Climate Futures for Lizards and Snakes in Western North America May Result in New Species Management Issues
Funding: This project was funded by the Western Association of Fish and Wildlife Agencies (WAFWA).
ABSTRACT
We assessed changes in fundamental climate-niche space for lizard and snake species in western North America under modeled climate scenarios to inform natural resource managers of possible shifts in species distributions. We generated eight distribution models for each of 130 snake and lizard species in western North America under six time-by-climate scenarios. We combined the highest-performing models per species into a single ensemble model for each scenario. Maps were generated from the ensemble models to depict climate-niche space for each species and scenario. Patterns of species richness based on climate suitability and niche shifts were calculated from the projections at the scale of the entire study area and individual states and provinces, from Canada to Mexico. Squamate species' climate-niche space for the recent-time climate scenario and published known ranges were highly correlated (r = 0.81). Overall, reptile climate-niche space was projected to move northward in the future. Sixty-eight percent of species were projected to expand their current climate-niche space rather than to shift, contract, or remain stable. Only 8.5% of species were projected to lose climate-niche space in the future, and these species primarily occurred in Mexico and the southwestern U.S. We found few species were projected to lose all suitable climate-niche space at the state or province level, although species were often predicted to occupy novel areas, such as at higher elevations. Most squamate species were projected to increase their climate-niche space in future climate scenarios. As climate niches move northward, species are predicted to cross administrative borders, resulting in novel conservation issues for local landowners and natural resource agencies. However, information on species dispersal abilities, landscape connectivity, biophysical tolerances, and habitat suitability is needed to contextualize predictions relative to realized future niche expansions.
1 Introduction
Reptiles are a diverse (Tingley, Meiri, and Chapple 2016; Roll et al. 2017) but relatively understudied group of vertebrates that are likely to respond to changing climates because of their ectothermic physiology, relatively small body size, and limited dispersal capabilities (Ceia-Hasse et al. 2014; Winter et al. 2016; Diele-Viegas et al. 2020). In western North America, the highest reptile diversity occurs in the south, where they are well adapted to the hot, dry desert environments (Pilliod et al. 2020; Whiting and Fox 2021). However, reptiles also occupy nearly all ecosystems from Canada to Mexico, apart from some of the highest elevations and latitudes (i.e., reptiles currently do not occur north of 60° north latitude).
As ectotherms, reptiles are strongly associated with, and highly sensitive to, environmental temperatures (Whiting and Fox 2021). Thus, upper elevation limits and northern boundaries of species' ranges are often established because excessive cold or temperature variability limit successful reproduction and survival (Sperry et al. 2010; Hoffmann, Chown, and Clusella-Trullas 2013), or because climate interacts with other factors (e.g., prey, predators, behavior) to restrict ranges (Wilson and Cooke 2004). Hence, if a reptile species has an innate adaptive capacity, whereby it can either persist-in-place or shift-in-space (e.g., Thurman et al. 2022) in response to a warming climate, then climate-based changes to species distributions may be variable but predictable. This enables modeling and mapping of species distributions at broad spatial extents and predicting changes in distributions under future climate change scenarios. Such information can be used by Tribal, state, provincial, and federal natural-resource managers who are responsible for the management and conservation of these non-game wildlife species or their habitats. Species-level assessments of potential shifts in distribution may provide an early warning for agencies managing for squamate species persistence, informing them how they might alter management to account for projected species gains (via colonization, translocation) or losses (local extirpation, extinction).
Like most other vertebrates, reptiles are experiencing elevated extinction risk in the Anthropocene, when human activities have significant effects on the global environment (Böhm, Williams, et al. 2016). Changes in species' distributions and abundance have been attributed to a complex mix of interacting factors (Saha et al. 2018). Global threats to reptiles include habitat loss and degradation, introduced invasive species, pollution, disease, unsustainable use and exploitation, and climate change (Gibbons et al. 2000). Climate change is particularly concerning because reptiles are ectothermic animals with life-history functions and corresponding diel-and-seasonal behaviors that are highly sensitive to changes in environmental temperature; climatic shifts will variably affect populations directly through changing climate regimes and indirectly by creating ecological mismatches, novel or vacant niches, and ecological traps (Cadby et al. 2010; Dell, Pawar, and Savage 2014; Ockendon et al. 2014; Hale and Swearer 2016; Winter et al. 2016). For example, temperature has been documented to alter the phenology of offspring production (e.g., Ockendon et al. 2014), which may shift to a suboptimal time of year that does not align with foraging opportunities (Sinervo et al. 2010; Dell, Pawar, and Savage 2014; Vafidis, Smith, and Thomas 2019) or seasonal weather trends (Wheeler et al. 2015).
Concern for reptiles in western North America is increasing because the region has been susceptible to prolonged drought and is currently experiencing increasing temperatures and altered moisture patterns as the climate changes (Huang et al. 2017; Williams et al. 2020). Recent estimates suggest that at least 20% of all snake and lizard species are highly vulnerable to climate change (Sinervo et al. 2010; Case, Lawler, and Tomasevic 2015; Böhm, Cook, et al. 2016; Jezkova and Wiens 2016; Griffis-Kyle et al. 2018). Some species may be particularly vulnerable because their close affiliation with specific substrates for thermoregulation and reproduction and relatively limited dispersal abilities can result in small or patchy distributions. For example, a study of 48 Sceloporus lizard species across 200 populations in Mexico reported a 12% population-level extinction rate between 1975 and 2009 (Sinervo et al. 2010). The authors suggested these local extinctions may be the result of altered thermal niches, whereby lizards were unable to forage adequately to permit viable growth and reproduction (Sinervo et al. 2010).
To begin understanding the potential effects of climate change on snake and lizard species in western North America, we first projected the current climate niche of each species and then assessed how the locations of those niches may change in the future. We used species occurrence points and climate variables to model the geographic distribution of each species' fundamental or potential climate-niche space under current or recent climate conditions and projected these relationships using past and future climate scenarios. Under each scenario, we generated maps depicting overall species richness and richness change (i.e., on the basis of climate suitability) between climate scenarios. We evaluated each species for future range changes and summarized information at the range-wide level and for each of 47 state or provincial jurisdictions in western North America from Canada to Mexico. Given existing models and known reptile climate sensitivities, we hypothesized that: (1) species climate-niche space would exhibit patterns of northern expansion and southern contraction in future scenarios, and (2) species would be more likely to occupy higher elevations in the future owing to warming temperatures and changing precipitation regimes (Cunningham et al. 2016).
2 Methods
2.1 Reptile Occurrence Points
We acquired snake and lizard occurrence points from open-source data when available and from government-owned data sets accessible through data-sharing agreements (Appendix 1). Data were formatted and combined using ArcGIS software (ESRI, Redlands, CA, USA). We used Crother (2017) for species nomenclature, and we grouped sub-species because of the potential for unreliable identification across time and observers. We filtered the dataset to include only data from the years 1981 to 2018. We removed redundant data by location (i.e., 1-km climate grid cell) and species (i.e., identical species records from different data sources or multiple captures of the same species from a specific grid cell). We flagged and discarded records within the United States (U.S.) that fell ≥ 200 km from their documented Gap Analysis Project (GAP) range polygons (U.S. Geological Survey 2013b, 2018b), when available. We also discarded records that fell within 300 m of a state or county centroid (i.e., geographic center of a polygon) because examination found that the centroid coordinates had been used as an approximate location by various data sources for records without specific location information. We discarded records that did not have a year attribute and those with uncertain location or quality.
Candidate reptile species were selected for modeling if we had > 50 occurrence points (133 of 195 species: 68.2%; Appendix 2). Data-deficient species that were not included in analyses were primarily found in the southern and on the eastern edge of our study extent (e.g., Mexico and southeastern U.S.). We combined four Hypsiglena species into one species group (i.e., genus) because of taxonomic uncertainty of historical records (Crother 2017), which resulted in 129 species and 1 genus for modeling (Appendix 2). Hereafter, we refer to these as 130 taxa as “species” for convenience.
2.2 Climate Data
We used the ClimateNA v5.5 tool to generate climate surfaces from a Digital Elevation Model (DEM) raster (U.S. Geological Survey and ESRI 2005; Wang et al. 2016). For each 1-km grid cell across western North America, we generated average monthly temperature and precipitation values, resulting in 24 variables (Appendix 3). The climate variables were generated for four periods: past (1901–1930), recent (1981–2010), future mid-century (2040–2069), and future late-century (2070–2100). Future climate was derived using a 15-model ensemble approach under two future emission scenarios (RCP 4.5 and RCP 8.5; Wang et al. 2016). Thus, six time-by-climate scenarios were examined for each species (one past, one recent, and four future). The tabular output data from ClimateNA were converted into rasters for subsequent climate niche modeling procedures.
2.3 Climate Niche Modeling
We performed the following analyses in R 3.4.3–3.5.1 and RStudio 1.1.383 (RStudio Team 2016; R Core Team 2017). We used the R package “biomod2 3.3-7” for species modeling (Thuiller et al. 2009, 2016; Guisan, Thuiller, and Zimmermann 2017). Because of the size of the datasets and computations required for this analysis, we processed all models with the Yeti computer cluster (i.e., “supercomputer”) hosted by the USGS Core Science Systems Advanced Research Computing center (https://www.usgs.gov/advanced-research-computing). We chose to exclude geographic (e.g., latitude), topographic (e.g., elevation, slope, aspect), and biophysical (e.g., soils, rock outcrops, vegetation cover types) variables in our models for the following reasons: (1) temperature and precipitation covary with many of these variables (Bonan 2002; Daly et al. 2008; Harrison, Spasojevic, and Li 2020); (2) not all variables will change under future climate scenarios and those that do are mostly not currently available across our study area (Rigge et al. 2023); (3) the intent of this research was to assess species climate niches and predict future climate suitability, not to develop the best-fitting species distribution models; and (4) we required datasets of commonly derived attributes that were available from Mexico to Canada. We ran 32 single models and one ensemble model for each species, resulting in 4290 total model runs.
We generated two replicates of pseudo-absences using 50,000 random points for each species from our presence-only dataset. For model evaluation, we withheld 30% of the occurrence points for each species' model run. This process was conducted twice for each of the two pseudo-absence generations, resulting in four runs for each analytical technique. We modeled the climate niche for each species using eight analytical techniques, including artificial neural networks (ANN), classification tree analysis (CTA), functional data analysis (FDA), generalized additive model (GAM), generalized linear model (GLM), multivariate adaptive regression splines (MARS), random forest (RF), and surface range envelope (SRE). We evaluated the single models based on their true skill statistic (TSS). To reduce model-based bias, we created a single ensemble model for each species by scenario combination using a weighted means approach. We included models in the aggregating procedure when they had TSS > 0.7 (Guisan, Thuiller, and Zimmermann 2017). Ensemble model performance was evaluated by the TSS score, specificity, and sensitivity. Variable importance values for the 24 climate variables were extracted for each species from the ensemble model.
2.4 Climate-Niche Space Projections
We generated binary projections for all six climate scenarios with the ensemble models using the default methodology within “biomod2,” which selects a threshold that will maximize specificity and sensitivity. When available, we correlated our recent (1981–2010) climate-niche space projections with published range and distribution information (U.S. Geological Survey 2013a, 2013b, 2018a, 2018b) to identify any modeling anomalies. We evaluated species' range changes by comparing the binary projection of suitable climate-niche space for each of the six climate scenarios. We used the biomod_rangesize function to create a summary table of projection comparisons for each species.
2.5 Changes in Species' Climate-Niche Space
For each species and scenario comparison, we identified whether its climate-niche space: (1) had a net increase of at least 10%; (2) had a net decrease of at least 10%; or (3) remained stable with a change between −10% and 10%. We then compared the recent (1981–2010) climate-niche space to the late-century future RCP 8.5 (2070–2100) scenario to evaluate if a species' suitable climate-niche space was projected to expand (little loss of suitable locations, with gains elsewhere), contract (loss of suitable locations, without gains elsewhere), remain stable (little change in suitable locations), or shift (expand or contract, while losing suitable locations). The predicted climate-niche space centroid for each scenario was calculated using the R package “sdmtools 1.1-221” and cogravity function (VanDerWal et al. 2014). We assessed range shifts between recent and future scenarios by evaluating the percent of pixels occupied in the recent scenario that were no longer predicted to be occupied in the future scenario in conjunction with the angle and distance between centroids of scenarios.
We then generated potential species richness rasters by stacking and summing the binary projections of climate-niche space for each species. These maps represent the cumulative climate suitability locations of all modeled reptiles in our dataset and not true estimated species richness. However, for ease of communication we refer to the maps as potential richness maps and we generated one for each of the six climate scenarios to visualize changes through time and under different climate-change outcomes. To evaluate richness change, we subtracted richness rasters between time steps. We identified “hotspots” and their changes visually.
2.6 Species Management Within and Across Jurisdictional Boundaries
We summarized which species were predicted to occur within each of 47 U.S. and Mexico states and districts and Canadian provinces (hereafter, “states”) per climate scenario. Presence was defined as having > 50 pixels of predicted suitable climate-niche space within a state. For U.S. states, we compared our model-derived, predicted state occurrence to those reported by Partners in Amphibian and Reptile Conservation (PARC; Pilliod and Wind 2008, Jones, Halama, and Lovich 2016), which are derived from Nature Serve (natureserve.org), regional herpetological expert opinion, and state biologist consultation. Changes in suitable climate area and location between scenarios were calculated as previously described. We then quantified elevation shifts within state boundaries by comparing elevation distributions within the predicted suitable locations between scenarios. Specifically, we compared the 25th and 75th percentile values to establish significance (Tukey 1977). For simplicity, in this paper, we focus on comparisons between the recent (1981–2010) and late-century future RCP 8.5 (2070–2100) scenarios, but comparisons between all scenarios are available for download or interactive visualization (Jeffries et al. 2024a, 2024b).
3 Results
3.1 Climate Niche Modeling and Projections
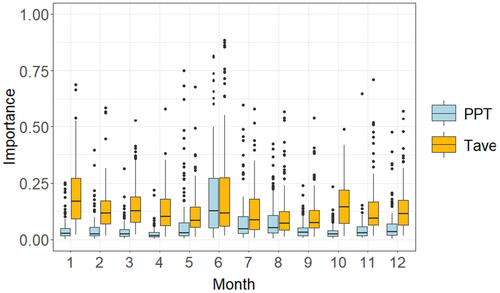
All 130 species yielded at least three single models with a TSS > 0.7; thus we created ensemble models for all 130 species for six climate scenarios: past (1901–1930), recent (1981–2010), mid-century future RCP 4.5 (2040–2069), mid-century future RCP 8.5 (2040–2069), late-century future RCP 4.5 (2070–2100), and late-century future RCP 8.5 (2070–2100) (Figure 1a,b; model performance summaries: Appendices 4 and 5; Jeffries et al. 2024a, 2024b). The top three climate variables across all models tended to include January average temperature (average variable importance ±SD: 0.21 ± 0.14), June average temperature (average variable importance ±SD: 0.20 ± 0.21), and June average precipitation (average variable importance ±SD: 0.19 ± 0.18; Appendix 6; Jeffries et al. 2024a).

3.2 Changes in Species' Climate-Niche Space
Summaries of suitable climate area and change in location between scenarios are available for each species in Jeffries et al. (2024a, 2024b, https://geonarrative.usgs.gov/reptileclimateniche). Relative to the recent (1981–2010) climate-niche projections, 68% of species are predicted to gain climate-niche space regardless of time or climate scenario. On average, by late-century (2070–2100), these species are predicted to gain an additional 70% (range: 20%–732%) of suitable climate space relative to their recent climate niche under the RCP 4.5 scenario and 116% (range: 10.5%–1513%) under the RCP 8.5 scenario. Conversely, only 8.5% of species are projected to lose climate-niche space in all future climate scenarios. On average, by late century, these species are predicted to lose 41% (range: 13%–100%) of their recent climate-niche space under the RCP 4.5 scenario and 65% (range: 17%–100%) under the RCP 8.5 scenario. Only six species (4.6%) are projected to maintain stable climate-niche space under all future emission scenarios.
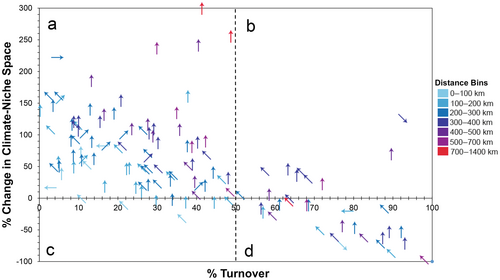
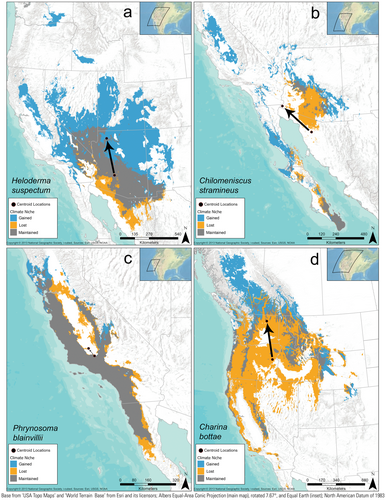
When we accounted for whether recently suitable climate space was maintained through time and climate scenario, we found that under the late-century future RCP 8.5 scenario, most species (85/130) are predicted to expand their climate space by at least 10%, while maintaining at least 50% of their original niche space (e.g., Figure 2a). In contrast, nine species are predicted to increase suitable space by at least 10%, but also to lose at least 50% of their recently suitable area (i.e., shift; Figure 2b). Twenty-one species are predicted to decrease suitable space by at least 10% while losing at least 50% of their original niche space (i.e., contract; Figure 2d, Table 1). Two additional species are projected to have range reductions of at least 10% overall, but also to lose less than 50% of their recently suitable area (i.e., contract; Figure 2c). Five species had stable climate-niche area sizes, but shifting centroid locations (i.e., Figure 2d near the x-axis). The remaining eight species had relatively stable climate-niche sizes and centroid locations (i.e., Figure 2b near the x-axis). The centroid values of suitable climate distributions largely shifted northward an average of 313 ± 18 km. To visualize these patterns, we show examples using the Gila Monster (Heloderma suspectum) as a species that is predicted to have an expanding climate-niche space (Figure 3a), the Variable Sandsnake (Chilomeniscus stramineus) as a shifting species (Figure 3b), the Blainville's Horned Lizard (Phrynosoma blainvillii) as a stable species (Figure 3c), and the Northern Rubber Boa (Charina bottae) as a species that is predicted to lose suitable climate area (Figure 3d).
| Scientific name | Range size | Percent change in future scenario | Range change | |||||
|---|---|---|---|---|---|---|---|---|
| Current | Future | Loss | Gain | Maintain | Lost | Gained | ||
| Common name | ||||||||
|
Sceloporus arenicolus Dunes Sagebrush Lizard |
4322 | 0 | 4322 | 0 | 0 | 100.00 | 0.00 | −100.00 |
|
Phrynosoma douglasii Pygmy Short-horned Lizard |
188,650 | 4897 | 184,792 | 1039 | 3858 | 97.96 | 0.55 | −97.40 |
|
Anniella pulchra Northern Legless Lizard |
28,923 | 3722 | 25,896 | 695 | 3027 | 89.53 | 2.40 | −87.13 |
|
Thamnophis couchii Sierra Gartersnake |
76,300 | 18,958 | 58,877 | 1535 | 17,423 | 77.17 | 2.01 | −75.15 |
|
Aspidoscelis flagellicauda Gila Spotted Whiptail |
36,205 | 10,057 | 31,429 | 5281 | 4776 | 86.81 | 14.59 | −72.22 |
|
Sceloporus virgatus Striped Plateau Lizard |
22,963 | 6606 | 21,362 | 5005 | 1601 | 93.03 | 21.80 | −71.23 |
|
Crotalus pricei Twin-spotted Rattlesnake |
46,652 | 17,456 | 38,514 | 9318 | 8138 | 82.56 | 19.97 | −62.58 |
|
Crotalus willardi Ridge-nosed Rattlesnake |
21,943 | 8226 | 19,176 | 5459 | 2767 | 87.39 | 24.88 | −62.51 |
|
Sceloporus jarrovii Spiny Lizard |
178,006 | 68,042 | 133,645 | 23,681 | 44,361 | 75.08 | 13.30 | −61.78 |
|
Sceloporus slevini Slevin's Bunchgrass Lizard |
25,788 | 12,126 | 22,968 | 9306 | 2820 | 89.07 | 36.09 | −52.98 |
|
Aspidoscelis uniparens Desert Grassland Whiptail |
70,471 | 33,148 | 64,910 | 27,587 | 5561 | 92.11 | 39.15 | −52.96 |
|
Crotalus lepidus Rock Rattlesnake |
130,401 | 63,573 | 93,115 | 26,287 | 37,286 | 71.41 | 20.16 | −51.25 |
|
Sceloporus graciosus Common Sagebrush Lizard |
458,409 | 245,176 | 367,842 | 154,609 | 90,567 | 80.24 | 33.73 | −46.52 |
|
Charina bottae Northern Rubber Boa |
440,424 | 248,029 | 339,587 | 147,192 | 100,837 | 77.11 | 33.42 | −43.68 |
|
Crotalus molossus Western Black-tailed Rattlesnake |
321,340 | 230,966 | 190,524 | 100,150 | 130,816 | 59.29 | 31.17 | −28.12 |
|
Aspidoscelis sonorae Sonoran Spotted Whiptail |
47,145 | 36,709 | 26,835 | 16,399 | 20,310 | 56.92 | 34.78 | −22.14 |
|
Senticolis triaspis Green Ratsnake |
86,733 | 69,096 | 67,975 | 50,338 | 18,758 | 78.37 | 58.04 | −20.34 |
|
Phrynosoma mcallii Flat-tailed Horned Lizard |
15,033 | 12,475 | 13,200 | 10,642 | 1833 | 87.81 | 70.79 | −17.02 |
|
Elgaria kingii Madrean Alligator Lizard |
115,286 | 96,979 | 65,054 | 46,747 | 50,232 | 56.43 | 40.55 | −15.88 |
|
Lampropeltis splendida Desert Kingsnake |
119,582 | 106,743 | 81,925 | 69,086 | 37,657 | 68.51 | 57.77 | −10.74 |
|
Coluber constrictor North American Racer |
454,753 | 406,524 | 309,533 | 261,304 | 145,220 | 68.07 | 57.46 | −10.61 |
- Note: Species are ordered by range change (%). Size values represent the count of pixels with a 1-km grid size. Current range sizes include the pixels predicted to be occupied in the recent (1981–2010) scenario, whereas the future sizes are those predicted to be occupied in the late-century future RCP 8.5 scenario. Loss is the number of pixels no longer predicted to be occupied in the future scenario. Gain represents pixels not predicted to be occupied in the first scenario but are occupied in the future scenario. Maintain pixels are predicted to be occupied in both scenarios. Percent lost is the percent of currently occupied pixels that are lost (Loss/(Loss + Maintain)). The percent gained is the percent of new pixels in relation to the current range size (Gain/(Loss + Maintain)). Species Range Change is calculated by subtracting the percent gained from the percent lost. These data are available for all species and scenario comparisons and within the interactive graphical supplement (Jeffries et al. 2024a, 2024b).
In the recent projection of richness, the highest values were found in southern Arizona and northern Mexico (Figure 1). By mid-century, under the RCP 8.5 emission scenario, richness shifts northward, with increases in northern Arizona and northward. The trend continued in the late-century future scenario, with richness losses being most evident in southern Arizona and northern Mexico, as species richness expands and shifts northward. The largest increases in species richness are projected in northeastern Arizona, southeastern Utah, western Utah, northwestern New Mexico, and southwestern Colorado. In contrast, fewer new climate-niche spaces expand into Canadian provinces (Figure 1).
3.3 Species Management Within and Across Jurisdictional Boundaries
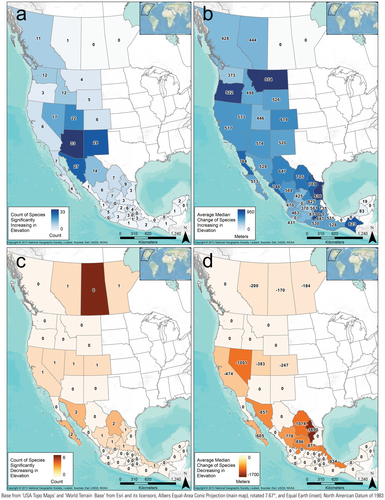
Species occurrence patterns within geographically defined jurisdictions were complex, thus here we report comparisons only between the recent (1981–2010) and late-century future RCP 8.5 (2070–2100) scenarios. Modeled species occurrences for the recent scenario were correctly predicted in a state an average of 83.1% of the time. When species were predicted to occur in the recent scenario, they were also predicted to occur (based on climate suitability) in that state in the future scenario 86% of the time (Figures 4b and 5b), and we designated those species as having a stable occurrence in the state (e.g., gray portions in Figures 4b and 5b). Those “stable-occurrence species” lost an average of 38 ± 1.08% (median: 28.7, range: 0–100) of their originally occupied climate-niche space but had an average overall change of 1231 ± 173% (median: 34%, range: −99 to 72,053) of suitable area in those states. In addition, 26% of the stable-occurrence species/state combinations are predicted to significantly increase in elevation within the state. In contrast, the majority (72%) are predicted to occupy significantly similar elevations, and only 2% were predicted have suitable climate ranges that decreased in elevation (Figure 6).
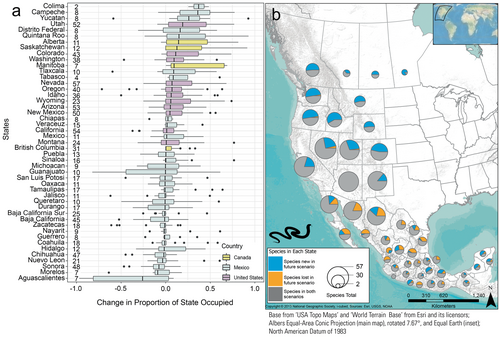
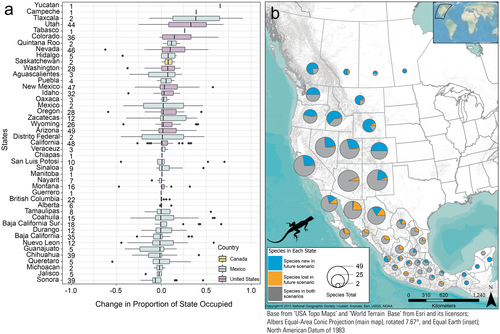
The 14% of species-by-state combinations where the species was predicted to occur recently and subsequently predicted to have no suitable climate in future scenarios tended to have small areas of suitable climate recently (i.e., fewer recent occurrence pixels: mean: 2594 ± 534, median: 523, range: 54–72,678) than those species predicted to persist (mean: 20,066 ± 800, median: 9194, range: 51–137,774). This value (14%) represents 176 of 1251 species predicted to occur in a state (note that 1251 is greater than the total number of distinct species modeled [i.e., 130] because most species occur in more than one state). Most species in Baja California Sur, Mexico, are expected to lose suitable climate area in the state during the future scenario, and half of those species had very small areas of suitable climate (< 500-occurrence pixels or about 1.8% of the state) recently. Some currently widespread species are also predicted to lose all suitable climate-niche space in a few states. For example, the Pygmy Short-horned Lizard (Phrynosoma douglasii) is projected to lose its entire climate-niche space in Alberta, Canada, and the U.S. states of California, Idaho, Montana, Nevada, Oregon, Utah, and Wyoming, while retaining only a small portion in Washington, USA and British Columbia, Canada. The North American Racer (Coluber constrictor) is projected to lose its entire climate-niche space in Baja California, Mexico, Nevada, and Utah, but maintain suitable areas in 10 other states. The Dunes Sagebrush Lizard (Sceloporus arenicolus) was predicted to lose all suitable climate-niche space across the entire study area, though its persistence in Texas, to the east of the study area, was not modeled (U.S. Geological Survey 2018a, 2018b; Jeffries et al. 2024a). This species was recently listed as Endangered by the U.S. Fish and Wildlife Service (Federal Register 2024).
Overall, 40 states (85%) are predicted to lose at least one species under the future climate scenario. Sonora and Chihuahua, Mexico, are predicted to lose suitable areas for the largest numbers of species (18 and 16, respectively). Conversely, nearly all states (89%) are predicted to gain suitable areas for additional species in the future. In the U.S., Idaho and Colorado are predicted to gain suitability for the most species, 40 and 38, respectively.
4 Discussion
Lizards and snakes are an understudied group of tetrapods in North America, which creates a challenge for natural-resource managers tasked with managing species, habitats, and planning mitigation actions in response to myriad threats including changing climate. By taking a taxonomically comprehensive approach to assessing recent and potential future reptile niches in western North America, we aimed to provide useful information to inform natural-resource managers across 47 geographically defined jurisdictions that have responsibility for species-conservation and habitat-management decisions. Our findings are congruent with several other studies suggesting a northward or upward climate-based shift for many species (Araújo, Thuiller, and Pearson 2006; Barrows 2011; Morena-Rueda et al. 2012). Furthermore, in western North America, our results suggest potentially widespread changes in squamate species distributions with species-scale losses and gains at state and provincial scales. With an uncertain capacity for individual lizard and snake species to persist-in-place or shift-in-space in response to climate change (Thurman et al. 2022), our results may help inform natural resource agencies within specific jurisdictional boundaries to prepare for new species arriving, current species extirpation, possibly assisted migration, and shifts in species distributions within jurisdictions, including into areas at higher elevations.
We identified several species with a shifted or contracted climate-niche space within the next 50 years, which could create new species-conservation challenges (Table 1). Interestingly, some of these species have not been previously identified as particularly rare and climate sensitive, thus they have not drawn management attention in prior assessments using different analysis approaches and spatial scales (e.g., Phrynosoma douglassi, Sceloporus graciosus, Charina bottae, Coluber constrictor: Mims et al. 2018). Hence, the current analyses identify potentially underappreciated broad-scale effects of climate change. Although range shifts and distributional changes are common over the evolutionary history of most species (Kafash et al. 2020), many species exist in isolated habitat “islands” (Jones, Kepner, and Martin 1985). Furthermore, the modern world has new barriers to dispersal due to anthropogenic activities that reduce landscape connectivity (Tan, Herrel, and Rödder 2023), and thus species that are predicted to shift-in-space from their current range may not be able to disperse into new areas of suitable climate because of anthropogenic barriers (Driscoll 2004; Lasky, Jetz, and Keitt 2011; Kay et al. 2016; Brehme, Hathaway, and Fisher 2018; Paterson et al. 2019). Even if species can disperse, they may not be able to find climate refugia or meet basic physiological requirements when dispersing through suboptimal habitat conditions (Hillman et al. 2014; Boyle et al. 2016). Despite dispersal limitations, some squamate species have been documented to shift their range with shifting climate regimes (Frishkoff et al. 2019). A meta-analysis of several species found shifts occurring at the rate of 6.1–19.9 km/decade (Parmesan and Yohe 2003; Chen et al. 2011). In addition to distance, elevation shifts have also been observed for some squamate species (Raxworthy et al. 2008; Chen et al. 2011). Where topographic variation provides climate refuge, climate niches may be available for some species in relatively close proximity but at higher elevations, as indicated by our analysis. This type of refuge has its realized limits and is dependent on concomitant shifts in critical resources (e.g., vegetation, prey) and availability of critical resources (e.g., hibernacula).
Across 130 squamate species, the most commonly important variables defining species' climate-niche space in our analyses were January temperature, June temperature, and June precipitation. The importance of January temperature is consistent with prior research (Williams, Henry, and Sinclair 2015; Hatten et al. 2016), and changes in these January and June conditions could shift the timing of life-history transitions, such as hibernation, breeding, and foraging, across seasons. Western North America has considerable seasonal and spatial climate variability. We might expect species found in areas with more heterogeneous climate conditions to be better suited to adjust to future changes, whereas species found in climate zones with more homogeneous conditions (e.g., equatorial) could be more sensitive to baseline changes. A recent meta-analysis, however, found that climate-vulnerable species occur in a variety of locations and from distinct phylogenies (Diele-Viegas et al. 2020).
Inference and prediction using climate-niche models can be influenced by the parameters selected and variables used (Zurell et al. 2020). There are several ways to characterize climate and seasonality, and we chose to use monthly climate data instead of mean annual temperature, annual temperature range, and total annual precipitation to identify possible mechanistic relationships with seasonal changes in animal life-history functions and processes. Although we evaluated monthly climate data, we were unable to assess more nuanced effects of climate change, such as seasonality or the occurrence and duration of extreme weather events (e.g., droughts; Zani and Stein 2018; regional extreme temperature events, e.g., 2021 “heat dome”: U.S. Department of Agriculture 2021). These episodic events are difficult to predict spatially under future scenarios, except as probabilities, yet could have significant effects on biota.
The effects of shifting climates on colonization and extinction processes are complex and difficult to predict because of the plurality of organismal or population-level factors that influence species' adaptive capacity, including but not limited to genetic capacity for adaptation, physiology, morphology, and phenotypic plasticity. Furthermore, many effects will be context dependent because of quality of habitats and site-to-microsite conditions such as thermal-and-moisture characteristics of microhabitats used for breeding, foraging, and overwintering, habitat conditions in dispersal areas, prey, and predators.
Daily temperature amplitude and nighttime temperatures have been shown to affect reptiles, primarily through embryonic development and metabolic pathways (Booth 2018; Rutschmann et al. 2024). For example, studies of Side-blotched Lizards (Uta stansburiana), a widespread species in western North America, indicated that warmer temperatures, especially nighttime minimum temperatures, during egg development could increase reproductive output and thus, lizard fitness (Clarke and Zani 2012). However, warm-and-dry conditions, such as during extended droughts, can reduce body size and condition in females and lead to reproductive failure and no population recruitment (Zani and Stein 2018). This effect was not associated with a lack of prey but rather a lack of surface water for drinking. Autumn growing-season length can increase body size in side-blotched lizards; larger lizards are more likely to survive winter (because of their higher energy storage prior to dormancy) and larger females are more likely to reproduce successfully (Zani 2005, 2008). Finally, warmer winter temperatures can reduce survival, apparently by depleting glycogen levels in the liver (Zani et al. 2012). These results demonstrate the ecological complexity of changing environmental conditions associated with climate and indicate that for side-blotched lizard species or populations at the northern limits of their ranges, certain conditions, such as warmer nighttime summer temperatures and longer growing seasons, may offset negative effects of drought or warmer winters by altering the timing of reproduction, reproductive output, growth, and recruitment (Zani and Rollyson 2011). This illustrates how the long-term persistence of some populations may depend on how specific aspects of a changing climate affect certain life stages or timing in an animal's life history (Thurman et al. 2020). These effects can be further confounded by local adaptations and animal traits (Smith, Zani, and French 2019).
Additional research would be needed to understand thermal tolerances, abiotic tolerances, and adaptive capacities of most squamate species across our study extent because the temporal and spatial scales of our analyses are not sufficient to describe proximate mechanisms as the interactions between climate change and life-history functions. Nevertheless, the broadscale climate-related range changes we project could be used to generate downscaled hypotheses that could be tested at spatial scales relevant for individual species. Such empirical studies could provide important contexts for natural resource planning of species and habitats at potential risk. For example, for each management jurisdiction, managers could use study results to identify areas of overlapping present and future species-specific climate-niche space and prioritize areas for targeted conservation activities (Jeffries et al. 2024a). Wildlife managers could also use study results to identify hotspots of climate suitability for multiple species and areas that provide climate refugia (i.e., climate stable, or areas suitable now and in the future) and may be particularly important for species persistence (Morelli et al. 2016). Interstate to international scale planning may be needed for species with large ranges or those predicted to shift long distances. As management actions that cross jurisdictions become necessary or common, larger-scale “top-down” planning can also be informed by our results (e.g., Popescu et al. 2013). Ultimately, our results can be used to assess landscape connectivity between climate scenarios and inform development of conservation priorities that sustain squamate species experiencing climate-driven range shifts (Littlefield et al. 2019; Zhu et al. 2021).
Author Contributions
David S. Pilliod: conceptualization (lead), funding acquisition (lead), project administration (lead), resources (lead), supervision (lead), writing – original draft (equal), writing – review and editing (equal). Michelle I. Jeffries: conceptualization (equal), data curation (lead), formal analysis (lead), investigation (lead), methodology (lead), project administration (supporting), software (lead), validation (lead), visualization (lead), writing – original draft (equal), writing – review and editing (equal). Robert S. Arkle: conceptualization (supporting), formal analysis (supporting), methodology (supporting), writing – review and editing (equal). Deanna H. Olson: conceptualization (supporting), data curation (supporting), investigation (supporting), writing – review and editing (equal).
Acknowledgments
We are grateful for the reviews and comments from Kathryn Ronnenberg and Sean Finn. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix 1
A list of data sources and contributors. Data sources were publicly available (HerpNet2 2012; GBIF 2016; BISON 2017), except for those with an asterisk, which were available upon approval or a data-sharing agreement with the data owners.
| Data sources and contributors |
|---|
| A. Linder* |
| A. Safranek* |
| A. St. John* |
| Alberta Fish and Wildfire Service* |
| American Museum of Natural History* |
| Arizona Game and Fish Department* |
| B. Bean* |
| B. Budd* |
| B. Ralston* |
| B. Schneider* |
| Berkeley Museum of Vertebrate Zoology* |
| Biodiversity Information Serving our Nation |
| British Columbia Ministry of Environment* |
| Bureau of Land Management* |
| C. & R. Grove* |
| C. Peterson* |
| California Academy of Sciences* |
| California Department of Fish and Wildlife* |
| Carnegie Museum* |
| D. Bilsland* |
| D. Fitcher* |
| D. Miller* |
| D. Quinney* |
| Douglas County Museum* |
| F. Uhler* |
| G. Beauvais* |
| G. Harold* |
| G. Pendlebury* |
| Global Biodiversity Information Facility |
| H. Fitch* |
| Hayden-Wing* |
| HerpNet2 (now VertNet) |
| Idaho Citizen Science Data* |
| Idaho Department of Fish and Game* |
| Idaho Fish and Wildfire Service* |
| Idaho Fish and Wildlife Information Systems* |
| Idaho Herp Database* |
| Idaho Natural Heritage Program* |
| Idaho Power Company* |
| Idaho State University* |
| iNaturalist |
| J. Beck* |
| J. Cossel* |
| J. Engle* |
| J. Hawk* |
| J. Munger* |
| J. Weaver* |
| J. Yeo* |
| Kanisku National Forest* |
| Kansas University Natural History Museum* |
| L. Diller* |
| L. Murphy* |
| L. Powers* |
| Los Angeles County Museum of Natural History* |
| M. Mathis* |
| Montana Natural History Database* |
| National Parks Service* |
| Natural Heritage New Mexico* |
| Natural Resource Information System* |
| Nevada Department of Wildlife* |
| Orchard Combat Training Center* |
| Oregon Biodiversity Information Center* |
| Oregon Bureau of Land Management* |
| Oregon Department of Fish and Wildfire* |
| Oregon State University* |
| P.M. de Laubenfels* |
| Pacificorps* |
| Portland State University* |
| R. & D. Brokken* |
| R. Clark* |
| R. Davis* |
| R. Hoyer* |
| R. Jones* |
| R. Kiester* |
| R. Mason* |
| R. Nussbaum* |
| Saskatchewan Ministry of Environment* |
| Smithsonian Institution's National Museum of Natural History* |
| Southern Oregon University* |
| U.S. Geological Survey Gap Analysis Project* |
| University of Michigan* |
| University of Puget Sound* |
| University of Texas at Arlington* |
| Utah Natural Heritage Program* |
| Utah State University* |
| W. Haas* |
| W. Turner* |
| Washington Department of Fish and Wildlife* |
| Washington Department of Natural Resources* |
| Wyoming Cooperative Fishery and Wildlife Research Unit* |
| Wyoming Department of Fish and Game* |
| Wyoming Natural Diversity Database* |
Appendix 2
The list of species that occurrence point-location data was collected for (Appendix 1). The “Count of All Species Points” column totals the number of points we collected, regardless of quality. The “Points with No Quality Flag, Any Date” subsets to those points without a known or obvious data quality issue. The “Points with No Quality Flag, 1981–2010” subsets the data to those from the years from 1981 to 2010. Lastly, the “Points with No Quality Flag, 1981–2010, One per Climate Grid-cell” further reduces list to include only one point per-species in each 1-km climate grid cell used for modeling. The final column indicates if the species was included for climate-niche modeling and spatial projection.
| Scientific name | Common name | Count of all species points | Points with no quality flag, any date | Points with no quality flag, 1981–2010 | Points with no quality flag, 1981–2010, one per climate grid-cell | Species niche modeled and projected? |
|---|---|---|---|---|---|---|
| Anniella pulchra | Northern Legless Lizard | 1096 | 766 | 228 | 157 | Yes |
| Arizona elegans | Glossy Snake | 3610 | 2676 | 1078 | 861 | Yes |
| Aspidoscelis exsanguis | Chihuahuan Spotted Whiptail | 112 | 112 | 99 | 60 | Yes |
| Aspidoscelis flagellicauda | Gila Spotted Whiptail | 264 | 143 | 109 | 77 | Yes |
| Aspidoscelis gularis | Common Spotted Whiptail | 585 | 302 | 144 | 116 | Yes |
| Aspidoscelis hyperythra | Orange-throated Whiptail | 1193 | 775 | 306 | 211 | Yes |
| Aspidoscelis inornata | Little Striped Whiptail | 614 | 400 | 214 | 143 | Yes |
| Aspidoscelis motaguae | Giant Whiptail | 1 | 1 | 0 | 0 | No |
| Aspidoscelis neomexicana | New Mexico Whiptail | 237 | 131 | 32 | 27 | No |
| Aspidoscelis neotesselata | Colorado Checkered Whiptail | 52 | 19 | 13 | 13 | No |
| Aspidoscelis scalaris | Plateau Spotted Whiptail | 98 | 92 | 1 | 1 | No |
| Aspidoscelis sexlineata | Six-lined Racerunner | 291 | 62 | 34 | 33 | No |
| Aspidoscelis sonorae | Sonoran Spotted Whiptail | 874 | 678 | 498 | 267 | Yes |
| Aspidoscelis stictogramma | Giant Spotted Whiptail | 379 | 255 | 180 | 113 | Yes |
| Aspidoscelis tesselata | Common Checkered Whiptail | 410 | 162 | 59 | 52 | Yes |
| Aspidoscelis tigris | Tiger Whiptail | 10,111 | 8863 | 4952 | 2984 | Yes |
| Aspidoscelis uniparens | Desert Grassland Whiptail | 1030 | 816 | 606 | 328 | Yes |
| Aspidoscelis velox | Plateau Striped Whiptail | 684 | 430 | 244 | 197 | Yes |
| Aspidoscelis xanthonota | Red-backed Whiptail | 39 | 36 | 28 | 23 | No |
| Bogertophis rosaliae | Baja California Ratsnake | 28 | 27 | 8 | 8 | No |
| Bogertophis subocularis | Trans-Pecos Ratsnake | 377 | 90 | 30 | 24 | No |
| Callisaurus draconoides | Zebra-tailed Lizard | 22,216 | 19,901 | 11,598 | 3185 | Yes |
| Charina bottae | Northern Rubber Boa | 2726 | 2199 | 1389 | 1192 | Yes |
| Charina umbratica | Southern Rubber Boa | 65 | 10 | 4 | 4 | No |
| Chilomeniscus stramineus | Variable Sandsnake | 592 | 467 | 216 | 113 | Yes |
| Chionactis occipitalis | Mohave Shovel-nosed Snake | 2487 | 2154 | 996 | 534 | Yes |
| Chionactis palarostris | Sonoran Shovel-nosed Snake | 127 | 99 | 30 | 15 | No |
| Cnemidophorus lemniscatus | Rainbow Whiptail | 1 | 1 | 0 | 0 | No |
| Coleonyx brevis | Texas Banded Gecko | 388 | 171 | 63 | 57 | Yes |
| Coleonyx switaki | Switak's Banded Gecko | 47 | 30 | 18 | 18 | No |
| Coleonyx variegatus | Western Banded Gecko | 4855 | 4339 | 2801 | 1441 | Yes |
| Coluber bilineatus | Sonora Whipsnake | 588 | 444 | 356 | 204 | Yes |
| Coluber constrictor | North American Racer | 5186 | 4527 | 3174 | 2313 | Yes |
| Coluber flagellum | Coachwhip | 3541 | 3129 | 1764 | 1362 | Yes |
| Coluber fuliginosus | Baja California Coachwhip | 228 | 207 | 43 | 40 | No |
| Coluber lateralis | Striped Racer | 1140 | 867 | 451 | 386 | Yes |
| Coluber taeniatus | Striped Whipsnake | 1887 | 1396 | 722 | 574 | Yes |
| Contia longicauda | Forest Sharp-tailed Snake | 27 | 27 | 12 | 12 | No |
| Contia tenuis | Common Sharp-tailed Snake | 978 | 818 | 473 | 392 | Yes |
| Cophosaurus texanus | Greater Earless Lizard | 2251 | 1566 | 611 | 397 | Yes |
| Crotalus adamanteus | Eastern Diamond-backed Rattlesnake | 2 | 1 | 0 | 0 | No |
| Crotalus atrox | Western Diamond-backed Rattlesnake | 5503 | 4311 | 3307 | 1580 | Yes |
| Crotalus cerastes | Sidewinder | 3504 | 3048 | 1608 | 1113 | Yes |
| Crotalus cerberus | Arizona Black Rattlesnake | 208 | 203 | 169 | 98 | Yes |
| Crotalus lepidus | Rock Rattlesnake | 1003 | 719 | 438 | 213 | Yes |
| Crotalus mitchellii | Speckled Rattlesnake | 1412 | 1152 | 558 | 444 | Yes |
| Crotalus molossus | Western Black-tailed Rattlesnake | 2181 | 1733 | 1309 | 686 | Yes |
| Crotalus oreganus | Western Rattlesnake | 6983 | 6123 | 4211 | 2786 | Yes |
| Crotalus pricei | Twin-spotted Rattlesnake | 566 | 483 | 321 | 86 | Yes |
| Crotalus ruber | Red Diamond Rattlesnake | 1332 | 1023 | 335 | 278 | Yes |
| Crotalus scutulatus | Mohave Rattlesnake | 3247 | 2601 | 1826 | 1092 | Yes |
| Crotalus sp. | Rattlesnake | 190 | 185 | 105 | 92 | No |
| Crotalus stephensi | Panamint Rattlesnake | 269 | 269 | 269 | 215 | Yes |
| Crotalus tigris | Tiger Rattlesnake | 1304 | 1209 | 1095 | 189 | Yes |
| Crotalus viridis | Prairie Rattlesnake | 9116 | 6308 | 4757 | 3038 | Yes |
| Crotalus willardi | Ridge-nosed Rattlesnake | 309 | 251 | 151 | 75 | Yes |
| Crotaphytus bicinctores | Great Basin Collared Lizard | 21,570 | 21,330 | 20,597 | 3627 | Yes |
| Crotaphytus collaris | Eastern Collared Lizard | 2720 | 1892 | 606 | 464 | Yes |
| Crotaphytus nebrius | Sonoran Collared Lizard | 157 | 113 | 66 | 35 | No |
| Crotaphytus vestigium | Baja California Collared Lizard | 134 | 119 | 28 | 24 | No |
| Diadophis punctatus | Ring-necked Snake | 2870 | 2206 | 1140 | 911 | Yes |
| Dipsosaurus dorsalis | Desert Iguana | 11,348 | 10,931 | 9447 | 1438 | Yes |
| Elgaria coerulea | Northern Alligator Lizard | 3805 | 3260 | 1835 | 1478 | Yes |
| Elgaria kingii | Madrean Alligator Lizard | 670 | 491 | 284 | 203 | Yes |
| Elgaria multicarinata | Southern Alligator Lizard | 5881 | 5172 | 3093 | 2208 | Yes |
| Elgaria panamintina | Panamint Alligator Lizard | 67 | 51 | 28 | 21 | No |
| Gambelia copeii | Cope's Leopard Lizard | 92 | 67 | 11 | 11 | No |
| Gambelia sila | Blunt-nosed Leopard Lizard | 620 | 281 | 50 | 44 | No |
| Gambelia wislizenii | Long-nosed Leopard Lizard | 30,228 | 29,531 | 27,668 | 5357 | Yes |
| Gyalopion canum | Chihuahuan Hook-nosed Snake | 292 | 165 | 75 | 67 | Yes |
| Gyalopion quadrangulare | Thornscrub Hook-nosed Snake | 171 | 155 | 26 | 19 | No |
| Heloderma suspectum | Gila Monster | 2296 | 2073 | 1760 | 755 | Yes |
| Hemidactylus turcicus | Mediterranean Gecko | 442 | 300 | 250 | 200 | Yes |
| Heterodon kennerlyi | Mexican Hog-nosed Snake | 185 | 176 | 122 | 102 | Yes |
| Heterodon nasicus | Plains Hog-nosed Snake | 1212 | 833 | 476 | 399 | Yes |
| Heterodon platirhinos | Eastern Hog-nosed Snake | 15 | 1 | 0 | 0 | No |
| Holbrookia elegans | Elegant Earless Lizard | 339 | 294 | 123 | 98 | Yes |
| Holbrookia lacerata | Spot-tailed Earless Lizard | 9 | 1 | 0 | 0 | No |
| Holbrookia maculata | Common Lesser Earless Lizard | 2850 | 2074 | 706 | 451 | Yes |
| Hypsiglena chlorophaea | Desert Nightsnake | 1273 | 1177 | 923 | 619 | Yesa |
| Hypsiglena jani | Chihuahuan Nightsnake | 321 | 109 | 67 | 65 | Yesa |
| Hypsiglena ochrorhyncha | Coast Nightsnake | 290 | 197 | 98 | 92 | Yesa |
| Hypsiglena torquata | Night Snake | 2243 | 1659 | 547 | 451 | Yesa |
| Lampropeltis alterna | Gray-banded Kingsnake | 63 | 8 | 8 | 7 | No |
| Lampropeltis californiae | California Kingsnake | 1000 | 866 | 580 | 533 | Yes |
| Lampropeltis calligaster | Prairie Kingsnake | 3 | 0 | 0 | 0 | No |
| Lampropeltis getula | Eastern Kingsnake | 3109 | 2468 | 1008 | 755 | Yes |
| Lampropeltis holbrooki | Speckled Kingsnake | 3 | 2 | 2 | 1 | No |
| Lampropeltis knoblochi | Madrean Mountain Kingsnake | 11 | 11 | 11 | 11 | No |
| Lampropeltis pyromelana | Arizona Mountain Kingsnake | 642 | 430 | 265 | 158 | Yes |
| Lampropeltis splendida | Desert Kingsnake | 103 | 92 | 62 | 55 | Yes |
| Lampropeltis triangulum | Eastern Milksnake | 1205 | 836 | 417 | 318 | Yes |
| Lampropeltis zonata | California Mountain Kingsnake | 847 | 472 | 243 | 195 | Yes |
| Lichanura orcutti | Rosy Boa | 123 | 122 | 104 | 97 | Yes |
| Lichanura trivirgata | Three-lined Boa | 693 | 562 | 196 | 162 | Yes |
| Micruroides euryxanthus | Sonoran Coralsnake | 541 | 358 | 199 | 115 | Yes |
| Nerodia erythrogaster | Plain-bellied Watersnake | 78 | 51 | 12 | 10 | No |
| Nerodia fasciata | Southern Watersnake | 6 | 6 | 5 | 2 | No |
| Nerodia rhombifer | Diamond-backed Watersnake | 11 | 10 | 10 | 9 | No |
| Nerodia sipedon | Common Watersnake | 76 | 37 | 8 | 8 | No |
| Opheodrys vernalis | Smooth Greensnake | 400 | 233 | 146 | 118 | Yes |
| Oxybelis aeneus | Brown Vinesnake | 401 | 329 | 143 | 105 | Yes |
| Pantherophis emoryi | Great Plains Ratsnake | 232 | 47 | 37 | 36 | No |
| Petrosaurus mearnsi | Mearn's Rock Lizard | 445 | 385 | 102 | 61 | Yes |
| Phrynosoma blainvillii | Blainville's Horned Lizard | 2005 | 1199 | 538 | 429 | Yes |
| Phrynosoma cornutum | Texas Horned Lizard | 2173 | 1446 | 765 | 536 | Yes |
| Phrynosoma douglasii | Pygmy Short-horned Lizard | 1526 | 764 | 454 | 341 | Yes |
| Phrynosoma goodei | Goode's Horned Lizard | 41 | 31 | 28 | 19 | No |
| Phrynosoma hernandesi | Greater Short-horned Lizard | 4087 | 3244 | 2068 | 1122 | Yes |
| Phrynosoma mcallii | Flat-tailed Horned Lizard | 2056 | 1594 | 1410 | 281 | Yes |
| Phrynosoma modestum | Round-tailed Horned Lizard | 1344 | 983 | 348 | 238 | Yes |
| Phrynosoma platyrhinos | Desert Horned Lizard | 36,111 | 35,492 | 33,697 | 5554 | Yes |
| Phrynosoma solare | Regal Horned Lizard | 1227 | 1001 | 658 | 322 | Yes |
| Phyllodactylus nocticolus | Peninsula Leaf-toed Gecko | 64 | 58 | 25 | 22 | No |
| Phyllorhynchus browni | Saddled Leaf-nosed Snake | 387 | 291 | 102 | 70 | Yes |
| Phyllorhynchus decurtatus | Spotted Leaf-nosed Snake | 1725 | 1411 | 431 | 334 | Yes |
| Pituophis catenifer | Gophersnake | 17,968 | 15,645 | 10,899 | 7846 | Yes |
| Plestiodon callicephalus | Mountain Skink | 141 | 113 | 42 | 22 | No |
| Plestiodon gilberti | Gilbert's Skink | 1217 | 1075 | 339 | 283 | Yes |
| Plestiodon multivirgatus | Many-lined Skink | 398 | 277 | 90 | 69 | Yes |
| Plestiodon obsoletus | Great Plains Skink | 762 | 513 | 199 | 158 | Yes |
| Plestiodon septentrionalis | Prairie Skink | 1 | 0 | 0 | 0 | No |
| Plestiodon skiltonianus | Western Skink | 3505 | 3135 | 1802 | 1269 | Yes |
| Plestiodon tetragrammus | Four-lined Skink | 89 | 22 | 10 | 8 | No |
| Podarcis muralis | Common Wall Lizard | 33 | 32 | 32 | 21 | No |
| Podarcis sicula | Italian Wall Lizard | 18 | 18 | 18 | 12 | No |
| Rena dissecta | New Mexico Threadsnake | 64 | 47 | 30 | 25 | No |
| Rena dulcis | Texas Threadsnake | 176 | 123 | 66 | 58 | Yes |
| Rena humilis | Western Threadsnake | 880 | 665 | 246 | 183 | Yes |
| Rhinocheilus lecontei | Long-nosed Snake | 4190 | 3245 | 1560 | 1130 | Yes |
| Salvadora grahamiae | Eastern Patch-nosed Snake | 605 | 371 | 187 | 161 | Yes |
| Salvadora hexalepis | Western Patch-nosed Snake | 2790 | 2196 | 1195 | 917 | Yes |
| Sauromalus ater | Common Chuckwalla | 5997 | 5733 | 4806 | 1203 | Yes |
| Sceloporus arenicolus | Dunes Sagebrush Lizard | 1254 | 1192 | 1147 | 332 | Yes |
| Sceloporus bimaculosus | Twin-spotted Spiny Lizard | 28 | 24 | 24 | 19 | No |
| Sceloporus clarkii | Clark's Spiny Lizard | 2240 | 1802 | 849 | 423 | Yes |
| Sceloporus consobrinus | Prairie Lizard | 188 | 164 | 49 | 43 | No |
| Sceloporus cowlesi | Southwestern Fence Lizard | 489 | 485 | 395 | 275 | Yes |
| Sceloporus graciosus | Common Sagebrush Lizard | 6196 | 5017 | 2912 | 1909 | Yes |
| Sceloporus jarrovii | Yarrow's Spiny Lizard | 1884 | 1552 | 941 | 367 | Yes |
| Sceloporus magister | Desert Spiny Lizard | 4121 | 3317 | 1378 | 809 | Yes |
| Sceloporus merriami | Canyon Lizard | 260 | 64 | 20 | 17 | No |
| Sceloporus occidentalis | Western Fence Lizard | 22,822 | 21,336 | 16,807 | 7152 | Yes |
| Sceloporus orcutti | Granite Spiny Lizard | 950 | 831 | 307 | 184 | Yes |
| Sceloporus poinsettii | Crevice Spiny Lizard | 999 | 681 | 211 | 158 | Yes |
| Sceloporus scalaris | Bunch Grass Lizard | 86 | 83 | 30 | 19 | No |
| Sceloporus slevini | Slevin's Bunchgrass Lizard | 219 | 200 | 149 | 81 | Yes |
| Sceloporus tristichus | Plateau Fence Lizard | 925 | 579 | 284 | 230 | Yes |
| Sceloporus undulatus | Eastern Fence Lizard | 3907 | 3019 | 893 | 627 | Yes |
| Sceloporus uniformis | Yellow-backed Spiny Lizard | 9697 | 9694 | 9694 | 2497 | Yes |
| Sceloporus virgatus | Striped Plateau Lizard | 520 | 437 | 258 | 108 | Yes |
| Senticolis triaspis | Green Ratsnake | 443 | 381 | 178 | 114 | Yes |
| Sistrurus catenatus | Eastern Massasauga | 228 | 165 | 103 | 81 | Yes |
| Sistrurus miliarius | Pygmy Rattlesnake | 2 | 2 | 0 | 0 | No |
| Sonora semiannulata | Western Groundsnake | 1353 | 889 | 380 | 292 | Yes |
| Storeria dekayi | Dekay's Brownsnake | 103 | 40 | 16 | 16 | No |
| Storeria occipitomaculata | Red-bellied Snake | 57 | 24 | 16 | 16 | No |
| Tantilla atriceps | Mexican Black-headed Snake | 29 | 26 | 4 | 4 | No |
| Tantilla hobartsmithi | Smith's Black-headed Snake | 588 | 365 | 216 | 110 | Yes |
| Tantilla nigriceps | Plains Black-headed Snake | 567 | 354 | 145 | 124 | Yes |
| Tantilla planiceps | Western Black-headed Snake | 310 | 236 | 59 | 55 | Yes |
| Tantilla wilcoxi | Chihuahuan Black-headed Snake | 63 | 53 | 20 | 17 | No |
| Tantilla yaquia | Yaqui Black-headed Snake | 91 | 73 | 41 | 30 | No |
| Thamnophis atratus | Aquatic Gartersnake | 1324 | 1115 | 649 | 479 | Yes |
| Thamnophis couchii | Sierra Gartersnake | 1132 | 818 | 385 | 290 | Yes |
| Thamnophis cyrtopsis | Black-necked Gartersnake | 1779 | 1344 | 700 | 484 | Yes |
| Thamnophis elegans | Terrestrial Gartersnake | 12,674 | 10,590 | 6684 | 4826 | Yes |
| Thamnophis eques | Mexican Gartersnake | 702 | 607 | 342 | 180 | Yes |
| Thamnophis gigas | Giant Gartersnake | 411 | 50 | 16 | 16 | No |
| Thamnophis hammondii | Two-striped Gartersnake | 941 | 638 | 235 | 206 | Yes |
| Thamnophis marcianus | Checkered Gartersnake | 1304 | 837 | 401 | 265 | Yes |
| Thamnophis ordinoides | Northwestern Gartersnake | 2489 | 1872 | 801 | 576 | Yes |
| Thamnophis proximus | Western Ribbonsnake | 478 | 294 | 110 | 97 | Yes |
| Thamnophis radix | Plains Gartersnake | 2749 | 2379 | 1971 | 1608 | Yes |
| Thamnophis rufipunctatus | Narrow-headed Gartersnake | 840 | 785 | 671 | 175 | Yes |
| Thamnophis sirtalis | Common Gartersnake | 8146 | 6906 | 4436 | 3149 | Yes |
| Thamnophis sp. | Gartersnake, Ribbonsnake | 52 | 43 | 43 | 34 | No |
| Trimorphodon biscutatus | Western Lyresnake | 1089 | 835 | 238 | 186 | Yes |
| Trimorphodon lambda | Sonoran Lyresnake | 540 | 534 | 494 | 121 | Yes |
| Trimorphodon lyrophanes | California Lyresnake | 23 | 21 | 5 | 5 | No |
| Trimorphodon vilkinsonii | Texas Lyre Snake | 55 | 5 | 4 | 4 | No |
| Tropidoclonion lineatum | Lined Snake | 133 | 81 | 45 | 45 | No |
| Uma inornata | Coachella Fringed-toed Lizard | 374 | 171 | 28 | 28 | No |
| Uma notata | Colorado Desert Fringe-toed Lizard | 420 | 312 | 58 | 43 | No |
| Uma rufopunctata | Yuman Desert Fringe-toed Lizard | 48 | 48 | 30 | 27 | No |
| Uma scoparia | Mohave Fringe-toed Lizard | 424 | 315 | 146 | 73 | Yes |
| Urosaurus graciosus | Long-tailed Brush Lizard | 857 | 670 | 263 | 202 | Yes |
| Urosaurus nigricaudus | Baja California Brush Lizard | 774 | 681 | 151 | 139 | Yes |
| Urosaurus ornatus | Ornate Tree Lizard | 4461 | 3299 | 1565 | 927 | Yes |
| Uta stansburiana | Common Side-blotched Lizard | 18,102 | 15,887 | 8984 | 4834 | Yes |
| Xantusia arizonae | Arizona Night Lizard | 52 | 41 | 9 | 8 | No |
| Xantusia bezyi | Bezy's Night Lizard | 20 | 19 | 17 | 10 | No |
| Xantusia gracilis | Sandstone Night Lizard | 6 | 5 | 4 | 3 | No |
| Xantusia henshawi | Granite Night Lizard | 513 | 428 | 88 | 71 | Yes |
| Xantusia riversiana | Island Night Lizard | 185 | 126 | 21 | 12 | No |
| Xantusia vigilis | Desert Night Lizard | 1777 | 1467 | 604 | 413 | Yes |
| Xantusia wigginsi | Wiggins' Night Lizard | 137 | 131 | 31 | 29 | No |
| 427,846 | 367,953 | 257,427 | 110,121 |
- a The species was modeled at the genus level.
Appendix 3
Climate variables used in the species climate-niche modeling. These variables were derived from the ClimateNA v5.5 tool (Wang et al. 2016). Variables were generated for each period and climate projection scenario: past (1901–1930), recent (1981–2010), future Representative Concentration Pathway (RCP) 4.5 (2040–2069 and 2070–2100), and future RCP 8.5 (2040–2069 and 2070–2100).
| Acronym | Climate variable | Definition |
|---|---|---|
| PPT01–PPT12 | Monthly precipitation | The average total precipitation for each of the months January–December (mm) |
| Tave01–Tave12 | Monthly average temperature | The average mean temperature for each of the months January–December (°C) |
Appendix 4
True skill statistic (TSS) score summaries for eight modeling techniques across all squamate species (n = 130) and runs (four per species; Jeffries et al. 2024a, 2024b).
| Model | Mean | Standard deviation | Median | Min | Max | Count non-converged | Count converged |
|---|---|---|---|---|---|---|---|
| ANN | 0.84 | 0.09 | 0.85 | 0.49 | 0.99 | 2 | 518 |
| CTA | 0.87 | 0.07 | 0.88 | 0.55 | 0.99 | 0 | 520 |
| FDA | 0.84 | 0.10 | 0.85 | 0.001 | 0.99 | 142 | 378 |
| GAM | 0.84 | 0.10 | 0.86 | 0.23 | 0.99 | 16 | 504 |
| GLM | 0.85 | 0.11 | 0.87 | 0.002 | 0.99 | 1 | 519 |
| MARS | 0.88 | 0.09 | 0.89 | 0.07 | 0.99 | 0 | 520 |
| RF | 0.89 | 0.07 | 0.90 | 0.64 | 0.99 | 0 | 520 |
| SRE | 0.65 | 0.09 | 0.66 | 0.27 | 0.90 | 0 | 520 |
- Abbreviations: ANN, Artificial neural network; CTA, Classification tree analysis; FDA, Flexible discriminant analysis; GAM, Generalized additive model; GLM, Generalized linear model; MARS, Multiple adaptive regression splines; RF, Random forest; SRE, Surface range envelope.
Appendix 5
Receiver operating characteristic (ROC) and true skill statistic (TSS) summary across all modeled species (Jeffries et al. 2024a, 2024b).
| Evaluation statistic | Mean evaluation score | Evaluation range | Mean sensitivity | Sensitivity range | Mean specificity | Specificity range |
|---|---|---|---|---|---|---|
| ROC | 0.99 | 0.94–1.00 | 98.4 | 90.4–100 | 95.4 | 85.9–99.9 |
| TSS | 0.94 | 0.77–0.99 | 98.4 | 90.4–100 | 95.4 | 85.9–99.9 |
Appendix 6
Open Research
Open Research Badges
Data Availability Statement
The species occurrence data used in the analysis for this study were collected with appropriate data-sharing agreements and from sources openly available to the public. See Appendix 1 for a list of data sources. Climate data were derived using the Climate North America tool (ClimateNA v5.5; http://climatena.ca/) and a Digital Elevation Model raster (https://www.sciencebase.gov/catalog/item/50db7222e4b061270600b8a4). Summary data and model outputs can be found at https://doi.org/10.5066/P9ZGRGE4. The interactive graphical supplement is available at https://geonarrative.usgs.gov/reptileclimateniche. Full citations can be found in the References.
This article has earned an Open Data badge for making publicly available the digitally-shareable data necessary to reproduce the reported results. The data is available at https://doi.org/10.5066/P9ZGRGE4.