Root reinforcement to soils provided by common Ethiopian highland plants for gully erosion control
Abstract
Grasses and trees are often used to stabilize gully banks. However, the effectiveness of such biological conservation measures has not been investigated for the Ethiopian highlands. This study investigates the reinforcement that plant roots may provide to strengthen gully banks in Ethiopia. The root systems of 26 indigenous and exotic plant species of 3 plant life forms (grasses, shrubs, and trees) were sampled, and root tensile strength and distribution were determined. The RipRoot model was used to quantify the additional cohesion derived from the plant roots. Among all tested roots, Eleusine floccifolia (grass), Tephrosia vogelii (tree), and Rosa abyssinica (shrub) had the strongest roots for each life form. The root volumetric ratio (root volume divided by soil volume) in the top 0.6 m of soil ranged from 0.03% to 0.46%. Roots of Digitaria abyssinica provided the maximum added cohesion (10.6 kPa) over this depth. For a given plant species, root volumetric ratio had a greater effect on additional cohesion than root tensile strength. Plant species with a fibrous root system provided greater additional cohesion values in the top 0.6 m (on average 3.4 vs. 1.7 kPa for tap root systems) and could potentially enhance gully bank stability for shallow gullies more than plants having a tap root system. To effectively rehabilitate larger gullies, plants should be integrated with other gully rehabilitation measures, such as regrading of gully banks and structural measures such as check dams to trap sediments, thereby reducing the effective gully bank height relative to rooting depth.
1 INTRODUCTION
Gully channels increase runoff and sediment loads in landscapes where equilibrium conditions have been disturbed (Schumm, 1979). In the Ethiopian highlands, increasing population pressure and associated land use change have caused gully formation (Billi, 2015; Frankl, Poesen, Haile, Deckers, & Nyssen, 2013; Guzman et al., 2017; Tebebu et al., 2015), which has resulted in severe land degradation (Tebebu et al., 2010) and accelerated reservoir sedimentation (Haregeweyn et al., 2005) at great economic cost (Ayele et al., 2015). These gullies occur mainly in the periodically saturated bottomlands and are caused by channel head seepage erosion (Dietrich & Dunne, 1993).
In the past, check dams have been the preferred method to control gully expansion with limited success (Poesen, 2011). Although biological measures have been integrated with the structural measures, their contribution to enhance the effectiveness of physical soil and water conservation (SWC) measures for controlling soil erosion has been rarely studied. For example, grass and forage trees have been planted on the banks of gullies and bunds in the valley bottoms and uplands, respectively (Dagnew et al., 2015). Amare et al. (2014) found on experimental plots that plants such as elephant grass (Pennisetum purpureum) integrated with soil bunds reduced soil erosion significantly compared with soil bunds only. According to Guto, Pypers, Vanlauwe, de Ridder, and Giller (2011), the nature of root and shoot structure of elephant grass helped to conserve soil and water. Also, Tephrosia vogelii, a nitrogen-fixing shrub native to Ethiopia, has shown wider adaptability with high biomass yield that could be used as a green manure. The effectiveness of vetiver grass (Chrysopogon zizanioides) to reduce soil erosion has been widely published (Babalola, Oshunsanya, & Are, 2007; Oshunsanya, 2013). It is known to be environment-friendly, and when planted in single rows, it will form a hedge that is very effective in slowing and spreading runoff water, thereby reducing soil erosion, conserving soil moisture, and trapping sediment and farm chemicals on site (Kebede & Yaekob, 2009). Vetiver is a perennial grass growing up to 2 m high and more than 3 m deep (Truong, Van, & Pinners, 2008). The extremely deep and massively thick root system of vetiver grass binds the soil, which makes it very difficult to be dislodged under high water velocity. The deep root system makes the vetiver plant also extremely drought tolerant (Hengchaovanich, Chomchalow, Limpichati, & Vessabutr, 1998). Similarly, vetiver is also highly resistant to pest, disease, and fire (West, Sterling, & Truong, 1996). As a limitation, Amare et al. (2014) reported that vetiver grass requires at least 3 years of establishment to conserve soil and water efficiently. Further, as vetiver is an exotic species and potentially invasive, it is important to evaluate its benefits in enhancing soil shear strength for gully stabilization relative to, for example, elephant grass.
Plant root systems play important roles in soil erosion and eco-hydrology as they affect the infiltration rate, aggregate stability, moisture content, and shear strength (Gyssels, Poesen, Bochet, & Li, 2005). Roots reduce runoff and soil erosion in both dry and wet seasons (De Baets et al., 2008). The effectiveness of plant roots in reducing runoff and soil erosion depends on the distribution and number of fibrous roots less than 1 mm in diameter (X.-L. Li, Marschner, & George, 1991), root surface area density (Zhou & Shangguan, 2005), and root length densities or root densities (De Baets, Poesen, Gyssels, & Knapen, 2006; Gyssels et al., 2006; Gyssels & Poesen, 2003; Vannoppen, Vanmaercke, De Baets, & Poesen, 2015). Studies have demonstrated that the root systems of plants play a critical role in stabilizing banks of gullies and streams by enhancing the soil shear strength both mechanically by reinforcement of roots and hydrologically by reducing soil water content (De Baets et al., 2008; Pollen & Simon, 2005; Pollen, Simon, & Collison, 2004; Simon & Collison, 2002). According to Simon and Collison (2002), plant roots increased bank factor of safety by an average of 39% for woody species and 70% for grasses. Plant roots may also provide a larger resistance to surface erosion by water (Y. Li et al., 2016; Thomas & Pollen, 2010). Y. Li et al. (2016) found that root architecture type determines effective resistance to water flow. For example, oblique root architecture types exhibit the largest resistance to erosion by water.
Roots reinforce the soil, which is commonly expressed as an additional shear resistance or as cohesion (Waldron, 1977). The magnitude of this cohesion depends on the plant root characteristics such as distribution, tensile strength, tensile modulus, the interface friction with the soil, and the orientation with respect to the principal direction of strain (De Baets et al., 2008; Gyssels et al., 2005; Pollen & Simon, 2005; Wu, McKinnell III, & Swanston, 1979). In the most simplistic models, the increase in shear strength depends entirely on the tensile strength of the roots and the root–area ratio (De Baets et al., 2008; De Baets, Poesen, Knapen, Barberá, & Navarro, 2007; Wu et al., 1979). However, Pollen and Simon (2005) found that such simplistic models tend to overestimate root reinforcement and therefore developed a dynamic fibre bundle model, RipRoot, accounting for the progressive manner in breaking of roots.
Although several experimental and modelling studies have demonstrated the role of the root systems of grasses, shrubs, and trees in stabilizing soils (Kokutse, Temgoua, & Kavazović, 2016; Oorschot, Kleinhans, Geerling, & Middelkoop, 2015; Veylon, Ghestem, Stokes, & Bernard, 2015; Zaimes & Schultz, 2015), studies investigating the contribution of the root systems of indigenous and exotic plant species of the Ethiopian highlands in increasing soil shear strength are lacking. It is important to study these because gully erosion is rampant and stabilizing banks with plants would be a cost-effective practice of protecting gullies (Ayele et al., 2015; Zegeye et al., 2016). The objective of our study was therefore to investigate which plant species, commonly found in the subhumid Ethiopian highlands, are most appropriate to strengthen the gully banks, and thereby reduce gully erosion, and to quantify the additional cohesion by roots using the RipRoot model.
2 MATERIALS AND METHODS
2.1 Study area
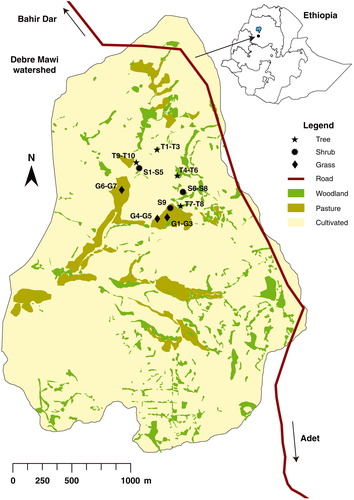
This study was conducted in the Debre Mawi watershed located 30 km south of Lake Tana along the road from Bahir Dar to Adet between latitudes 11o20′13″ and 11o21′58″ N and longitudes 37o24′07″ and 37o25′55″ E in the northwestern Ethiopian highlands (Figure 1). The Debre Mawi watershed covers an area of 608 ha. The semi-humid climate is characterized by a major dry phase lasting from October until May with an average annual rainfall of 1,280 mm. Ninety-two percent of the area is crop land and used for the cultivation of teff (Eragrostis abyssinica), finger-millet (Eleusine coracana), maize (Zea mays), and wheat (Triticum aestivum). The steep slopes in the upper part of the watershed, where the top soil is too shallow to sustain crop growth, are covered by small indigenous bushes and shrubs.
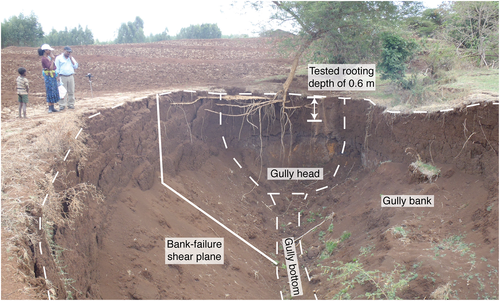
Though the valley bottoms are potentially the most productive, rapidly developing gullies are fragmenting the fertile land and aggravating soil losses (Tebebu et al., 2010). More than 20 ha or 3% of land in the Debre Mawi watershed is affected by gully erosion (Zegeye et al., 2016). In response to the problems of land degradation, the government of Ethiopia with aid of local farmers carried out a large-scale SWC campaign in the Debre Mawi watershed in 2012 (Dagnew et al., 2015). However, the campaign work was mainly focused on controlling sheet or rill erosion, with little attention paid to gully erosion control. Thus, despite this effort, sediment load and concentration at the watershed outlet have not decreased (Dagnew et al., 2015). Further, efforts to rehabilitate gullies using check dams, runoff diversions, and vegetative treatments were not successful as such practices did not take into account the actual gully formation mechanics (Dagnew et al., 2015; Langendoen, Tebebu, Steenhuis, & Tilahun, 2013). The continued gully incision, lateral expansion, and headwards extension are governed by both the high ground water causing the collapse of the gully head and sidewalls and the subsequent removal of the failed materials by flowing water (Zegeye et al., 2016). Figure 2 shows a typical eroding gully in the Debre Mawi watershed.
2.2 Sampling and experimental design
The most abundant plant species in the Debre Mawi watershed were identified by interviewing the local community and through transect walks. Roots of 26 plant species comprising 7 grass, 10 shrub, and 9 tree species were sampled to determine both tensile strength and root volumetric ratio (RVR). Of the 26 sampled plants, 18 (70%) were indigenous, and eight (30%) were exotic (Table 1), and their average age was approximately 3 years.
| Species local name | Scientific name | Life form | Origin | Total roots measured | Number of successfully tested roots (number of roots tested) | Tr, ave. (MPa) | RVR (%) | Diameter (mm) | a | b | R2 | Cr at depth of 0.6 m (kPa) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Godir | Digitaria abyssinica | Grass | I | 907 | 9 (12) | 44 | 0.46 | 0.43–1.17 | 37 | −0.48 | 0.52 | 10.6 |
| Akirma | Eleusine floccifolia | Grass | I | 419 | 9 (12) | 47.7 | 0.26 | 0.28–0.86 | 26 | −1.09 | 0.78 | 3.8 |
| Senbelet | Hechynomene dregeana | Grass | I | 241 | 10 (11) | 30.2 | 0.18 | 0.39–1.50 | 26 | −0.97 | 0.93 | 3.6 |
| Vetiver | Chrysopogon zizanioides | Grass | E | 244 | 8 (10) | 25.9 | 0.24 | 0.41–1.82 | 23 | −0.29 | 0.67 | 3.5 |
| Elephantgrass | Pennisetum purpureum | Grass | E | 110 | 10 (18) | 36.7 | 0.11 | 0.41–1.33 | 36 | −0.73 | 0.61 | 2.2 |
| Murie | Sporobolus natalensis | Grass | I | 158 | 7 (8) | 33 | 0.15 | 0.2–1.28 | 28 | −0.58 | 0.77 | 1.5 |
| Shenbeko | Arundo donax | Grass | I | 60 | 10 (16) | 36.6 | 0.06 | 0.71–2.02 | 35 | −0.3 | 0.47 | 1.2 |
| Simiza | Justica shimperiana | Shrub | I | 144 | 11 (16) | 18.4 | 0.28 | 0.57–2.84 | 23 | −0.64 | 0.89 | 5.0 |
| Dengorita | Vernonia auriculata | Shrub | I | 69 | 9 (11) | 14 | 0.28 | 0.14–3.27 | 21 | −0.67 | 0.68 | 3.3 |
| Yergib Ater | Cajans cajan | Shrub | I | 33 | 10 (12) | 39 | 0.11 | 0.19–3.46 | 38 | −0.48 | 0.67 | 3.3 |
| Grawa | Vernonia amygdalina | Shrub | I | 33 | 12 (17) | 10.9 | 0.11 | 0.30–5.00 | 18 | −0.94 | 0.77 | 1.3 |
| Gorjejit | Sida rhombifolia | Shrub | I | 53 | 9 (11) | 30.3 | 0.07 | 0.41–3.0 | 26 | −0.75 | 0.75 | 1.1 |
| Agam | Carissa edulis | Shrub | I | 51 | 11 (15) | 25.7 | 0.09 | 0.39–3.9 | 26 | −0.44 | 0.72 | 1.1 |
| Gesho | Rhamnus prinoides | Shrub | I | 14 | 4 (6) | 36.5 | 0.04 | 1.45–3.63 | 45 | −0.88 | 0.9 | 0.8 |
| Qega | Rosa abyssinica | Shrub | I | 13 | 7 (8) | 37.2 | 0.07 | 0.23–3.78 | 36 | −0.97 | 0.94 | 0.7 |
| Ambacho | Aechynomene elaphroxylon | Shrub | I | 8 | 7 (7) | 7.4 | 0.03 | 1.02–2.13 | 12 | −0.93 | 0.57 | 0.2 |
| Yabsha Girar | Acacia abyssinica | Tree | I | 25 | 8 (11) | 27.2 | 0.13 | 0.55–3.65 | 30 | −0.19 | 0.51 | 4.6 |
| Susbania | Sesbania sesban | Tree | E | 83 | 12 (14) | 52 | 0.19 | 0.33–6.34 | 53 | −0.5 | 0.86 | 3.9 |
| Yeferenj Girar | Acacia decurrens | Tree | E | 417 | 11 (17) | 38.8 | 0.21 | 0.33–2.93 | 34 | −0.38 | 0.63 | 3.9 |
| Tephrosia | Tephrosia vogelii | Tree | E | 88 | 12 (14) | 30.3 | 0.1 | 0.13–3.12 | 31 | −1.02 | 0.91 | 2.6 |
| Nech Girar | Acacia seyal | Tree | I | 14 | 13 (14) | 31.5 | 0.03 | 0.31–3.00 | 39 | −0.62 | 0.76 | 2.2 |
| Tree Lucerne | Cytisus proliferus | Tree | E | 48 | 6 (8) | 28.2 | 0.16 | 0.56–2.38 | 25 | 0.44 | 0.27 | 1.8 |
| Wanza | Cordia africana | Tree | I | 49 | 9 (13) | 25.4 | 0.16 | 0.55–6.20 | 22 | −0.28 | 0.59 | 1.6 |
| Bahir Zaf | Eucalyptus camaldulensis | Tree | E | 23 | 9 (14) | 17.5 | 0.12 | 0.24–3.20 | 18 | −0.57 | 0.49 | 1.1 |
| Misana | Croton macrostachyus | Tree | I | 14 | 9 (11) | 15.9 | 0.05 | 0.48–3.65 | 19 | −0.43 | 0.45 | 1.0 |
| Gravilia | Gravilea robusta | Tree | E | 32 | 9 (13) | 26.5 | 0.07 | 0.37–2.28 | 22 | −1.09 | 0.92 | 0.4 |
- Note. The species origin is labelled E for exotic species and I for indigenous species. Tr is root tensile strength, RVR is root volumetric ratio, a, b, and R2 are values for the power relationships Tr = aD−b, and D is root diameter
The excavation of each plant (one individual plant per each plant species) was carried out manually within an area delineated by the vertical projection of the aboveground biomass (De Baets et al., 2007) and to a depth of 0.6 m (Figure 3). The vertical projection of the aboveground biomass may underestimate the lateral extent of reinforcement by roots whose lateral spread exceed the vertical projection, especially for mature vegetation. However, the tested species were approximately 3 years old, and the number of roots that had grown beyond the plant's crown width was negligible compared with the total number of roots. Care was taken to avoid any damage to roots during the excavation process; such damage was not observed during the excavation process. After excavation, the roots were washed by water and packed immediately in a plastic bag to preserve their moisture content and then transported to the Adet Agricultural Research Center where they were stored at 4 °C, and RVR for each plant was determined (Figure 4).


2.3 Determination of root volumetric ratio and root tensile strength
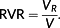 (1)
(1)This depth was purposely selected to represent the rooting depths of all plant species having the age of 3 years. However, in practice, we found that this soil depth underestimated the maximum RVR of grasses, as most of their roots were found at depths less than 0.6 m. Figure 2 shows that this depth is also representative for the majority of roots for trees.
Following the determination of RVR of each plant, the roots were stored in a 15% ethanol solution at a temperature of 4 °C (De Baets et al., 2008) for 3 to 5 days until transported to the Addis Ababa Leather Industry Development Institute (LIDI) for determining root tensile strength. This conservation method was proposed by Bischetti et al. (2005) as an alternative method to preserve root moistures for several months and showed results equivalent with fresh root samples.
The root tensile strength tests were then carried out in the LIDI research and testing laboratory using a Testometric M350-20AT materials testing machine, which has a speed range of 0.001 to 500 mm min−1. Selecting an appropriate test speed is very important. Velocities recorded for the expansion or retreat of gully systems by rapid landslides or mass movements range from 1 to 300 mm min−1 (Cruden & Varnes, 1996). According to De Baets et al. (2008), a smaller test speed has to be performed when a critical root tensile strength has to be assessed for very slow soil erosion processes. Therefore, a speed of 10 mm min−1 was selected (Bischetti et al., 2005; De Baets et al., 2008). The tested root length was 0.1 m for all roots as shown in Table S1.
From a total of 3350 roots collected from all selected plant species, 319 samples that represent the range of diameters were tested, and data were collected from these roots (Table S1). Before testing, root diameter was measured at three points, that is, near the upper grip, at the middle, and near the bottom grip, using a digital calliper. Clamping is the most critical issue when measuring root tensile strength (De Baets et al., 2008). Two hundred forty-one specimens (76%) broke successfully near and at the middle of their test length. Seventy-eight specimens (24%) broke near or at the position of clamping or slipped out of the clamps. These tests were considered as invalid and not used in the data analysis (Table S1).
2.4 Determination of additional cohesion from roots
To determine the additional shear resistance to the soil, we used the root reinforcement model RipRoot (Pollen, 2007; Pollen & Simon, 2005), which is included in the bank stability and toe erosion model (BSTEM) v5.4 (Simon, Pollen-Bankhead, & Thomas, 2011). RipRoot is a global load-sharing fibre-bundle model that calculates root reinforcement by evaluating two processes: (a) root reinforcement based on progressive root breaking, which depends on the number, sizes, and tensile strength of roots present in the bank; and (b) root pull-out based on the friction at the root–soil interface, which is a function of root and soil properties and pore-water pressures. The Ethiopian indigenous and exotic plant species are not included in RipRoot's built-in database of vegetation species. Thus, we used our measured root tensile strength–diameter relationship and root distribution data to run the model.
BSTEM was set up based on the data collected at one of the large gullies in the Debre Mawi watershed. To calculate the forces exerted by the soil on the roots, the tested plant species were located on a gully bank with a height of 5 m, which was inclined at 85° except for the bottom 0.8 m (that is, the bank toe area) where the inclination was 45°. Further, the groundwater table was 0.5 m below the ground surface, and the measured soil shear strength values were used (Figure S1; cohesion ranged from 10 to 16 kPa and internal friction angles ranged from 14° to 25° for five bank soil layers; see also Zegeye et al., 2014). The measured number of roots for each plant species was used in the model and grouped into six root diameter classes: 0–1, 1–2, 2–3, 3–5, 5–10, and 10–20 mm (Table S2). In addition, the values of coefficient “a” and exponent “b” obtained from the power relationship between root diameter and root tensile strength (Table 1) were inputs for the RipRoot model. The reported additional cohesion (Table 1) is an average for the top 0.6 m of the soil profile, which fieldwork has shown to generally include the majority of fine roots that contribute to the reinforced root–soil matrix (see also Figure 2). In addition, the potential of one of the tested species (Digitaria abyssinica, which could add a cohesion of about 10.6 kPa over a depth of 0.6 m, Table 1) to stabilize gully head and banks was evaluated over a range of bank slopes (45–85°) with the BSTEM.
2.5 Statistical analysis
Tests for normality (Kolmogorov–Smirnov D statistic) and equality of variance (Levene statistic) were conducted, and it was found that root tensile strength, RVR, and cohesion were not normally distributed. Hence, a nonparametric test, a Kruskal–Wallis test (Daniel, 1990) was performed to test the significance of the difference in root tensile strength, RVR, and cohesion between life forms (grasses, shrubs, and trees). Also, we tested the significance of differences in root tensile strength, RVR, and cohesion within a life form (i.e., within grass, shrub, and tree species). Further, the differences in the above three parameters between fibrous (the root system usually formed by thin, moderately branching roots growing from the stem) and tap root (the root system consisting of a large, central, and dominant root that grows directly downward from which other roots sprout laterally) systems and between native (four species) and exotic (six species) tree species were tested using the same statistical test. Because, only two grass species and no shrub species were exotic, the comparisons of the three parameter tests between native and exotic species for both life forms were not made. Using a least square error method, power law equations were fitted through the measured root tensile strength as a function of root diameter. Correlation tests using Excel were conducted to determine the relationships between additional cohesion (Cr) and RVR and root tensile strength.
3 RESULTS AND DISCUSSION
3.1 Root systems
All grasses, the trees Acacia decurrens and Cytisus proliferus, and shrub species Justica shimperiana had a dense and fibrous root system. Other investigated shrubs and trees had a tap root system (Figure 4 and Table 1). On average, the seven grass species had just over 300 roots per plant, and the number of roots varied from 907 for Godir (D. abyssinica) to 60 for Shenbeko (Arundo donax, Table 1). The 10 tree and 9 shrub root systems had fewer roots with an average of 40 roots per shrub and 70 roots per tree (Table 1). The number of roots of Yeferenj Girar (A. decurrens) was similar to grass (Table 1).
3.2 Root volumetric ratio
The volume of roots per soil volume (RVR) of all tested plants was less than 1% (Table 1). The maximum RVR measured was 0.46% for D. abyssinica (grass) roots. In general, the grass species had greater RVR compared with shrub and tree species. The species A. decurrens and the species Vernonia auriculata and J. shimperiana had the largest RVRs in the tree and shrub vegetation groups, respectively. Using the Kruskal–Wallis test, we found no significant difference in RVR between the three groups of plant life forms (p > .05). Similarly, there was no significant difference between native and exotic tree species (p > .05), but plants with a fibrous root system had significantly (p < .05) higher RVR than plants with a tap root system.
3.3 Root tensile strength
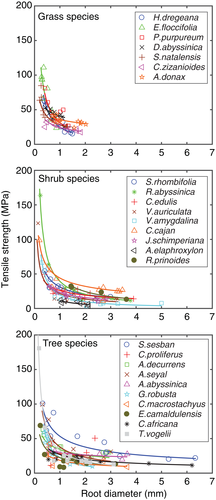
The results demonstrated that smaller roots are stronger per unit area than large roots, resulting in decreasing root tensile strength (Tr) with increasing root diameter (D) for all but one of the tested plant species (C. proliferus), see Figure 5 and Table 1. For all tested plant roots, tensile strength was strongly correlated to root diameter (mean R2 = 0.67 ± 0.18). The weakest relationship (R2 = 0.27) between tensile strength and diameter was found for C. proliferus, whereas the strongest correlation (R2 = 0.94) between tensile strength and diameter was found for Rosa abyssinica (Table 1). The mean tensile strength of the investigated roots of grass species ranged from 25 to 48 MPa (n = 86 roots tested), of shrub species from 7 to 39 MPa (n = 103 roots tested), and of tree species from 16 to 52 MPa (n = 129 roots tested). Within a life form, Eleusine floccifolia (grass), T. vogelii (tree), and R. abyssinica (shrub) had relatively the strongest roots, whereas C. zizanioides (grass), Cordia africana (tree), and Aechynomene elaphroxylon (shrub) had the weakest roots. The maximum root tensile strength value (384 MPa) of all 26 tested plant life forms was recorded for T. vogelii with a root diameter of 0.13 mm.
The median root tensile strength between the three groups of plant life forms was not significantly different (p > .05). Our results indicated that plants with a fibrous root system have significantly (p < .05) higher root tensile strength (mean = 47 MPa) than plants with a tap root system (mean = 25 MPa). Similarly, the root systems of exotic tree species are significantly (p < .05) stronger (mean = 40 MPa) than those of native tree species (mean = 27 MPa).
Our results are comparable with those of previous studies (Burylo, Rey, Mathys, & Dutoit, 2012; De Baets et al., 2008; Genet et al., 2005; Pollen et al., 2004; Pollen & Simon, 2005; Wu et al., 1979), in that root tensile strength and root diameter are inversely related (Figure 5). The maximum measured root tensile strength value of 354 MPa for a 0.13-mm diameter T. vogelii root was similar to that found in previous studies. For example, Genet et al. (2005) observed strength values of 132–201 MPa for roots <0.9 mm in diameter for three tree species, and Bischetti et al. (2005) found extremely high values (up to 750 MPa) for root diameters of 0.2–0.5 mm of several tree species.
3.4 Root reinforcement
The RipRoot submodel of BSTEM (Pollen & Simon, 2005) was used to calculate additional cohesion (Cr) values for each studied plant over the excavated depth of 0.6 m (Table 1). Additional cohesion from roots varied between 0.2 (A. elaphroxylon; shrub) and 10.6 kPa (D. abyssinica; grass). The values of additional cohesion from roots of grasses were generally twice those of shrubs and approximately 50% greater than those of trees (Table 1). However, the median Cr value of the three groups of plant life forms was not significantly different (p > .05). Similarly, there was no significant difference in additional cohesion from roots between native and exotic tree species (p > .05). Plant species having fibrous root systems with a large number of fine, strong roots (e.g., all grasses and the tree species A. decurrens) contributed the highest amount of soil reinforcement (on average 3.4 kPa) as compared with plants with tap root systems (on average 1.7 kPa; e.g., C. africana [wanza], Eucalyptus camaldulensis, and R. abyssinica [Qega]), which is in line with the study by De Baets et al. (2008).
The additional cohesion from roots (Cr) calculated using RipRoot for each plant species was significantly correlated with the plant's RVR (R2 = 0.76, p < < .05; Figure 6a), whereas the relationship between Cr and root tensile strength (Tr) for a species was not significant (R2 = 0.15, p > .05; Figure 6b). Generally, plants with a larger RVR provided greater soil root-reinforcement compared with plants having higher root tensile strength (Table 1 and Figure 6). For example, though the roots of R. abbysinica (shrub), Gravilea robusta (tree), and Sporobolus natalensis (grass) have greater root tensile strength, their smaller value of RVR limits their effectiveness in increasing the strength of soils.
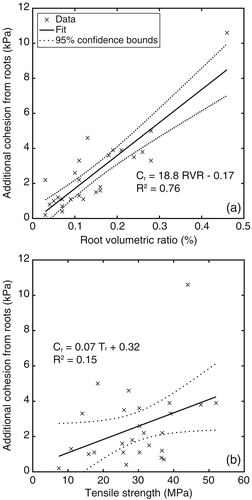
Previous model studies that did not use the RipRoot model reported higher Cr values. For example, trees and rush plants increased cohesion up to 304 kPa (De Baets et al., 2008). This may be due to the difference in the estimating ability of the models used. Most published research used Wu et al.'s (1979) model, which has shown to overpredict additional cohesion from roots relative to the RipRoot model (Pollen et al., 2004; Pollen & Simon, 2005). Because of this, Schwarz, Preti, Giadrossich, Lehmann, and Or (2010) developed a correction factor of 0.44, which indicates that the Wu et al. (1979) model may overestimate the additional cohesion by 227%. Pollen and Simon (2005) validated the RipRoot estimates by performing direct shear tests on soil samples with and without Switchgrass roots. They found that the Wu et al. (1979) model overestimated the shear strength by 6 to 14-fold. The estimates of the RipRoot model were considerably more accurate. The different assumptions of root failure underlying the Wu et al. (1979) equation and RipRoot model, for example, catastrophic failure of all roots at their peak tensile strength (Wu et al., 1979) versus progressive breaking and stress redistribution (RipRoot), are the main reason for the respective difference in additional cohesion.
3.5 Mechanical and hydrologic effect of plant roots on soil shear strength and erosion control strategies
Gully bank erosion in the subhumid northern highlands of Ethiopia is often caused by the interaction of gravitational forces operating on the bank mass and hydraulic forces operating at the bank toe that result in undercutting and steepening of the toe. Improving the stability of gully banks and heads is a matter of either decreasing the driving forces operating on the soil mass and/or increasing the shear resistance of the soils to gravitational failure. Soil shear strength is highly sensitive to pore-water pressure within the bank material (Simon, Curini, Darby, & Langendoen, 2000). When soils are saturated, pore-water pressure reduces soil shear strength. Positive pore-water pressures not only reduce soil shear strength as described above but also increase the weight of the bank material mass and contribute to bank failure. Thus, maintaining negative pore-water pressure by artificial or other means could decrease the driving forces and increase the shear strength of bank material resulting in greater bank stability and reduced frequency of mass failure (Simon, Curini, Darby, & Langendoen, 1999).
Vegetation is an important contributor to bank stability for two reasons: (a) additional cohesion of the soil through root reinforcement and (b) increased apparent cohesion by reduced pore-water pressures caused by the lowering of groundwater table and soil water content through transpiration. As reported by Coppin and Richards (1990), about only 10–20% of the rainfall can be intercepted by the canopy of deciduous tree species. This was confirmed by Simon and Collison (2002) that most bank failures occur when deciduous vegetation is dormant and canopies have been shed. Simon and Collison (2002) investigated that the bank factor of safety (Fs) for a bank covered by eastern gamagrass (Tripsacum dactyloides) was increased by 35% due to root reinforcement effects but reduced by 10% due to the detrimental effects of wetter soil conditions, to give a net increase of 25%. Similarly, these authors reported that the hydrologic effects (enhanced matric suction) for mixed trees were more beneficial than the mechanical effects, increasing Fs by 71%. In comparison, grasses produced a detrimental hydrologic effect due to increased infiltration rate and delivery of water to depth during rainfall events, and Fs was reduced by 10%.
Although the root distribution with depth was not studied in this research, our physical observation showed that the number of plant roots decreased with soil depth, indicating that in the case of high banks, like those along larger rivers or deeply incised gully channels, potential slip surfaces are located below the root zone, and vegetation probably has little effect on soil strength (Abernethy & Rutherford, 2001). This finding is in line with Langendoen et al. (2013) who found through computer modelling that root reinforcement provided by vegetation has a limited stabilizing effect for deep gullies. Cammeraat, van Beek, and Kooijman (2005) and De Baets et al. (2008) reported similar findings for Mediterranean vegetation, in that the root reinforcement effect is limited and only present in the upper 0.4 to 0.5 m of top soil. This implies that gully sidewall stabilization with vegetation should be integrated with other protective measures such as a combination of gully bottom grade control to prevent further incision and toe protection to prevent sidewall steepening (Langendoen et al., 2013).
Deep rooted shrubs, such as J. shimperiana, V. auriculata, and trees such as A. abyssinica, can be used to stabilize gully walls as they provide an added cohesion exceeding soil depths of 1 m. Grasses, such as D. abyssinica, E. floccifolia, C. zizanioides, Hechynomene dregeana, or P. purpureum (in the order of decreasing additional cohesion from 10.6–2.6 kPa), can be planted on small gullies to stabilize the 0–0.30 m of topsoil (Addisie et al., 2017; Ayele et al., 2015). These species have also other benefits. P. purpureum (elephant grass) yields high biomass for livestock feed and revegetates very quickly to provide continuous added shear resistance (Amare et al., 2014). H. dregeana (Senbelet) is used for animal fodder and for covering the roof of a house and can increase economical return. D. abyssinica and E. floccifolia can be planted as well in natural drainage lines or on gully bottoms to prevent soil detachment by water as they have strong roots.
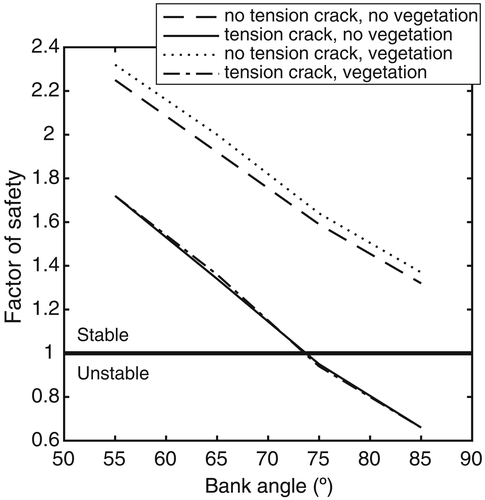
The BSTEM simulation results showed that planting grasses on the bank top of the gully had no effect on bank stability when there is a tension crack at the gully bank (Figure 7). However, vegetation can prevent the occurrence of a tension crack and therefore could play a greater role in increasing the stability of banks (Fs was increased by a value of 0.6) than discussed above (Figure 7). Further, the tested grass and shrub species could reduce the probability of gully initiation and could stabilize the banks of gullies whose depths are reduced, either by filling the channel or through constructing check dams to trap sediments. Grass and shrub species can then be planted or sowed on a regraded bank or on the deposited silt.
4 CONCLUSIONS
A study was conducted to investigate the root reinforcement to soil by indigenous and exotic plants in the northwestern subhumid Ethiopian highlands. The main findings are as follows. Root tensile strength for all tested species but one (C. proliferus) was inversely correlated to root diameter. The mean tensile strength of the investigated roots ranged from 7 to 52 MPa (319 roots tested). Plants with a fibrous root system had significantly higher root tensile strength than plants with a tap root system. The RVR over a soil depth of 0.6 m varied between 0.03% and 0.46%. Grass species had higher RVRs compared with shrub and tree species. The tested exotic tree species are stronger than native tree species. The additional cohesion by roots to the soil over a depth of 0.6 m varied between 0.2 (A. elaphroxylon; shrub) and 10.6 kPa (D. abyssinica; grass). The RVR had a greater effect on soil root-reinforcement than root tensile strength. There was a significant difference in mean additional cohesion from roots between plant species having fibrous root systems and tap root systems, which was not observed between native and exotic plant species, and between the three plant life forms.
Although vegetation provides additional cohesion to soil and could reduce the groundwater elevation and soil water content in gully banks through transpiration, the findings suggest that it needs to be integrated with other gully rehabilitation measures such as reshaping or regrading of gully banks and constructing physical structures (e.g., check dams) to effectively rehabilitate larger gullies. Grass and shrub species could be planted or sowed on a regraded bank or on a raised gully bottom. Our findings could contribute to the success of the nationwide Ethiopian SWC campaign by providing science-based evidence on (a) effectiveness of different plant species in controlling gully erosion, (b) technical requirements that should be considered when using biological conservation measures, and (c) identifying the better integration of physical and biological conservation measures.
ACKNOWLEDGEMENTS
This research was supported financially by The Norman E. Borlaug Leadership Enhancement in Agriculture Program (Borlaug LEAP-016258-82), International Foundation for Science (IFS-W/5407-1), Cornell University (Hudson H. Lyon fund, Bradfield Research award), Ethiopian Ministry of Water, Irrigation and Energy, and Ethiopian Road Authority. We would like to thank Addis Ababa Leather Industry Development Institute (LIDI), especially Petros, Kibrom, and Tsegab, for allowing us to use the materials testing machine for the root tensile strength tests. This manuscript was greatly improved by comments from Dr Alexia Stokes and six anonymous reviewers for which we are very grateful.




