al., 2012). Hence, we predict that if frequent climate events occur during the growing season, the niche distribution of all coexisting plants will likely be dynamic and adaptable to the circumstances. Therefore, from a long-term perspective, the precipitation patterns would affect the adaptability of coexisting plants, which might have further implications on the distributions of tree species in South China.
5 CONCLUSION
In this study, we explored the water use patterns and strategies of five co-occurring trees species by investigating their seasonal variations in sap flux, leaf water potential, and related hydraulic traits. Without soil water limitation, L. formosana, Q. griffithii, and P. massoniana transpired more water than S. superba and Q. acutissima, whereas the daily EL of P. massoniana rapidly decreased from the early dry seasons. Significant differences in the leaf water potential and mean hydraulic conductance were observed among the species for the wet August and the dry October. Specifically, Q. griffithii mainly owned the highest K values, whereas the Q. acutissima possessed the minimum K. Linear relationships between daily EL and VPD were established for the five species in August and October. The slopes for L. formosana and Q. griffithii were much higher than those for the other three species, and clear difference in the slopes was found between the 2 months (p < .05), indicating species-specific water strategies under the changing water conditions. In conclusion, our finding emphasizes the importance of the temporal changes in patterns of water use and may facilitate the coexistence of different tree species. The results can provide insight into the possible impact of environmental changes on plant biodiversity and ecosystem services in the studied region.
ACKNOWLEDGEMENT
This study was finally supported by National Natural Science Foundation of China (31700334, 41630752, 41172313) and National Key Research and Development Program (2016YFC0500106-02).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.




