Odorant receptor phylogeny confirms conserved channels for sex pheromone and host plant signals in tortricid moths
Abstract
The search for mates and food is mediated by volatile chemicals. Insects sense food odorants and sex pheromones through odorant receptors (ORs) and pheromone receptors (PRs), which are expressed in olfactory sensory neurons. Molecular phylogenetics of ORs, informed by behavioral and functional data, generates sound hypotheses for the identification of semiochemicals driving olfactory behavior. Studying orthologous receptors and their ligands across taxa affords insights into the role of chemical communication in reproductive isolation and phylogenetic divergence. The female sex pheromone of green budworm moth Hedya nubiferana (Lepidoptera, Totricidae) is a blend of two unsaturated acetates, only a blend of both elicits male attraction. Females produce in addition codlemone, which is the sex pheromone of another tortricid, codling moth Cydia pomonella. Codlemone also attracts green budworm moth males. Concomitantly, green budworm and codling moth males are attracted to the host plant volatile pear ester. A congruent behavioral response to the same pheromone and plant volatile in two tortricid species suggests co-occurrence of dedicated olfactory channels. In codling moth, one PR is tuned to both compounds, the sex pheromone codlemone and the plant volatile pear ester. Our phylogenetic analysis finds that green budworm moth expresses an orthologous PR gene. Shared ancestry, and high levels of amino acid identity and sequence similarity, in codling and green budworm moth PRs offer an explanation for parallel attraction of both species to the same compounds. A conserved olfactory channel for a sex pheromone and a host plant volatile substantiates the alliance of social and habitat signals in insect chemical communication. Field attraction assays confirm that in silico investigations of ORs afford powerful predictions for an efficient identification of behavior-modifying semiochemicals, for an improved understanding of the mechanisms of host plant attraction in insect herbivores and for the further development of sustainable insect control.
1 INTRODUCTION
Olfactory perception of food cues and sex signals is intimately interconnected in insects (Borrero-Echeverry, Bengtsson, Nakamuta, & Witzgall, 2018; Conchou et al., 2019; Lebreton et al., 2017; Reddy & Guerrero, 2004; Rouyar et al., 2015; Varela, Avilla, Gemeno, & Anton, 2011). Deciphering the chemicals encoding food and mates is basic to understanding insect ecology and evolution. Moreover, the knowledge of such behavior-modifying chemicals can be applied for detection and environmentally safe control of insects (Evenden & Silk, 2016; Gregg, Del Socorro, & Landolt, 2018; Reddy & Guerrero, 2010; Ridgway, Silverstein, & Inscoe, 1990; Suckling et al., 2014; Witzgall, Kirsch, & Cork, 2010; Witzgall, Stelinski, Gut, & Thomson, 2008).
New tools for insect management are needed in the wake of a changing climate that accelerates insect invasions and outbreaks, aggravating food insecurity (Deutsch et al., 2018). Recent efforts to deregulate the most toxic compounds have left growers with few efficient insecticides (Chandler et al., 2011; Jactel et al., 2019). The overwhelming majority of insect species, however, do not feed on our food crops. Including pollination services, insects are integral to all terrestrial food webs. The overuse of synthetic pesticides affects nontarget and beneficial insects and other arthropods and is a contributing cause of the biodiversity apocalypse. This has been a point of debate since DDT (Carson, 1962), yet despite this, the currently most widely used insecticides, the neonicotinoids show severe side effects (Chmiel, Daisley, Burton, & Reid, 2019; Longing et al., 2020; Seibold et al., 2019; Wagner, 2020; Yamamuro et al., 2019).
The development of pheromones and other semiochemicals as a species-specific and environmentally safe alternative to conventional insecticides has always been the rationale for chemical ecology research. Air permeation with synthetic pheromones, for disruption of premating sexual communication, is used against key orchard and forest insects (Reddy & Guerrero, 2010; Witzgall, Kirsch, et al., 2010; Evenden & Silk, 2016). Pheromone lures for specific and sensitive detection are available for hundreds of species. Such lures, in combination with traps, insect pathogens or insecticides, may even achieve population control, when the female sex becomes attracted (El-Sayed, Suckling, Byers, Jang, & Wearing, 2009; Ridgway et al., 1990; Suckling et al., 2014). In stark contrast to pheromones attracting insects for mating, only few semiochemicals have been identified that attract gravid females for oviposition. Designing female or bisexual lures is therefore a current challenge toward a more widespread use of behavior-modifying chemicals for insect control.
Identification of many hundreds of sex pheromones, across all insect orders (El-Sayed, 2019), has been facilitated by a mutual coordination of production and response in both sexes. Pheromones are produced in dedicated glands, produce strong antennal responses, and immediately trigger a sequence of distinctive behaviors.
Identification of semiochemicals, or kairomones, that mediate oviposition behavior meets substantial methodological difficulties. Synthetic plant volatile blends that have been found to attract insect herbivores typically build on compounds found across many plant species (Bruce & Pickett, 2011; Lu, Wang, Wang, Luo, & Qiao, 2015; Najar-Rodriguez, Galizia, Stierle, & Dorn, 2010; Tasin et al., 2010). The attractant power of such ubiquitous plant volatiles is sometimes faint, compared with sex pheromones.
Some plant compounds, that are unique or characteristic for larval food plants, have been found to mediate significant attractancy. One such key host plant compound is ethyl (E,Z)-2,4-decadienoate, pear ester, a bisexual attractant for codling moth Cydia pomonella (Lepidoptera, Tortricidae) (Light & Knight, 2005; Light et al., 2001). Pear ester is efficient for population monitoring (Knight, Light, & Chebny, 2013; Knight, Mujica, Herrera, & Tasin, 2019) and for behavioral disruption of codling moth larvae and adults, alone or combined with sex pheromone (Knight & Light, 2013; Knight et al., 2012; Light & Beck, 2012; Light & Knight, 2011). The discovery of pear ester demonstrates the potential of kairomones to both improve pheromone-based techniques and to design stand-alone applications. That pear ester is released only in trace amounts from green apples (Gonzalez et al., 2020) underlines that the abundance of volatiles in plant headspace does not correlate with their behavioral saliency. Compounds released in large amounts often stem from main biosynthetic pathways shared by many plants and cannot account for specific host plant finding.
The most widely employed tool for studying plant compounds mediating host attraction is gas chromatography coupled to electroantennographic detection (GC-EAD). GC-EAD measures the response of the entire antenna to odorants (Arn, Städler, & Rauscher, 1975), and biases compounds occurring in large amounts in headspace collections. An active compound such as pear ester, on the other hand, has been overlooked in GC-EAD recordings due to its low abundance.
The discovery of the genetic code of insect odorant receptors (ORs) (Clyne et al., 1999) enables a new approach. The ligand binding specificity of ORs determines the spectrum of volatile chemicals transmitted by OSNs from the antenna to olfactory centers in the brain. Sequencing antennal RNA extracts and gene transcript annotation provides OR expression data and a first functional differentiation, between pheromone receptors (PRs) and ordinary ORs, responding to environmental odorants. Subsequent phylogenetic analysis groups orthologous ORs from related species and provides leads on putative ligands, through comparison with an accumulating database of deorphaned insect ORs (Fleischer, Pregitzer, Breer, & Krieger, 2018; Robertson, 2019; Zhang & Löfstedt, 2015). Single ORs are accordingly a tool of choice to interrogate the plant and microbial odorscape for bioactive compounds. A powerful experimental approach is to express ORs singly in defined sensilla of the antenna of Drosophila melanogaster (Dobritsa, Van Naters, Warr, Steinbrecht, & Carlson, 2003; Hallem, Ho, & Carlson, 2004), where they can be addressed with single sensillum electrophysiological recordings, coupled to gas chromatography (GC-SSR).
In codling moth Cydia pomonella (Lepidoptera, Tortricidae), CpomOR3 has been deorphaned, following transcriptome analysis (Bengtsson et al., 2012; Walker, Gonzalez, Garczynski, & Witzgall, 2016) and heterologous expression (Bengtsson et al., 2014; Cattaneo et al., 2017; Wan et al., 2019). The main ligand of CpomOR3, which belongs to the PR clade, is the plant volatile pear ester (Bengtsson et al., 2014; Light & Knight, 2005; Light et al., 2001). A recent assembly of the codling moth genome reveals presence of two paralogues of CpomOR3, which, according to functional characterization in Xenopus oocytes, respond to a lesser extent also to codling moth sex pheromone, codlemone (Wan et al., 2019). A seemingly conserved response in a closely related species underscores this deeply rooted interconnection of pheromone and plant volatiles: Green budworm moth Hedya nubiferana (Lepidoptera, Tortricidae) is attracted to codlemone (Arn, Schwarz, Limacher, & Mani, 1974; El-Sayed, 2019) and to pear ester (Jósvai, Koczor, & Tóth, 2016; Schmidt et al., 2007).
We have reinvestigated sex pheromone production by green budworm moth H. nubiferana females and the male response to codling moth C. pomonella sex pheromone and to pear ester, in laboratory and field bioassays. A comparative phylogenetic analysis of ORs in the antennal transcriptome of green budworm and codling moth aligns with the behavioral evidence and suggests the presence of a conserved olfactory channel dedicated to these compounds, in both species. This demonstrates how functional characterization of ORs in model species such as codling moth (Bengtsson et al., 2014; Gonzalez, Witzgall, & Walker, 2016), followed by in silico studies of antennal transcriptomes in the taxonomically related species will advance the identification of insect kairomones, and the development of insect management.
2 MATERIALS AND METHODS
2.1 Insects
Green budworm moth Hedya nubiferana Haworth (dimidioalba Retzius) (Lepidoptera, Tortricidae) (Figure 1) is a polyphagous leafroller on Rosacean trees and shrubs and co-occurs with codling moth Cydia pomonella on apple, throughout the Northern Hemisphere. The larvae feed on fruit in autumn and on flower buds in the spring (Bradley, Tremewan, & Smith, 1979). For pheromone analysis, last-instar larvae were field-collected in apple orchards in Scania (Sweden) during May. Larvae were fed with apple leaves and a semisynthetic agar-based diet (Rauscher, Arn, & Guerin, 1984). Pupae and adults were kept under a 18:6 hr light–dark cycle in screen cages and were supplied with fresh apple branches and sucrose solution.

2.2 Pheromone gland extraction and chemical analysis
Female abdominal sex pheromone glands were dissected at the onset of the calling period, toward the end of the scotophase. Glands of 2- to 4-day-old females were extracted in batches of 5 to 15 in 7 µl of redistilled hexane for 1 min (Bäckman, Bengtsson, & Witzgall, 1997). Identification of female gland compounds by coupled gas chromatography-mass spectrometry (GC-MS) was done on a Hewlett Packard 5970 B instrument, with electron impact ionization (70 eV), interfaced with a Hewlett Packard 5890 GC. Helium was used as carrier gas on a 30 m × 0.25 mm DB-Wax column (J&W Scientific), programmed from 80°C (hold 2 min) at 10°C/min to 230°C. The compounds were identified by comparing retention times and mass spectra of natural and synthetic compounds. Double bond position was determined by co-injection with synthetic samples and by evaluation of mass spectra.
2.3 Field trapping
The geometric isomers of E8,E10-12Ac and E8,E10-12OH were synthesized (Witzgall, Bengtsson, Unelius, & Löfqvist, 1993). All other compounds were purchased from S. Voerman (Institute for Pesticide Research, Wageningen, The Netherlands). Purity of synthetic pheromone compounds was ≥96.2% (chemical) and ≥99.7% (isomeric). Compounds in hexanic solution were formulated on red rubber septa (Merck ABS, Dietikon, Switzerland), which were replaced every 2 weeks. Tetra traps (Arn, Rauscher, & Schmid, 1979) were hung in apple trees at eye level and were ca. 5 m apart within one replicate. Traps were placed in untreated apple orchards at Alnarp, Scania (Sweden), and at Halásztelek, Pest county (Hungary), and checked twice a week. Further traps were placed in orchards treated with commercial pheromone dispensers for mating disruption of codling moth. These dispensers were polyethylene tubes containing 87 mg E8,E10-12OH, 49 mg 12OH, and 10 mg 14OH (Shin-Etsu Chemical Co., Tokyo), and they were applied at a rate of 1000/ha. For statistical analysis, trap captures were transformed to log(x + 1) and submitted to a 2-way ANOVA, followed by Tukey's test.
2.4 Wind tunnel
The wind tunnel had a flight section of 63 × 90 × 200 cm (Witzgall et al., 2001). Air was blown by a horizontal fan onto an array of activated charcoal cylinders. The wind tunnel was lit diffusely from above at 6 lux, the wind speed was 30 cm/s, and the temperature ranged from 22 to 24°C. Two-day-old males were transferred to glass tubes (2.5 × 12.5 cm) stoppered with gauze before testing. Males were flown individually, in batches of 15. Two batches of 15 males were tested on one day, 1 to 3 hr after onset of the scotophase, each blend was tested four times (n = 60 males), on different days. The following types of behavior were recorded: taking flight, flying upwind over 100 cm toward the source, and landing at the source.
2.5 Phylogenetic analysis
Sequences of predicted pheromone receptors from C. pomonella (Walker et al., 2016) were used for direct comparison with putative PRs of H. nubiferana (Gonzalez, Witzgall, & Walker, 2017). All amino acid sequences were aligned using MAFFT online (Katoh, Rozewicki, & Yamada, 2019; version 7.220; http://mafft.cbrc.jp/alignment/server/phylogeny.html) with the FFT-NS-i iterative refinement method, with JTT200 scoring matrix, and default parameters. Aligned sequences were used to calculate the best fitting model for comparison in MEGA6 software (Tamura, Stecher, Peterson, Filipski, & Kumar, 2013). The analysis involved 23 amino acid sequences, with a total of 564 positions in the final dataset. An initial tree for the heuristic search was obtained by applying the neighbor-joining method to a matrix of pairwise distances estimated using a JTT model. Then, a Maximum Likelihood Tree was generated using a JTT matrix-based model with bootstrap support inferred from 600 replicates. A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+G, parameter = 3.3624)). The tree is drawn to scale, with branch lengths measured in the number of substitutions per site.
3 RESULTS
3.1 Sex pheromone identification
Analysis of green budworm moth H. nubiferana pheromone gland extracts by GC and GC-MS showed eight further compounds, in addition to the previously identified acetates (Frerot, Priesner, & Gallois, 1979). The major compound codlemone acetate E8,E10-12Ac was accompanied by the monounsaturated 8- and 10-dodecenyl acetates, its three geometric isomers (EZ, ZE, and Z8,Z10-12Ac) as well as the analogous alcohol codlemone, E8,E10-12OH (Table 1).
| Compound | Short form | ng/female | % |
|---|---|---|---|
| Decyl acetate | 10Ac | 0.2 | 2 |
| Dodecyl acetate | 12Ac | 1.0 | 15 |
| (Z)-5-dodecenyl acetate | Z5-12Ac | 0.1 | 2 |
| (E)-8-dodecenyl acetate | E8-12Ac | 0.7 | 10 |
| (Z)-8-dodecenyl acetate | Z8-12Ac | 3.6 | 56 |
| (E)-10-dodecenyl acetate | E10-12Ac | 0.7 | 11 |
| (Z)-10-dodecenyl acetate | Z10-12Ac | 0.1 | 2 |
| (Z,E)-8,10-dodecadienyl acetate | Z8,E10-12Ac | 0.3 | 4 |
| (E,E)-8,10-dodecadienyl acetate | E8,E10-12Ac | 6.5 | 100 |
| (E,Z)-8,10-dodecadienyl acetate | E8,Z10-12Ac | 0.4 | 6 |
| (Z,Z)-8,10-dodecadienyl acetate | Z8,Z10-12Ac | <0.01 | trace |
| (E,E)-8,10-dodecadienol | E8,E10-12:OH | 0.4 | 6 |
Field attraction of H. nubiferana males to compounds identified from the female gland confirms that the sex pheromone of H. nubiferana is a blend of E8,E10-12Ac and Z8-12Ac (Table 2; Frerot et al., 1979). The main compound, E8,E10-12Ac, by itself was not attractive, while addition of Z8-12Ac had a strong synergistic effect (F(7,72) = 61.95, p < .0001). Addition of E8-12Ac further increased male attraction in untreated apple orchards, but the difference was not significant. Blends of E8,E10-12Ac and the ∆10–12 monoenes or the analogous alcohol, codlemone, did not produce significant captures. Adding these compounds to the 3-component acetate blend slightly diminished trap catch (Table 2).
| Compound | μg/trap | |||||||
|---|---|---|---|---|---|---|---|---|
| E8,E10-12:OAc | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| E8-12:OAc | 1 | 1 | 1 | 1 | ||||
| Z8-12:OAc | 5 | 5 | 5 | 5 | ||||
| E10-12:OAc | 1 | 1 | 1 | |||||
| Z10-12:OAc | 0.2 | 0.2 | 0.2 | |||||
| E8, E10-12:OH | 1 | 1 | ||||||
| Number of males/trap | ||||||||
| Untreated | 0 b | 0 b | 6.1 a | 6.9 a | 6.3 a | 4.7 a | 0.1 b | 0 b |
| Mating disruption | 0 a | 0 a | 0.2 a | 0.3 a | 0.3 a | 0.3 a | 0 a | 0 a |
Note
- Means followed by the same letter are not significantly different (Tukey test, F(7,72) = 61.95, p < .0001).
The gland compounds identified from female glands with no apparent effect on attraction may be biosynthetic by-products or precursors. A study of the female effluvium will show whether they are released at all, and at which ratio. The full blend of compounds may also carry information that cannot be revealed by a field trapping test.
3.2 Attraction to codlemone and pear ester
Wind tunnel observations and a trap test in an apple orchard, adjacent to a pea field, corroborate that codlemone acetate E8,E10-12Ac as a single compound does not attract green budworm moth. Attraction of pea moth C. nigricana confirms that the trap lures released E8,E10-12Ac at high isomeric purity (Table 3; Witzgall et al., 1993, 1996). In comparison, traps baited with codlemone alone regularly captured few green budworm moth males, in addition to codling moth. Blends of codlemone and codlemone acetate attract far fewer codling moths and no green budworm moths at all (Table 3).
| Compound | μg/trap | ||||
|---|---|---|---|---|---|
| E8,E10-12Ac | 10 | 10 | 10 | 1 | |
| E8-12Ac | 1 | 1 | |||
| Z8-12Ac | 5 | 5 | |||
| E8,E10-12OH | 10 | 10 | 10 | ||
| Number of males/trap | |||||
| H. nubiferana | 0 c | 57.5 a | 53.9 a | 0.4 bc | 1.4 b |
| C. nigricana | 20.1 a | 3.4 b | 0 | 0 | 0 |
| C. pomonella | 0 | 0 | 0 | 3 b | 12.2 a |
| Male H. nubiferana wind tunnel behavior (%) | |||||
| Taking flight | 48 a | 51 a | 47 a | –* | – |
| Upwind flight | 0 b | 39 a | 33 a | – | – |
| Landing at source | 0 b | 22 a | 17 a | – | – |
Note
- Field traps attracted also codling moth C. pomonella and pea moth Cydia nigricana. Means followed by the same letter are not significantly different (Tukey test, p < .05).
- * Not tested.
Interestingly, a blend of codlemone and its three geometric isomers significantly increased green budworm moth captures over codlemone alone (Table 4; F(7,72) = 2.62, p = .04413). In contrast, this isomer blend captured fewer codling moth males (Table 4; F(7,72) = 4.22, p = .02135; El-Sayed et al., 1998).
| Compound | μg/trap | |||||||
|---|---|---|---|---|---|---|---|---|
| E8,E10-12OH | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| E8,Z10-12OH | 0.5 | 2 | 2 | |||||
| Z8,E10-12OH | 0.5 | 2 | 2 | |||||
| Z8,Z10-12OH | 0.5 | 2 | 2 | |||||
| H. nubiferana | 2.0 b | 3.5 ab | 3.2 ab | 2.3 ab | 2.1 ab | 1.8 b | 3.8 ab | 6.6 a |
| C. pomonella | 8.0 a | 9.0 a | 4.8 ab | 10.5 a | 11.9 a | 10.2 a | 6.4 ab | 3.2 b |
Note
- Means followed by the same letter are not significantly different (Tukey test, p < .05).
Green budworm moth has also been reported to respond to pear ester (Jósvai et al., 2016; Schmidt et al., 2007). A further field test in Hungary confirmed this and showed that addition of codlemone to pear ester does not enhance attraction of either sex (Table 5).
| Compound | μg/trap | |||
|---|---|---|---|---|
| Pear ester | 6.000 | 6.000 | 6.000 | 6.000 |
| E8, E10-12OH | 1 | 3 | 10 | |
| Number of moths/trap | ||||
| Males | 0.3 a | 0.3 a | 0.1 a | 0.1 a |
| Females | 0.1 a | 0.1 a | 0.04 a | 0.2 a |
Note
- Means followed by the same letter Means followed by the same letter are not significantly different (Tukey test, p < .05).are not significantly different (Tukey test, p < .05).
Orchard mating disruption treatments with codlemone strongly diminished attraction of H. nubiferana males to synthetic pheromone (Table 2), corroborating a behavioral effect of codlemone via a dedicated olfactory channel. This supports the idea that communication disruption in moths may be achieved with single pheromonal compounds or incomplete pheromone blends (Carde & Minks, 1995; Porcel et al., 2015), which is of practical importance for the implementation of pheromonal control of codling moth and leafrollers in European orchards.
3.3 Phylogenetic analysis and antennal expression
Hedya nubiferana Haworth and Hedya dimidioalba Retzius are synonymous taxonomic names for green budworm moth. The National Center for Biotechnology Information (NCBI) lists OR sequences (including PRs) as "HnubOR##."
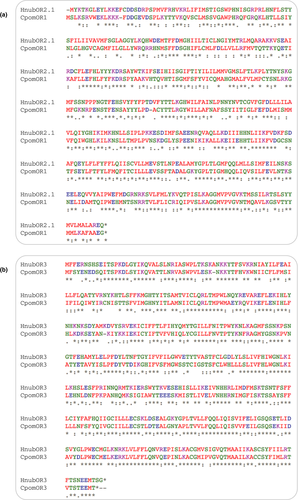
Predicted putative PRs from H. nubiferana displayed orthology to PRs in Cydia pomonella (CpomOR3, CpomOR6, and CpomOR22; Figure 2a). Notably, HnubOR6 was >50% similar to CpomOR6. Sequence comparison analysis revealed that CpomOR1 and HnubOR2.1 shared 49% amino acid identity and 66% similarity, while the OR3 orthologs of both species shared 64% and 76% identity and similarity, respectively. Amino acid differences between these putative PRs are observed across the entire length of the protein sequences (Figure 3).
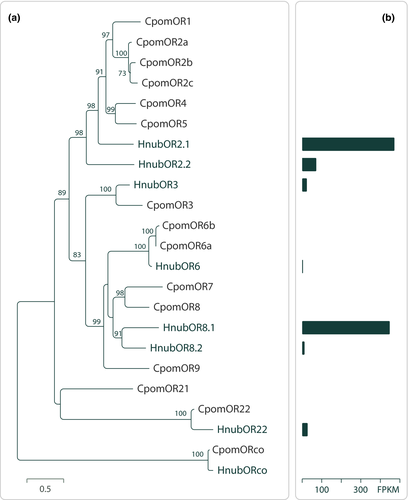
Abundance estimation of the predicted sequences showed that the most highly expressed were HnubOR2.1 and HnubOR8.1 (Gonzalez et al., 2017). The other 3 putative PRs detected in male antennae were one or two orders of magnitude lower (Figure 2b).
4 DISCUSSION
4.1 In silico identification of semiochemicals for the development of insect control
Semiochemicals are efficient tools for insect control by air permeation and mass trapping (El-Sayed et al., 2009; Witzgall, Kirsch, et al., 2010), and inform push-pull techniques and plant breeding (Khan et al., 2014; Stenberg, Heil, Åhman, & Björkman, 2015; Tamiru, Khan, & Bruce, 2015). A current bottleneck and main research challenge is the identification of the chemicals that mediate host plant recognition.
Availability of compounds that attract insects to mating sites, elicit oviposition or feeding in adults and larvae, enables multiple applications. Pear ester, for example, is efficient for monitoring codling moth males and females, is used to supplement pheromone-based communication disruption, and is a stand-alone tool for the disruption of larval host-finding and feeding (Knight & Light, 2013; Knight et al., 2012; Kovanci, 2015; Light, 2016; Light & Beck, 2012; Light & Knight, 2011; Schmidt, Tomasi, Pasqualini, & Ioriatti, 2008). Apple fruit moth, Argyresthia conjugella, mates outside apple orchards in forests, which precludes the use of sex pheromone for control. Specific attraction of gravid females to host plant volatiles has been translated into an efficient kairomone lure for monitoring and control (Bengtsson et al., 2006; Knudsen et al., 2008; Knudsen, Norli, & Tasin, 2017; Knudsen & Tasin, 2015).
Chemical analysis of plant or microbial volatomes returns a large number of compounds (Knudsen, Tollsten, & Bergström, 1993; Lemfack et al., 2018; Ljunggren et al., 2019), necessitating careful selection of candidate compounds for subsequent behavioral analysis (Figure 4a). The traditional and most widely used approach is to screen volatile collections, eluting from a gas chromatograph, with the entire insect antenna. GC-EAD was conceived for sex pheromone identification in moths (Arn et al., 1975), where mutually coordinated production and response lead to distinct male antennal signals to a few bioactive compounds in female pheromone glands.

GC-EAD suffers, however, from serious bias and produces false positives when screening plant or microbial headspace. Typically, ORs respond, to some extent, to diverse volatiles that are structurally similar to their cognate ligands. Ubiquitous compounds present in large amounts, for example, short aliphatic acetates or alcohols, farnesenes, linalools, and caryophyllenes, invariably elicit an antennal response, generated by the ensemble of olfactory sensory neurons (OSNs) on the antenna, expressing the entire olfactory receptor (OR) repertoire. Their behavioral relevance remains, however, uncertain.
On the other hand, GC-EAD potentially overlooks active compounds released in small amounts. Pear ester, the strongest known attractant for codling moth, is present in trace amounts in apple headspace and has not been detected by GC-EAD (Gonzalez et al., 2020). Recordings from single sensilla, instead of the entire antenna, provide higher resolution, but are technically demanding. Reliable replication is a main obstacle for recordings from sensillum types other than sensilla trichodea, containing pheromone-sensitive OSNs. In codling moth, SSR produced conclusive results when investigating sex pheromones, not plant volatiles (Ansebo, Ignell, Löfqvist, & Hansson, 2005; Bäckman et al., 2000).
Antennal transcriptomes and phylogenetic analysis of ORs, followed by heterologous expression (Figure 4b,c), emerge as an opportune methodological advancement to experimentally address single ORs. Functional characterization of ORs expressed in cultured cell lines, such as human embryonic kidney cells or Xenopus oocytes, depends, however, on the quality of the odorant panel used, where chemical purity (Schorkopf et al., 2019) and selection of test compounds are main limitations. An experimental difficulty is the aqueous delivery of solubilized volatiles. In comparison, heterologous expression of ORs in select sensory neurons in Drosophila enables in vivo single sensillum recordings (SSR). Coupled to a GC, GC-SSR eliminates differences in volatility of test compounds and makes it even possible to screen entire headspace collections (Dobritsa et al., 2003; Fleischer et al., 2018; Gonzalez et al., 2016; Hallem et al., 2004).
Nonetheless, attempts to deorphan ORs are far from always successful. For example, the orthologous CpomOR19 and SlitOR19 both respond best to indanone analogs (Gonzalez et al., 2015), but their behavioral role remains unclear. An intriguing idea is that the ligand is instead 1,4-dimethylindanyl acetate (W. Francke, pers. comm.), a rare floral compound (Braunschmid et al., 2017), which is unstable and not available as synthetic standard.
Chemical analysis and functional OR assays deliver candidate compounds for behavioral tests (Figure 4a,c). This selection of compounds may be incomplete, as outlined above, but candidate compounds may anyhow elicit behavioral responses and suffice the criterium of validly identified semiochemicals—while key compounds remain unknown. A prominent example is α-farnesene, early on identified as a codling moth kairomone (Sutherland & Hutchins, 1972). It is ubiquitously found in most green plants, has some effect on codling moth adult and larvae, but does not encode specific host plant recognition.
In silico identification of OR ligands now emerges as an additional experimental approach and opportune advancement in semiochemical research (Figure 4d). OR expression levels in the sexes, in adult versus larval stages, in combination with phylogenetic analysis and computational approaches (Caballero-Vidal et al., 2020; Chepurwar, Gupta, Haddad, & Gupta, 2019; De Fouchier et al., 2017), informed by a rapidly accumulating database of deorphaned insect ORs, afford powerful predictions of putative OR ligands and behavior-modifying chemicals.
Promising targets for future work include, for example, tephritid fruit flies, in view of our thorough knowledge of Drosophila ORs (Liu, Smagghe, Lei, & Wang, 2016; Muench & Galizia, 2016) or moths from several families, aided by a rapidly accumulating database of lepidopteran antennal transcriptomes (Cao et al., 2014; Cao, Huang, Shen, Liu, & Wang, 2020; Chang et al., 2017; Corcoran, Jordan, Thrimawithana, Crowhurst, & Newcomb, 2015; Dong, Song, Li, Shi, & Wang, 2016; Du et al., 2018; Feng, Guo, Zheng, Qin, & Du, 2017; Jia et al., 2016; Jia, Zhang, Liu, Wang, & Zhang, 2018; Jiang et al., 2014; Koenig et al., 2015; Li, Du, Li, & Wu, 2015; Park, Withers, Suckling, & Collaboration, 2015; Steinwender, Thrimawithana, Crowhurst, & Newcomb, 2015; Tian et al., 2018; Yang, Cao, Wang, & Liu, 2017; Zeng et al., 2015; Zhang et al., 2013, 2015, 2017; Zhang, Zhang, Wang, & Kong, 2014) Rojas et al. 2018).***
4.2 Green budworm moth response to codlemone and pear ester
We here employ a reverse approach, to interpret behavior in the light of transcriptome data and the tortricid OR phylogeny. The empirical finding that green budworm moth H. nubiferana males respond to codling moth C. pomonella sex pheromone and kairomone, codlemone and pear ester, correlates with the ORs found in antennal transcriptomes.
Functional characterization of CpomOR3, a codling moth OR, has established pear ester as its principal ligand. This was achieved through heterologous expression of CpomOR3 in olfactory sensory neurons of ab3 and T1 antennal sensilla in Drosophila melanogaster, followed by single sensillum electrophysiological recordings (SSR) (Bengtsson et al., 2014; Gonzalez et al., 2016), and has meanwhile been corroborated by luminescence assays after expression in human embryonic kidney cells and Xenopus oocytes (Cattaneo et al., 2017; Wan et al., 2019). CpomOR3, albeit tuned to a plant volatile compound, is part of the lepidopteran pheromone receptor (PR) clade (Bengtsson et al., 2012, 2014; Walker et al., 2016).
The hypothesis that H. nubiferana perceives pear ester via HnubOR3 is parsimonious. A PR phylogeny of H. nubiferana and C. pomonella (Figure 2a), together with sequence similarity analysis (Figure 3), show that CpomOR3 and HnubOR3 are orthologues, which is in line with the behavioral data (Table 5; Jósvai et al., 2016; Schmidt et al., 2007). This compares to the receptor orthologs CpomOR19 and SlitOR19 (Spodoptera littoralis). Following functional characterization of SlitOR19, ligand affinity of CpomOR19 was predicted on the basis of amino acid sequence similarity (Gonzalez et al., 2015).
Oriental fruit moth Grapholita molesta, although taxonomically closer to C. pomonella than to H. nubiferana (Bradley et al., 1979; Regier et al., 2012), is not known to respond to dienic pheromone compounds or pear ester, which is corroborated by PR phylogeny (Gonzalez et al., 2017; Li et al., 2015). The broad host range of G. molesta overlaps only partially with C. pomonella and H. nubiferana food plants.
4.3 Attraction to sex pheromone and codlemone employs distinct olfactory channels
Attraction of green budworm moth H. nubiferana to its multicomponent sex pheromone and to codling moth pheromone employs separate olfactory channels. Codlemone E8,E10-12OH does not mimic the H. nubiferana main pheromone compound codlemone acetate E8,E10-12Ac, since codlemone is active as single compound, while codlemone acetate is not (Tables 2, 3). Tortricid moths differentiate analogous alcohol from acetate pheromone compounds at high resolution (Witzgall et al., 1991, 1993, 1996, 2010), probably since the functional groups strongly affect receptor interactions (Bengtsson, Liljefors, Hansson, Löfstedt, & Copaja, 1990). From analysis of PR phylogeny and expression levels in H. nubiferana and C. pomonella (Figure 2; Walker et al., 2016), we hypothesize that CpomOR1 and HnubOR2.1 are tuned to codlemone, and CpomOR6 and HnubOR8.1 to codlemone acetate (Cattaneo et al., 2017). In codling moth, codlemone acetate is a pheromone synergist or antagonist, when added to the main pheromone compound codlemone at small and large amounts, respectively (Hathaway et al., 1974; Witzgall et al., 2001).
The presence of two pheromone channels in H. nubiferana males is reminiscent of the "hopeful monster" (Baker, 2002; Dietrich, 2003) and "asymmetric tracking" (Phelan, 1992) concepts, suggesting that new communication channels arise through saltational shifts in female pheromone production, which are subsequently tracked by the male sex. Such shifts are facilitated by redundancies in the PR repertoire.
Three related species, H. ochroleucana, H. pruniana, and H. salicella, are best attracted to the Z,E isomers of codlemone and codlemone acetate, and Z,E-codlemone is active in codling moth (El-Sayed et al., 1998; Witzgall, Trematerra, Liblikas, Bengtsson, & Unelius, 2010). A candidate PR for Z,E-codlemone is HnubOR2.2 (Figure 2). Regarding HnubOR8.1 and HnubOR8.2, which are close to GmolOR1 and GmolOR11 (Gonzalez et al., 2017; Li et al., 2015), we hypothesize that they respond to the minor acetate pheromone components (Z)- and (E)-8-dodecenyl acetate (Tables 2, 3), which are main pheromone compounds of Oriental fruit moth G. molesta (Carde, Baker, & Carde, 1979).
4.4 Interaction of plant volatiles and pheromones
Food and mate finding, the essential components of insect reproductive behavior, depend on a finite number of ORs encoding relevant odor signals. Peripheral olfactory perception employs 39 ORs in the fruit fly Drosophila melanogaster (Grabe, Strutz, Baschwitz, Hansson, & Sachse, 2015; Menuz, Larter, Park, & Carlson, 2014), 58 ORs in codling moth C. pomonella (Walker et al., 2016), and a similar number of ORs in other tortricids (Corcoran et al., 2015; Steinwender et al., 2015, Rojas et al., 2018). Evolution of host specialization in insects is associated with accelerated OR gene loss, combined with strong selection on the remaining, intact ORs (Arguello et al., 2016; McBride & Arguello, 2007; Robertson, 2019; Sánchez-Gracia, Vieira, & Rozas, 2009). Receptors that are conserved across taxonomic clades, such as CpomOR3 and HnubOR3 (Figure 2; Gonzalez et al., 2017), likely play adaptive roles.
Green budworm moth attraction to pear ester and codlemone is intriguing, because it provides further evidence for the association of olfactory channels dedicated to social and environmental signals in phytophagous insects. Transcriptome data and phylogenetic context confirm this association. CpomOR3 is tuned to the plant volatile pear ester, while it belongs to the pheromone receptor clade (Figures 2a, 3; Bengtsson et al., 2012, 2014; Walker et al., 2016). That PRs respond to pheromones and plant volatiles has even physiological consequences: OR genes with highest sequence similarity tend to be expressed in OSNs that project to neighboring glomeruli in the antennal lobe, facilitating interactions between the circuits encoding these signals (Couto, Alenius, & Dickson, 2005; Krieger et al., 2009; Ramdya & Benton, 2010). This has indeed been confirmed in codling moth, by intracellular recordings from olfactory projection neurons and functional imaging of the antennal lobe, showing a powerful synergistic interaction between codlemone and pear ester (Trona et al., 2013; Trona, Anfora, Bengtsson, Witzgall, & Ignell, 2010).
HnubOR3 has not been deorphaned, but the recent discovery that CpomOR3 responds to pear ester and to a lesser extent also to codlemone (Wan et al., 2019) provides an explanation for consistent attraction of H. nubiferana to codlemone (Tables 3, 4; Arn et al., 1974). Codling moth C. pomonella and H. nubiferana both feed on apple, but belong to different tortricid tribes (Bradley et al., 1979; Regier et al., 2012). Occurrence of conserved olfactory genes contributing to mate finding and host plant attraction lends further support to the concept that host plant recognition and sexual communication are interlinked (Borrero-Echeverry et al., 2018) and that a combination of natural and sexual selection gives rise to reproductive isolation in insect herbivores (Boughman, 2002; Paterson, 1978; Rosenthal, 2017). A more complete analysis of olfactory genes and their behavioral and ecological functions will contribute to the study of phylogenetic divergence in phytophagous insects. Equally rewarding is the perspective that this research also drives the development of semiochemicals for efficient and sustainable insect control.
ACKNOWLEDGEMENTS
This work was supported by Carl Tryggers Stiftelse för Vetenskaplig Forskning, Formas (project 2011-1370) and the Linnaeus environment “Insect Chemical Ecology, Ethology, and Evolution (IC-E3)” (Formas, SLU). The authors acknowledge support from Science for Life Laboratory, the National Genomics Infrastructure (NGI), and Uppmax for providing assistance in massive parallel sequencing and computational infrastructure. Reviewers provided constructive and valuable comments.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
Francisco Gonzalez: Formal analysis (equal); Investigation (equal); Validation (equal); Writing-review & editing (equal). Felipe Borrero-Echeverry: Formal analysis (equal); Investigation (equal); Validation (equal); Writing-review & editing (equal). Julia K. Josvai: Investigation; Writing-review & editing. Maria Strandh: Investigation. Rikard Unelius: Investigation; Writing-review & editing. Miklos Toth: Investigation. Peter Witzgall: Conceptualization (equal); Data curation (equal); Formal analysis; Funding acquisition (equal); Investigation; Project administration (equal); Supervision (equal); Validation; Visualization (equal); Writing-original draft (equal). Marie Bengtsson: Data curation (equal); Funding acquisition (equal); Investigation; Supervision (equal); Validation; Writing-review & editing. William B. Walker: Conceptualization; Data curation; Formal analysis; Investigation; Supervision (equal); Validation; Visualization; Writing-review & editing.
Open Research
Data Availability Statement
Transcriptome raw reads sequence data are available through the NCBI Sequence Read Archive (Accession Number: SRX1741573). Putative pheromone receptor sequences identified from the H. nubiferana transcriptome assembly are available through NCBI, and are included in a Transcriptome Shotgun Assembly project that has been deposited at DDBJ/EMBL/GenBank (accession numbers KY283585.1, KY283590.1, and KY283600.1).




