American marten occupancy and activity patterns at the southern extent of their range in the eastern United States
Abstract
The relatively recent rediscovery of an American marten (Martes americana) population that was reintroduced over 30 years ago in southern Vermont provides an opportunity to investigate the relative importance of other mesocarnivores, and forest stand (e.g., DBH, downed logs, vertical structure) and habitat variables to their presence on the Green Mountain National Forest. Marten are state-listed as an endangered species in Vermont and occur there at the southern extent of their range in the eastern United States. We collected detection data from camera surveys in 5 km2 units between 2019 and 2021 (December–April; n = 40 units, 238 cameras). We examined activity patterns and applied an occupancy modeling framework to the detection data to assess the relative importance of covariates at unit and camera levels and assess interactions of marten with other mesocarnivores. We did not find any unit-level occupancy models with significant covariates that were better supported than the base model in the single-season unit-level analysis. Distance to the nearest release site was the covariate most supported for detectability at both spatial scales, and marten occupancy at the camera level was positively influenced by the amount of canopy cover. Two species interaction models did not indicate any positive or negative association beyond random with other mesocarnivores and activity patterns among mesocarnivores had substantial overlap. Marten recovery since the time of the reintroduction appears slow, and even 30 years later, the marten distribution is limited and suggests that dispersal is restricted at some level. We recommend a further investigation of the possible impact of other mesocarnivores to juvenile survival or other vital demographic rate (e.g., recruitment) in marten that were not explicitly measured in this study.
1 INTRODUCTION
Multiple drivers such as habitat type (Gompper et al., 2016), human disturbance (Kalle et al., 2013), climate (Zielinski et al., 2017), and species interactions (Guo et al., 2017), govern the occurrence of carnivores in complex ways that vary in importance between and within species and operate at different spatial scales (Mitchell & Hebblewhite, 2012). Further, occurrence is closely associated with specific life history activities such as breeding and foraging (Dechner et al., 2018; Uboni et al., 2017) or can reflect resource availability (Grassman Jr. et al., 2005).
Range boundaries often have markedly different conditions compared to interior parts of a species range and present notable challenges for resident carnivores. Their body sizes can be smaller at range boundaries compared to interior portions of their range and fundamental niches can be limited on at least one niche axis (Meiri et al., 2009). Carnivores that occur at range boundaries are less abundant (Brown et al., 1995) and have difficulty adapting to changing conditions (Brown, 1984; Whittington et al., 2022).
Biotic interactions such as competition and predation are predicted to be more important at range boundaries, particularly at low latitudes, and can constrain occurrence patterns (Aragón & Sánchez-Fernández, 2013; Louthan et al., 2015; Sirén & Morelli, 2020). Competitive generalist predators (e.g., coyotes; Canis latrans) at range boundaries can potentially limit prey species to areas of reduced predation (Mott, 2010). Generalist predators also behave opportunistically by altering their diet and behavior in response to changes in the availability of different prey types, which may feedback on other subordinate predators and force them to alter their realized niche spaces (Glasser, 1982; Smith et al., 2018). Competition for food and suitable habitat by a competitively superior species may force a subordinate species into suboptimal habitat (Hairston et al., 1987; Jaeger, 1971a, 1971b, 1972; Kyaw et al., 2021; Vanek et al., 2013). Coexistence among carnivores in these areas is potentially possible through mechanisms that minimize intraguild competition and predation including spatial avoidance, minimizing dietary overlap, and temporal shifts in daily or seasonal activity patterns in the search for and acquisition of resources (Grassel et al., 2015; St. Pierre et al., 2006).
American marten (Martes americana) are small, elusive carnivores that are broadly distributed throughout the boreal forest zones of North America (Powell et al., 2003). They were considered common and widely distributed prior to the 20th century (Moruzzi et al., 2003) and their historical range in the eastern United States once extended as far south as northern Pennsylvania (Rhoads, 1903). Like other native furbearers including fisher (Pekania pennanti) and mink (Neovison vison), their numbers and distribution declined significantly in the northeastern United States (hereafter, the Northeast) from overtrapping and habitat loss (Brander & Books, 1973; Silver, 1957) and by the early 1900s, they were extirpated from most areas of the Northeast (e.g. Vermont, New Hampshire) except the Adirondacks of New York and northern portions of Maine (Krohn, 2012).
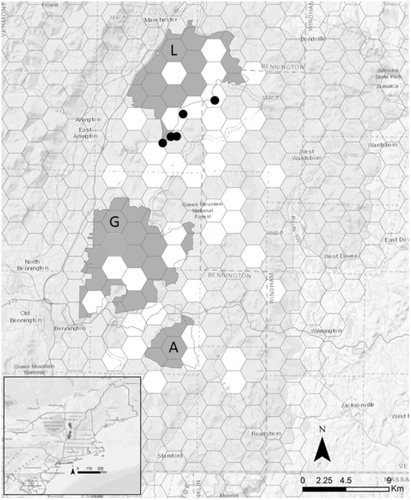
Vermont closed the marten trapping season and eventually listed the species as endangered in 1972 (10 V.S.A. Chap. 123-2012). Active recovery of marten in Vermont included the reintroduction of animals from Maine (n = 104) and New York (n = 11) between October 1989 and December 1991 at five sites in southern Vermont (Distefano et al., 1990; Moruzzi et al., 2003; Royar, 1992; Figure 1). Despite considerable monitoring effort of the reintroduced marten by Vermont Fish and Wildlife (Royar, 1992), camera surveys from 1994–1995 and 1997–1998 resulted in only a few marten detections, and the reintroduction effort was later deemed unsuccessful (Moruzzi et al., 2003). One plausible explanation proposed for the assumed failure of the reintroduction was competition and predation by fishers (Moruzzi et al., 2003). Winter camera surveys conducted between 2015 and 2017 revealed a population of marten in the southern part of the Green Mountain National Forest (O'Brien et al., 2018) that may be a remnant of the original reintroductions (Aylward et al., 2019). This population currently represents the southernmost extent of the marten distribution on the east coast. As a state-listed endangered species (10 V.S.A. Chap. 123-2012) in Vermont, marten residing there require a thorough understanding of their distribution, habitat requirements, and limiting factors to effectively manage them.
Preferred habitat characteristics of marten are variable and largely dependent on age, sex, season, and population density (Buskirk & Powell, 1994; Clark et al., 1987). Characteristics associated with home range and habitat use by marten vary at different spatial scales that are relevant for their conservation (Chapin et al., 1998; Smith & Schaefer, 2002). Across the eastern part of their distribution, marten demonstrate an avoidance of open areas (Cheveau et al., 2013; Gosse et al., 2005) and landscapes lacking structural complexity as a method of minimizing the risk of predation (Eriksson et al., 2019). Marten prefers vertical and horizontal structural complexity that may be beneficial for hunting and provide protection from predators (Evans & Mortelliti, 2022). They have an affinity for a higher elevation when available (Sirén et al., 2016) including mature forest, canopy closure, mixedwood and softwood, and coarse woody debris (Godbout & Ouellet, 2010; Jensen, 2012; Lambert et al., 2017; Payer & Harrison, 2004).
Most studies that examined habitat features associated with marten in eastern North America have occurred in either interior parts of their range or in populations at the northern extent of their range. Little is known about the importance of co-occurring mesocarnivores that interact with marten as competitors and predators at the southern extent of their range such as red fox (Vulpes), coyote, bobcat (Lynx rufus), and fisher (Bull & Heater, 2001; Pauli et al., 2022; Raine, 1981; Suffice et al., 2023; Vernam, 1987). Krohn et al. (1995, 1997) suggested that high fisher populations limit marten populations at the southern extent of their range. However, recent work by Manlick et al. (2017), Croose et al. (2019) suggests a large amount of spatial and temporal overlap between marten and fisher is possible.
In this study, we evaluated the relative importance of other mesocarnivores, forest stand variables, and habitat variables to occupancy and detectability (MacKenzie et al., 2005, 2006; MacKenzie & Royle, 2005) of marten at multiple spatial scales (Gompper et al., 2016; Lesmeister et al., 2015; Long et al., 2010). We focused primarily on marten habitat use at the 5 km2 unit level which coincided with the scale of the average home range size of male American marten home range and the development of a long-term monitoring program. The specific objectives were to (1) provide camera and unit level estimates of occupancy and detectability for marten, (2) identify the relative importance of other mesocarnivores, forest stand variables, and habitat variables to marten occurrence in the study area, (3) identify relationships between marten occurrence and the presence of other mesocarnivores in the study area, and (4) compare activity pattern overlap of marten with other mesocarnivores detected in the study. We predicted that occupancy models for marten that included covariates associated with other mesocarnivore occupancy or detections would have greater support than models that included forest stand variables and other habitat variables measured in this study (Louthan et al., 2015). We also predicted that marten occupancy and detectability would be lower further away from reported release sites given the limited number of anecdotal reports of marten in southern Vermont. We predicted that occupancy and detectability would be negatively associated with the presence of coyote, red fox, gray fox (Urocyon cinereoargenteus), and bobcat (Martin et al., 2022) but not fisher (Manlick et al., 2017). We also predicted that marten would show substantial overlap in daily activity patterns with fisher based on the recent work of Manlick et al. (2017) but not with other mesocarnivores in our study.
2 STUDY AREA
The study area was located within the southern extent of the Green Mountain National Forest (GMNF) in southern Vermont and northwestern Massachusetts in the United States (Figure 1). The Green Mountain National Forest encompassed more than 1620 km2 in southwestern and central Vermont, forming the largest contiguous block of public land in the state (United States Department of Agriculture Forest Service (USFS), 2006). The study area was mountainous with a maximum elevation of 1220 m and included 3 federally designated wilderness areas (Lye Brook, George D. Aiken, and Glastenbury). The forest was composed primarily of northern hardwoods (~80%) including red oak (Quercus rubra), American beech (Fagus grandifolia), yellow birch (Betula alleghaniensis), red maple (Acer rubrum), and sugar maple (Acer saccharum). Coniferous stands were predominantly balsam fir (Abies balsamifera) and spruce (Picea spp.) and occur near wetland areas. Understory vegetation was highly variable and includes hobblebush (Viburnum alnifolium) and common witchhazel (Hamamelis virginiana; Siccama, 2014).
3 METHODS
3.1 Data collection
We collected detection data from camera surveys in 40 (n = 238 cameras), 5 km2 sample units over three winter field seasons (December–April) between 2019 and 2021. We surveyed each unit only once during the time we surveyed (units sampled yearly n = 15, 19, 6). The primary focus of the surveys was to detect marten, so we scaled the units to represent an area equivalent to the average home range size of male American marten (5 km2; Jensen, 2012). Several species were also important to document because of their potential for predation on or competitive interactions with marten including coyote, fisher, bobcat, and red and gray fox. We selected units within the study area based on random sampling with the constraint that the overall sample had to include units where the probability of marten was non-zero and occurrence ranged from high to low (Jensen, 2012).
Within each sample unit, we used simple random sampling to place six motion-triggered cameras (Moultrie 990is, P150s; Moultrie Products, Birmingham, AL and Bushnell Trophy Cam Aggressors; Bushnell Outdoor Products, Overland, KS) at sites to survey for marten. We expected that the use of camera arrays within the sample units would increase the likelihood of detecting marten during the surveys (O'Connor et al., 2017). At camera each site, we mounted cameras on trees one and a half to two meters above ground surface and aimed them toward the base of a bait tree to maximize the field of view. On the bait tree, we placed a single can of sardines in water wired approximately 1 m above the ground, and emptied another can at the base of the tree. To each bait tree, we applied a mixture of skunk essence (Wildlife Control Services, Granby, CT) and petroleum jelly (<15 mL) and separately, a curiosity scent (“Marten Magic”; Minnesota Trapping Supplies, Inc., Pennock, MN) containing peppermint-anise oil extracts (<1.5 mL).
Camera surveys in units lasted 15 days divided into three consecutive 5-day periods (total = 3 survey periods, 15 days), and following each 5-day survey period, we re-scented, re-baited, and exchanged the data cards. The data resulted in detection histories with a 0 or 1 for each of the three survey periods (e.g., 010) for each unit. To maintain the independence of our units, we avoided carrying out surveys in adjacent units at the same time. We set cameras to operate for 24 h per day and recorded date, time, barometric pressure, and temperature at the time of all detections. Cameras were set to minimum delay with a three-shot photo burst. Images were stored in Colorado Parks and Wildlife (CPW) Photo Warehouse 4.3 and reviewed by a minimum of two individuals; that recorded the date, time of day, and frequency of detections for each species. We also recorded notable behaviors (e.g. vigilance antipredator behavior such as upright posture, and looking around), weather conditions, and circumstances observed in the photos or at the time of data collection in the field. We listed images as unknown species in the CPW database and did not include them in the analysis if we could not identify the species.
3.2 Covariate data
We collected data on 14 covariates that were potentially associated with marten presence and detectability (Table 1 and Table S2). Covariate data was collected within a 30 m2 buffer around camera sites and within the entire units by extracting covariate data from polygons in ArcMap (Table 1). We recognize that simple detection or non-detection of a mesocarnivore during a survey does not account for imperfect detection, so we included site occupancy estimates of mesocarnivores generated in the program PRESENCE 2.1.3.47 (Hines, 2006) as covariates. Data for the five covariates related to forest stand metrics (Table 1) was collected in the field at each camera site during summer 2019 following the Common Stand Exam Field Guidelines (USFS, 2015).
| Covariate | Level | Prediction | Description |
|---|---|---|---|
| (1) PerFor | U (ψ) | Positive | Percent (Per) of unit classified as foresta |
| (2) Habitat type e.g. PerDec, PerMix | U (ψ, p) | Positive for forest types | Percent (Per) of 30 m raster cells within units classified as a specific habitat typea including Deciduous (PerDec), Mixed, Conifer, Human Disturbance, Wet Woody, Water |
| (3) PerCC | U (ψ, p) | Positive | Canopy cover (CC) percent derived from percent tree canopy cover layer, values range 0%–100%a |
| (4) Can_Clos | U, C (ψ) | Positive | Value of canopy closure between 0–100 using a spherical densiometerb |
| (5) Vert_Struc | U, C (ψ) | Positive | Classification of vertical distribution of tree size from Common Stand Exam Field Guide USFS (2015)b |
| (6) DBH | U, C (ψ) | Positive | Mean value (cm) for trees within a radius of camera with radius defined using BAF 20 prismb |
| (7) D_Logs | U, C (ψ) | Positive | Number of downed logs of decay classes 1–2 from Common Stand Exam Field Guide USFS (2015)b |
| (8) BAF | U, C (ψ) | Positive | Value in m2/ha using a Basal Area Factor (BAF) 20 prismb |
| (9) Dist | U, C (ψ, p) | Negative | Distance from each units centroid or camera location to the closest reported release site (km)c |
| (10) Elev | U (ψ) | Positive | The 2-D mean elevation within each sampling unitd |
| (11) TRI | U (ψ) | Positive | Topographic Relief Index. The 3-D slope of the landscape within each sampling unit. Index is derived using ArcGIS 10.8 (ESRI) to extract raster cell values from the USGS National Elevation Dataset (NED) 1/3 arc second Digital Elevation Model 10 m rasterd |
| (12) Snow | U, C (ψ, p) | Positive | Unit locations overlaid on daily climate data maps from the National Weather Service to collect average historic snow depth values at each location for the duration of each survey periodf |
| (13) Mesocarnivores | Fisher (Fish), coyotee (Coy), fox (fox), bobcat (Bob) | ||
| e.g. FishPres, CoyPres | U (ψ, p) | Negative except fisher | Whether a species was detected (e.g. Coy Pres) during any survey period (1 present, 0 absent)g |
| e.g. FishOcc, CoyOcc | U, C (ψ, p) | Negative except fisher | Site occupancy estimate from base model Psi(.), p(.) for other mesocarnivores at cameras and in unitsg. Site estimates produced using the program Presence 2.13.47 Hines (2006) |
| Site estimates produced using the program Presence 2.13.47 Hines (2006) |
- Note: Level (U, Unit; C, Camera) indicates the scale at which the covariate data was included in the modeling process. The parameter for the covariate is either ψ (Psi) or p (Detectability). Positive or Negative, predicted relationship of covariate to occupancy or detectability (e.g., Positive implies that as covariate values increase so do values for occupancy or detectability).
- Data sources: a—National Land Cover Database 30 m resolution, b—Field current study, c—Historical reintroduction map, d—USGS National Elevation Dataset, e—Vermont Center for Geographic Information, f—https://www.weather.gov/btv/climatemaps, g—Camera.
3.3 Occupancy analyses
We conducted the occupancy analyses at several different spatial scales. We initially performed a single-season, single-species occupancy analysis at the unit level with covariates. For this analysis, we pooled detection data from the six cameras within units to produce a single detection survey for the entire unit. We also performed a hierarchical analysis similar to Pavlacky Jr. et al. (2012) using the multiscale occupancy estimation (e.g., ψ(.), θ(.), p(.)) in MARK 10.1 (White & Burnham, 1999). This approach allowed us to simultaneously examine the impact of covariates on local occupancy (θ) and detectability (p) at the camera site level while holding unit-level occupancy (ψ) constant. We assessed species interactions by including detections of other mesocarnivores during any survey period and site occupancy estimates for each mesocarnivore in the single season, single species models at the unit level. Finally, we used the two-species conditional occupancy model of Richmond et al. (2010) to produce estimates of a Species Interaction Factor (SIF; Φ) where SIF = ψA × ψBA/(ψA × (ψA × ψBA + (1 − ψA) × ψBa)), ψA = occupancy probability for species A, ψBA = occupancy probability for species B given species A is present, and ψBa = occupancy probability for species B given species A is absent. Species are considered to be acting independently when SIF = 1.
3.4 Modeling approach
Due to the considerable number of covariates (and resulting occupancy models) in the study, we adopted a sequential modeling approach in the single-season and multiscale analyses using single covariates (standardized within MARK). For each analysis, we first modeled each focal parameter of interest (ψ, θ, or p) with covariates separately while other model parameters were held constant (e.g. ψ(Coyote), p(.); Figure S1). We then selected top models in this first stage using Akaike's Information Criterion adjusted for small sample size (AICc; Burnham & Anderson, 2002). We used a more relaxed inclusion threshold (ΔAICc < 5) to select the top models for each parameter of interest to prevent the removal of covariates that may be important in combination with other covariates in more complex models (Morin et al., 2020). Covariates from the top models selected in the first stage were then included in a second modeling step. In the second modeling step (Figure S1), we combined covariates in all possible combinations (Doherty et al., 2012) from top models in the first step to produce an overall set of models that included covariates on the combined parameters (e.g., occupancy and detectability; ψ(Coyote), p(PerCC)). We based our interpretations on the single season and multiscale analyses only from models in this overall set that were within ΔAICc < 5 of the top model.
We limited the complexity of the models to avoid overfitting by including no more than three covariates (in addition to intercepts) Donovan and Hines (2007) and did not include covariates in the same model that showed evidence of collinearity (r Pearson > .50; Wilson & Schmidt, 2015). We produced model-averaged estimates for each parameter in MARK using all models in the overall set with a ΔAICc < 5. We established the relative importance of each covariate in the overall model sets by summing the Akaike weights (w) of the models (ΔAICc < 5) where each covariate had a significant effect. We considered the effect of covariates to be significant if the 95% confidence interval for their beta estimates did not include zero.
3.5 Activity patterns
We used a slightly longer period of time for independence (>1 h apart) than Kautz et al., 2021 (30 min) to assess activity patterns of mesocarnivores from camera surveys for each species of interest (Foresman & Pearson, 1999). The detection times from the camera data that were entered into the CPW database for each species were exported as radians and imported into the Overlap package in R (Ridout & Linkie, 2009) for analysis. We assessed overlap among mesocarnivores using the Coefficient of Overlap (Dhat4 estimator) from Ridout and Linkie (2009). Species that have activity patterns that completely overlap have a value of 1 and with no overlap, a value of 0. The 95% confidence intervals around the Dhat4 estimates were developed using 200 bootstrap replicates using the bootCI function in Overlap.
4 RESULTS
4.1 Unit level
We detected marten at 18 of 40 units (45%) during 3600 camera trap nights. The overall top model set for the single-season analysis included five models with a ΔAICc < 5 (Table 2). Model averaged estimates of occupancy and detectability over the five models were ψ = 0.579 (SE = 0.113, 95% CI = 0.356–0.774) and p = .609 (SE = 0.109, 95% CI = 0.387–0.793). Beta estimates for model parameters are found in Table S1. The base model (.) that assumes occupancy does not vary among units carried the most weight on occupancy (0.640) when summed across models. Distance to release site had the greatest relative importance for detectability (0.608) when summing across models and had a significant beta estimate. Other covariates on detectability that had some support were the percentage of deciduous forest (+) and the percentage of coniferous forest (−) in the unit.
| Model | AICc | ΔAICc | Weights | #par | −2log(L) |
|---|---|---|---|---|---|
| Psi(.), p(Dist+PerDec) | 106.026 | 0 | 0.352 | 4 | 96.884 |
| Psi(Dist), p(PerCC) | 106.525 | 0.499 | 0.274 | 4 | 97.382 |
| Psi(.), p(Dist+PerCC) | 107.523 | 1.497 | 0.167 | 4 | 98.380 |
| Psi(.), p(Dist+PerCon) | 107.653 | 1.627 | 0.156 | 4 | 98.510 |
| Psi(Dist), p(PerDec) | 109.865 | 3.838 | 0.052 | 4 | 100.722 |
- Note: Psi = occupancy, p = detectability, AICc and ΔAICc = values for Akaike Information Criterion adjusted for small samples and relative to top model. Weights = model weight, and −2Log(L) = log-likelihood of model, #Par = number of parameters in model.
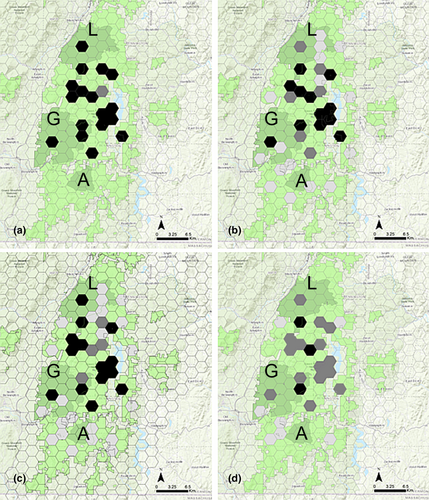
Mesocarnivores of interest were also detected in units during camera surveys including fisher (38/40 units), coyote (35/40 units), fox (28/40 units), and bobcat (10/40). Co-occurrence in units where marten were detected was greatest for fisher (17/18 units; Figure 2a) followed by fox (12/18; Figure 2b) coyote (13/18; Figure 2c), and bobcat (3/18; Figure 2d). The species interaction factor estimates (Φ) at the unit level from the species interaction models ranged from 0.563 between marten and bobcat to 0.994 between marten and fisher (Table 4). Confidence intervals for all estimates of phi (Φ) encompassed one and did not indicate any significant interactions with occupancy between marten and other mesocarnivores at the unit level in the study (Table 4).
4.2 Camera level
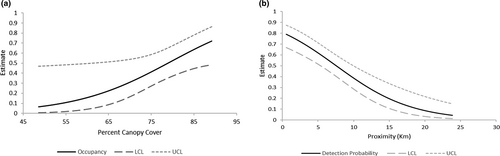
We detected marten at 93/238 cameras (39%). The analysis of local occupancy (θ) and detectability (p) from the multiscale models resulted in only a single model with a ΔAICc < 5 where covariates in the model were significant (Table 3; ψ(.), θ(PerCC), p(Dist)). Real parameter estimates from this model included ψ = 0.560 (SE = 0.099, 95% CI = 0.365–0.738), θ = 0.499 (SE = 0.066, 95% CI = 0.372–0.629), p = .389 (SE = 0.057, 95% CI = 0.283–0.507). Beta estimates for model parameters are found in Table S1. Percent canopy cover had a positive influence on local occupancy while camera level detections decreased the further cameras were located from documented release sites (Figure 3).
| Model | AICc | ΔAICc | Weights | −2Log(L) | #par |
|---|---|---|---|---|---|
| psi(.), theta(PerCC), p(Dist) | 378.589 | 0 | 0.568 | 1 | 5 |
| psi(.), theta(Vert_Struc), p(Dist) | 380.900 | 2.3115 | 0.179 | 0.315 | 5 |
| psi(.), theta(CoyOcc), p(Dist) | 381.109 | 2.5205 | 0.161 | 0.284 | 5 |
| psi(.), theta(.), p(Dist) | 382.221 | 3.6318 | 0.092 | 0.163 | 4 |
- Note: Detection data from American marten collected between 2019 and 2021 in the Green Mountain National Forest of southern Vermont.
- Psi = unit level occupancy, theta (θ) = local occupancy, p = camera level detectability, AICc and ΔAICc = values for Akaike Information Criterion adjusted for small samples and relative to top model. Weights = model weight, and −2Log(L) = log likelihood of model, #Par = number of parameters in model.
Species interaction factor estimates (Φ) at the camera site level from the species interaction models ranged from 0.968 between marten and fox to 2.125 between marten and bobcat (Table 4). Confidence intervals for all Φ estimates encompassed one and did not indicate any significant interactions with occupancy between marten and other mesocarnivores at the camera level in the study (Table 4).
| Species comparison | Level | Φ | SE | Lower | Upper |
|---|---|---|---|---|---|
| Marten-Bobcat | U | 0.563 | 0.431 | −0.281 | 1.407 |
| C | 2.125 | 0.949 | 0.265 | 3.984 | |
| Marten-Fisher | U | 0.993 | 0.042 | 0.910 | 1.078 |
| C | 1.084 | 0.075 | 0.936 | 1.231 | |
| Marten-Fox | U | 0.988 | 0.137 | 0.719 | 1.257 |
| C | 0.968 | 0.288 | 0.403 | 1.533 | |
| Marten-Coyote | U | 0.938 | 0.097 | 0.747 | 1.129 |
| C | 1.125 | 0.156 | 0.819 | 1.431 |
- Note: Estimates generated in MARK are from detection data collected between December and April during the years 2019 through 2021 in the Green Mountain National Forest of southern Vermont. Lower and Upper represent 95% confidence intervals around the Φ estimates. For each comparison, marten were assumed to be the subordinate predator. Level (U = Unit, C = Camera).
- Abbreviation: SE, standard error.
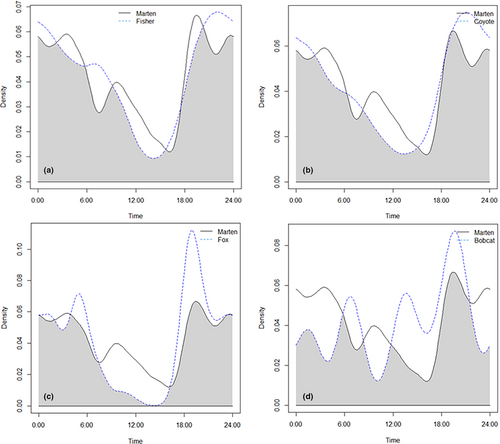
4.3 Activity
All mesocarnivores were predominantly nocturnal with peak activity occurring between early evening and midnight. Marten activity was high until approximately 04:00 (Figure 4). Overlap coefficients were generally high (Dhat4 = 0.701 to 0.929; Table 5). The largest overlap for marten with another species occurred with fisher (Dhat4 = 0.897; Table 5 and Figure 4).
| Comparison | Dhat4 | Lower CI | Upper CI |
|---|---|---|---|
| Marten-Fisher | 0.897 | 0.847 | 0.96 |
| Marten-Coyote | 0.891 | 0.849 | 1.00 |
| Marten-Fox | 0.801 | 0.737 | 0.894 |
| Marten-Bobcat | 0.715 | 0.565 | 0.883 |
| Fisher-Coyote | 0.929 | 0.907 | 1.00 |
| Fisher-Fox | 0.794 | 0.715 | 0.852 |
| Fisher-Bobcat | 0.705 | 0.554 | 0.895 |
| Coyote-Fox | 0.794 | 0.765 | 0.902 |
| Coyote-Bobcat | 0.735 | 0.605 | 0.925 |
| Fox-Bobcat | 0.701 | 0.576 | 0.899 |
- Note: Lower and upper CI = 95% confidence intervals on Dhat4 estimates. Detection data from cameras considered independent if photos were taken >1 h apart. Sample sizes for each species were marten (n = 160), fisher (n = 903), bobcat (n = 19), coyote (n = 123), and fox (n = 142).
5 DISCUSSION
We predicted that occupancy and detectability of marten at the southern edge of their distribution in Vermont would have a negative association with other mesocarnivores and those associations would be more important compared to forest stand variables and habitat variables measured in the study. We found little evidence to support these predictions; the majority of the top overall models in the various analyses did not contain covariates related to other mesocarnivore occupancy or detectability.
Distance to the nearest release site was the most important covariate to marten detectability at unit and camera levels. Given their reported dispersal distances (Johnson et al., 2009), we would have expected marten to have expanded further from the release sites in the GMNF in the 30 plus years since they were reintroduced. The survey results provide evidence that marten has been recovering in the central portion of the GMNF since the time of the reintroductions (1989–1991), but that recovery appears slow. Follow-up monitoring efforts after the reintroduction revealed that most marten dispersed within 16 km (average 6.6 km) from the release sites (Royar, 1992). We did not detect marten beyond 12 km from the release sites, and marten occupancy and detectability decreased with increasing distance from the nearest release site. Marten was also not detected during camera surveys in units further south in our study area (O'Brien et al., 2018) or at several sites further north of the study area, including another release site used during the 1989–1991 reintroduction (P. Hapeman 2018, unpublished data). A similar delayed recovery has been observed for marten in Wisconsin and has been attributed to the reliance of marten on less profitable or high-risk prey that could be impacting juvenile recruitment (Carlson et al., 2014). It is also possible that other mesocarnivores are limiting the distribution of marten by impacting juvenile survival or some other vital demographic rate (e.g., recruitment, growth; Louthan et al., 2015) that was not explicitly measured in the study, and this should be examined more fully in the future.
Local occupancy of marten within units was positively associated with the amount of canopy cover which may help limit their risk of predation (Eriksson et al., 2019). Detectability of marten was associated with higher percentages of deciduous forest in units and lower percentages of coniferous forest. The reasons for these associations are not entirely clear but it could be driven by differences in marten abundance in these two habitat types.
Interspecific competition and predation among co-occurring carnivores can play an important role in determining community structure (Schoener, 1985). Other mesocarnivores are known to negatively impact marten (Storch et al., 1990; Thompson, 1994) but we did not detect significant species interaction factors in the models. Our findings are more similar to several recent studies that did not detect significant associations between marten and other mesocarnivores (Croose et al., 2019; Evans & Mortelliti, 2022; Manlick et al., 2017).
Fisher may be important intraguild predators of marten (Hodgman et al., 1994; Raine, 1981) and their impact has been suggested as a contributing factor to the apparent failed reintroduction of marten in Vermont (Moruzzi et al., 2003). Co-occurrence patterns of marten and fisher (Zielinski et al., 2017) have been attributed to spatiotemporal segregation, differential habitat selection, and contrasting use of snow-cover characteristics (Fisher et al., 2013; Krohn et al., 1997; Pauli et al., 2022), yet these were not well-supported by the data. Fisher presence did not influence marten occupancy or detectability in the study area, and this supported our prediction. We observed a pattern of niche overlap between marten and fisher similar to other recent studies in the Great Lakes region and Maine that found substantial overlap in space and time, and neither species exhibited selection for separate habitats (Croose et al., 2019; Evans & Mortelliti, 2022; Manlick et al., 2017). Similar preferences during winter for a limited number of relatively abundant prey species could also explain the niche overlap seen in the present study between marten and fisher but to verify this, we would need to conduct additional dietary studies. Niche overlap is predicted to increase when resources are abundant (Schoener, 1982) and marten and fisher may simply synchronize nocturnal activity to their prey species (Carothers & Jaksić, 1984; Zielinski et al., 1983).
Marten may use several interacting mechanisms to reduce competition and predation. The pattern of marten detection times was multi-modal which could indicate they are timing activity when other mesocarnivores are less active (Figure 4). We also found that marten uses vigilant antipredator behaviors (e.g., upright body position and short times at cameras; Sharpe & Van Horne, 1998) that others have attributed to a larger strategy for predator avoidance (Kautz et al., 2021; Zielinski et al., 2017). Marten, like many small-bodied predators, may be forced to make a trade-off between time spent foraging and time spent performing vigilant behaviors to limit the risk of predation (Wikenros et al., 2014). This may be particularly important for marten whose activity period largely overlapped with fisher.
6 MANAGEMENT IMPLICATIONS
The marten population in southern Vermont is of considerable conservation concern given their endangered status in the state and localized presence on the GMNF (O'Brien et al., 2018) where land management is carried out for multiple uses and not specifically for marten recovery. The limited distance that marten have expanded away from past release sites suggests at least two possible and potentially related explanations relevant to management; that marten may not be abundant in southern Vermont or that mesocarnivores are limiting the distribution of marten by impacting juvenile survival or some other vital demographic rate. Future efforts to promote marten recovery should consider the potential ameliorating effects of adjusting current harvesting levels of fisher and other mesocarnivores on interspecific competition and predation of marten.
Marten may occur at low abundance in southern Vermont, but this would need to be verified with additional studies. At present, there is not enough information to verify that recovery goals have been met for the southern Vermont marten population or to consider the establishment of a regulated annual harvest. A recent genetic assessment of the southern Vermont marten population found substantially lower genetic diversity there compared to other marten populations in the Northeast (Aylward et al., 2019). Low genetic diversity is associated with small population size. Based on our distribution data, marten appears to be regionally isolated from the two closest marten populations in New Hampshire (150 km) and New York (97 km). Small, isolated populations are more susceptible to demographic stochasticity and the genetic effects of inbreeding associated with drift. Consideration should be given to the feasibility of maintaining connective corridors between marten populations in northern Vermont and neighboring states.
Finally, we recommend promoting canopy cover through uneven-aged forestry practices that have a positive association with marten presence including the maintenance of large tracts of undisturbed forest with structural complexity that promotes canopy cover. We view the parameter estimates from the models as a 3-year snapshot over the sample season. We recommend implementing a long-term monitoring program for marten using 3-year trends in marten occupancy and detectability to verify their status in the future.
AUTHOR CONTRIBUTIONS
Paul Hapeman: Conceptualization (lead); data curation (supporting); formal analysis (lead); funding acquisition (lead); investigation (supporting); methodology (lead); project administration (lead); supervision (lead); visualization (lead); writing – original draft (lead); writing – review and editing (equal). Sarah Ashbrook: Conceptualization (supporting); data curation (lead); formal analysis (supporting); funding acquisition (supporting); investigation (lead); methodology (supporting); project administration (supporting); supervision (supporting); visualization (supporting); writing – original draft (supporting); writing – review and editing (equal).
ACKNOWLEDGMENTS
We wish to thank C. Bernier and K. Royar for collaborating with us on this project and K. Crumley for her dedication to the field of collecting data. Forest stand variable data for Season 2 was collected by C. Plourde. Housing and access to many field sites were provided by S. Wixsom and the United States Forest Service. Additional thanks to P. O'Brien for his dedication to the marten project since the beginning and P. Jensen for providing us with his predictive model for American marten in the region, which guided the sampling design. D. Pavlacky provided valuable guidance on multiscale occupancy analysis. Funding for the project was provided by the A.V. Stout Fund, CCSU Foundation, and a P-R Enhancement Fund through Vermont Fish and Wildlife.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Requests for access to the data should be made to one of the authors.




