Conservation genetics of Notelaea lloydii (Oleaceae) in south-eastern Queensland, Australia
Abstract
Habitat fragmentation can increase the chance of population bottlenecks and inbreeding, and may ultimately lead to reduced fitness and local extinction. Notelaea lloydii is a native olive species endemic to Australia and listed as vulnerable due to its restricted distribution. A recent molecular systematics study has revealed there might be some geographic structuring among N. lloydii populations. Therefore, we undertook a genome-wide single nucleotide polymorphism (SNP) analysis to determine levels and patterns of genetic diversity, inbreeding and gene flow within and among N. lloydii populations in south-eastern Queensland. Furthermore, as the reproductive phase of a plant's life history has a profound influence on genetic diversity, life history reproductive traits were also studied. Our SNP analysis revealed low genetic diversity, inbreeding and significant genetic structuring even among proximate populations. Results of a flower and fruit bagging experiment in two consecutive seasons revealed that N. lloydii produced many flowers but only a few fruits survived to maturity. There were no differences in bagged and un-bagged flowering and fruiting rates, and therefore, we conclude that the high fruit abortion rate was probably due to inbreeding depression and/or suboptimal conditions, rather than pollinator availability and insect attack. Overall, results of this study indicate that the populations of N. lloydii are small, inbred and genetically isolated and represent unique management units that require local conservation management due to ongoing threats associated with urbanisation.
1 INTRODUCTION
Habitat loss and fragmentation are globally significant causes of biodiversity loss (Fahrig, 2003; Krauss et al., 2010; Wilson et al., 2016). As a result, previously widespread and connected plant populations can become small and isolated (Burrough et al., 2018; Field et al., 2008; Hobbs & Yates, 2003; Young et al., 1996). While conservation strategies for threatened plant species generally rely on protection of habitats, maintenance of genetic diversity and gene flow is critical for long-term persistence and should be factored into species conservation plans (Bezemer et al., 2019; Ottewell et al., 2016; Ralls et al., 2018). To maintain sufficient gene flow to prevent loss of genetic variation and inbreeding, plants rely on their pollination and seed dispersal mechanisms. Fragmentation and restricted population size can increase the chance of inbreeding (Leimu et al., 2010; Lowe et al., 2015) and the effects of genetic drift, which in turn can increase homozygosity and expression of deleterious alleles in a population, leading to reduced fitness and population bottlenecks (Breed et al., 2012). Habitat fragmentation can also alter the relative abundance of cross-compatible species within an area, which could increase the potential for inter-specific gene flow that results in the production of hybrids (Butcher et al., 2005; Field et al., 2008; Keppel et al., 2011).
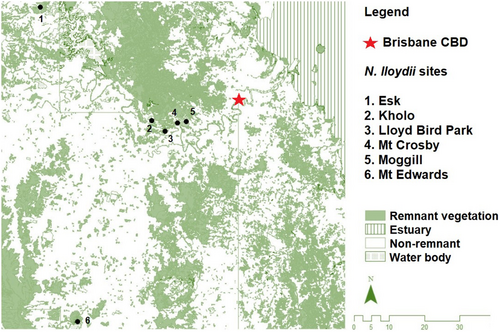
Notelaea lloydii (Guymer) is an endemic native olive species, currently listed as a threatened species due to its small population and restricted geographic distribution in south-eastern Queensland (SE-QLD), Australia (Appendix 1: Figure A1), a region subject to ongoing urban development (Approved conservation advice for Notelaea lloydii, 2008). It was first described as a new species by Guymer (1987), and at that time, it was known only from two locations that were 60 km apart. Several other disjunct populations have since been identified within an area of approximately 3700 km2 in SE-QLD (Approved conservation advice for Notelaea lloydii, 2008; Halford, 1998), resulting in N. lloydii being listed as vulnerable (Environment Protection and Biodiversity Conservation Act, 1999). A recent molecular systematics study of the genus Notelaea has revealed that another Notelaea species in the vicinity, N. ipsviciensis W. K. Harris, is a hybrid between N. lloydii and N. ovata (Manawaduge et al., 2022). Molecular data have revealed that hybrid backcrossing with N. lloydii is occurring at the site where they are sympatric. Hybridisation can reduce the viability of small populations through demographic and genetic swamping (Ellstrand & Elam, 1993; Ellstrand & Rieseberg, 2016) and may ultimately influence the time to local extinction (Wolf et al., 2001). Furthermore, this study did not find reciprocal monophyly of N. lloydii and N. microcarpa, i.e., two clades of N. lloydii were nested within different parts of the N. microcarpa clade, but some phylogeographic structure was reported (Manawaduge et al., 2022). Moreover, N. lloydii populations are small, each with less than 30 individuals, and limited recruitment has been observed (Approved conservation advice for Notelaea lloydii, 2008); therefore, further population genetic analysis is required.
In order to investigate the genetic diversity, inbreeding and spatial genetic structure among N. lloydii populations, we used genome-wide, single nucleotide polymorphisms (SNPs), produced using Diversity Array Technology sequencing (DArTseq) in the current study. This approach has been successfully used with minimal DNA sample requirements (Jaccoud et al., 2001) in numerous plant studies over the past decade (Pailles et al., 2017; Tang et al., 2015; Yang et al., 2016). We also investigated the genetic relatedness of N. lloydii to N. microcarpa. Furthermore, to understand the population genetic consequences of the rarity of N. lloydii, we undertook a comparative genetic analysis with one of other common species in the genus, N. punctata (Appendix 1: Figure A1), which occurs in sympatry with N. lloydii at some locations. Finally, because the reproductive phase (pollination, seed production, dispersal and survival) of a plant's life history can influence genetic diversity (Brown et al., 2003), we also conducted a two-year study of life history reproductive traits of N. lloydii.
2 MATERIALS AND METHODS
2.1 Sample collection and genotyping
A total of 123 individuals of Notelaea from five species were sampled in this study (Table 1 and Appendix 1: Table A1). Notelaea lloydii was sampled from all known populations throughout the range of the species and the number of accessions from each population varied (from 1 to 17) depending on the population size and site accessibility. Samples of N. microcarpa were also obtained throughout its range to assess genetic similarity between N. lloydii and N. microcarpa. Notelaea punctata samples were collected from locations that are sympatric or nearby to N. lloydii populations for comparative analysis of genetic diversity. Fresh leaf material was collected from plants located in SE-QLD where site access was granted and permits obtained (WIF418593017 and WITK18593017). Leaves were dried immediately in silica gel, and where fresh samples could not be obtained, dried leaf material from herbarium specimens was used.
| Species | Sample location | Population abbreviation | Number of samples |
|---|---|---|---|
| N. lloydii | Esk | LL_Esk | 17 |
| Mt Crosby | LL_MtCr | 17 | |
| Lloyd Bird Park | LL_LB | 16 | |
| Moggill | LL_Moggill | 16 | |
| Kholo | LL_Kholo | 5 | |
| Mt Edwards | LL_MtEd | 4 | |
| Ebbw Vale | LL_Ebbw | 1 | |
| N. microcarpa | Locker Valley | MCR_LV | 8 |
| Central highlands | MCR_Other | 2 | |
| Chinchilla | MCR_Other | 2 | |
| Ipswich | MCR_Other | 2 | |
| Tablelands | MCR_Other | 2 | |
| Balonne | MCR_Other | 1 | |
| Charters Towers | MCR_Other | 1 | |
| Etheridge | MCR_Other | 1 | |
| Flinders | MCR_Other | 1 | |
| Goondiwindi | MCR_Other | 1 | |
| Murweh | MCR_Other | 1 | |
| North Burnett | MCR_Other | 1 | |
| Somerset | MCR_Other | 1 | |
| Toowoomba | MCR_Other | 1 | |
| New South Wales | MCR_NSW | 2 | |
| N. punctata | Lloyd Bird Park | PNT_LB | 10 |
| Samford | PNT_SERF | 10 |
Genomic DNA was extracted as per methods described in Manawaduge et al. (2022), and DNA samples were sent to Diversity Arrays Technology Pty Ltd, Canberra, for DarTseq analysis. SNP callings were identified in each short sequence fragment (~20 bp) through proprietary DarT analytical pipelines as described in Tomkowiak et al. (2022), and the results of the SNP calls were returned as a matrix. DarTseq results were viewed and analysed using the DarTR package (Gruber et al., 2018) in the R platform (R Core Team, 2013). A single short sequence fragment with one SNP was considered as a locus, and the loci with call rates below 90% and average reproducibility below 95% were removed so that only high-quality informative data were retained.
2.2 Spatial genetic structure and gene flow among N. lloydii populations
A principal coordinate analysis (PoA) was performed on SNP data using DarTR to visualise the genetic relationship between N. lloydii and N. microcarpa. A phylogenetic tree was also constructed using the maximum likelihood method as described in Manawaduge et al. (2022). Pairwise Wright's Fst between N. lloydii populations were estimated using StAMPP (Pembleton et al., 2013) in R. Nei's pairwise Fst (Nei, 1987) and D genetic distance (Nei, 1972) were also estimated using the hierfstat (Goudet, 2005) and StAMPP (Pembleton et al., 2013) packages in R, respectively. A Mantel test (Mantel, 1967) was performed using ade4 (Dray & Dufour, 2007) implemented in R to test the correlation between pairwise Fst and geographic distance. The LEA (Frichot & François, 2015) R package was used to estimate individual admixture coefficients by implementing a sparse, non-negative matrix factorisation algorithm (sNMF) to estimate ancestry coefficients from large genotypic matrices and to evaluate the number of ancestral populations (K). The entire data set was run for K = 1 to 10, with 100 repetitions for each K value. The value of K that corresponded with the lowest cross-entropy criterion was selected as the value that best explained the results (Frichot & François, 2015).
CIRCUITSCAPE v 4.0.5 (McRae, 2006; McRae et al., 2008) was used to examine whether gene flow was a function of isolation by distance (IBD) or landscape resistance (IBR) associated with the cover of native vegetation (Figure 1) because native olive drupes are primarily dispersed by frugivorous vertebrates associated with forest vegetation. A remnant vegetation resistance surface (RS) was developed by transforming the Remnant Vegetation Cover (2017) vector layer (Queensland Spatial Catalogue: http://qldspatial.information.qld.gov.au/catalogue/) into a binary raster denoting the presence/absence of remnant vegetation at a cell size of 100 m. The RS was constrained to a 1 km buffer beyond the furthest sampling site in each direction. RS optimisation was carried out in R using ResistanceGA v4.0-14 (Peterman, 2018). ResistanceGA uses a genetic algorithm (Scrucca, 2013) and linear mixed effects model with maximum likelihood population effects (MLPE; Clarke et al., 2002) implemented in lme4 (Bates et al., 2014) in R. Linear mixed effects models fitted with MLPE account for non-independence among pairwise data (Clarke et al., 2002) and has been shown to perform better than alternative modelling techniques in landscape genetics model selection (Shirk et al., 2018). Optimisation of the remnant vegetation surface was performed using pairwise Fst as the response variable, and pairwise resistance distances were calculated in CIRCUITSCAPE using an eight-neighbour connection scheme as the predictor variable. Two additional models, a distance model where every RS cell is set to a resistance of 1 and an intercept-only model without any landscape structure, were also incorporated. Optimisation was performed twice to confirm convergence and the model performance was ranked based on Akaike Information Criterion (AIC), AIC corrected for small sample sizes (AICc) and for delta AICc (ΔAICc).
2.3 Comparative analysis of genetic diversity and relatedness of N. lloydii and N. punctata
Genetic diversity estimates were calculated from the SNP data for populations with a minimum of four individuals (six N. lloydii and two N. punctata populations). In SE-QLD, only scattered trees of N. microcarpa were located and genotyped, and therefore, these individuals were not included in the genetic diversity analysis. Observed heterozygosity (Ho), expected heterozygosity (He) and inbreeding coefficient (FIS) for each locus within each population were estimated using the hierfstat (Goudet, 2005) in R, and population comparisons between these parameters using ANOVA were conducted in IBM SPSS Statistics for Windows, version 26.0. Pairwise relatedness (kinship coefficients) between individuals within a population and among populations were estimated using the maximum likelihood estimation method in SNPRelate (Zheng et al., 2012) implemented in R.
2.4 Analysis of reproductive life history traits
Flowering and fruiting of N. lloydii was examined over two consecutive years (2017–2019) at two sites in SE-QLD (Lloyd Bird Park: LB-Park and Moggill Conservation Park: Moggill). The number of florets and/or fruits on five mature trees at each site was recorded once every fortnight between October and March. Since Notelaea produce numerous axillary inflorescences with many florets, counts were made for only 10 small terminal branches on each tree. At the beginning of each flowering season, five branches were labelled and bagged with see-through organza bags (225 × 165 mm2) before floral buds opened, while five other branches were labelled and left un-bagged. For each species, the mean number of flowers per branch, flower to fruit conversion rates and fruit survival rates over the sampling period were calculated. The percentage of developed fruit loss was assumed to be due to fruit removal by frugivores. Therefore, the difference in fallen fruits between bagged and un-bagged represents the number of fruits removed by frugivores, and percentage of fruit removal by frugivores was calculated using the equation; % fruit removal by frugivores = % fruit loss in un-bagged branches – % fruit loss in bagged branches. Where assumptions of normality were violated, Kruskal-Wallis non-parametric tests followed by Dunn's multiple comparison post-hoc tests were conducted using IBM SPSS Statistics for Windows, version 26.
The local distribution of N. lloydii (clumped, random or uniform) was assessed using the nearest-neighbour method (Krebs, 1989) to provide insight into germination pattern. Since no boundary strip for the study area was considered and the sample size was small (n < 100), the Donnelly (1978) modification of the Clark and Evans (1954) test was used to calculate the standard normal deviate (z) in order to check the significance of deviation from randomness (Krebs, 1989).
3 RESULTS
3.1 Spatial genetic structure and gene flow among N. lloydii populations
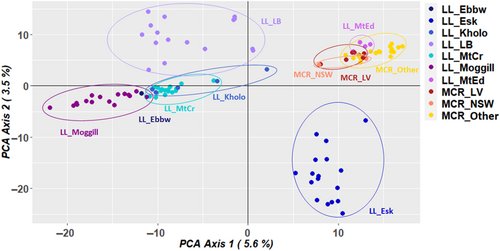
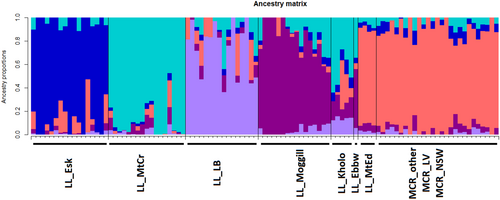
A PCoA was undertaken on the SNP data set containing 63,164 loci (call rate above 90%, average reproducibility above 95% and 3.7% missing data) for 103 individuals of N. lloydii and N. microcarpa (Figure 2). This included N. lloydii sampled from all known locations, N. microcarpa sampled from nearby locations in the Lockyer Valley region in Queensland (MCR_LV) and herbarium specimens to represent the wider distribution (MCR_other: from more distant locations in Queensland and MCR_NSW: from New South Wales). The first and second axes in the PCoA accounted for 5.6% and 3.6% of variation, respectively (Figure 2). All the N. microcarpa accessions cluster together indicating that there is little genetic variation among individuals, with overlap of samples from New South Wales and the Lockyer Valley in Queensland. Notelaea lloydii samples from Mt Edwards in Queensland also clearly overlap with N. microcarpa, indicating their close genetic affinity. Distinct clusters of N. lloydii samples from Esk and LB-Park were also evident in the PCoA. Our phylogenetic tree also supports these groupings (Appendix 1: Figure A2). The admixture analysis of these data supported K = 5 (i.e., 5 groups) as the values corresponding with the lowest cross-entropy from the sNMF algorithm in LEA (Figure 3). Almost all the samples of N. microcarpa across the eastern coast and a few N. lloydii cluster together, whereas the remaining N. lloydii samples exhibit some level of structure corresponding to sample location.
Pairwise Wright's Fst values and geographical distances are given in Table 2. Fst values revealed significant genetic differentiation between all N. lloydii populations. Mount Edwards and Esk populations of N. lloydii were most differentiated from all other populations (Table 2). A highly significant pattern of isolation-by-distance among N. lloydii populations was found (R = .8329, p = .0051). A comparison of Fst between sympatric populations of N. lloydii and N. punctata (at LB-Park) revealed higher Fst values for N. lloydii (0.1379) than N. punctata (0.0592, Appendix 1: Table A2). Among the three landscape models tested, pairwise resistance distance and Fst showed strongest support based on ΔAICc for the distance model (Table 3). The remnant vegetation RS showed the least support, behind the intercept-only model of no landscape structure.
| LL_Esk | LL_MtCr | LL_LB | LL_Moggill | LL_Kholo | LL_MtEd | |
|---|---|---|---|---|---|---|
| LL_Esk | – | 50.82 | 49.36 | 52.38 | 44.66 | 88.49 |
| LL_MtCr | 0.0922 | – | 4.19 | 2.46 | 7.47 | 62.5 |
| LL_LB | 0.1059 | 0.0720 | – | 6.57 | 4.51 | 59.2 |
| LL_Moggill | 0.1212 | 0.0686 | 0.0934 | – | 9.78 | 64.25 |
| LL_Kholo | 0.0874 | 0.0439 | 0.0663 | 0.0763 | – | 60.35 |
| LL_MtEd | 0.1277 | 0.1300 | 0.1379 | 0.1686 | 0.1295 | – |
| Surface | AIC | AICc | ΔAICc | Resistance | Parameters (k) |
|---|---|---|---|---|---|
| Distance | −72.812 | −69.466 | 0.000 | 2 | |
| Null | −64.711 | −63.711 | −5.756 | 1 | |
| Remnant vegetation | −73.466 | −61.466 | −8.000 | A-1; P-2.5 | 3 |
3.2 Genetic diversity and relatedness within and between N. lloydii and N. punctata populations
Genetic diversity estimates were calculated for N. lloydii and N. punctata populations that were represented by at least four individuals. The final data set of N. lloydii consisted of 75 individuals from six populations and 54,757 loci, while N. punctata consisted of 20 individuals and 56,252 loci. Each data set contained 2.2% and 1.8% missing data (following removal of low quality and monomorphic loci), respectively. Mean genetic diversity measures (Ho, He and Fis) for each N. lloydii population clearly indicate that all populations exhibit significant (p < .001) inbreeding (Table 4). In every population, a significant (p < .001) homozygote excess was found (Appendix 1: Table A3) and Ho ranged from 0.0983 to 0.1242. Fis values were positive and ranged from 0.1261 to 0.1857. Average genetic diversity measures (Ho, He and Fis) for each N. punctata population revealed no evidence of inbreeding (Table 4). In every population, a significant (p < .001) heterozygote excess was found (Appendix 1: Table A3b) and Ho ranged from 0.2508 to 0.22525. Fis values were negative and ranged from −0.0557 to −0.0449.
| Species | Population | H o | H e | F is |
|---|---|---|---|---|
| N. lloydii | LL_Esk | 0.1141 (0.0006) | 0.1471 (0.0007) | 0.1857 (0.0019) |
| LL_Kholo | 0.1142 (0.0008) | 0.1462 (0.0009) | 0.1517 (0.0029) | |
| LL_LB | 0.1242 (0.0007) | 0.1467 (0.0007) | 0.1261 (0.0018) | |
| LL_Moggill | 0.1160 (0.0007) | 0.1394 (0.0007) | 0.1350 (0.0019) | |
| LL_MtCr | 0.1197 (0.0007) | 0.1494 (0.0007) | 0.1590 (0.0017) | |
| LL_MtEd | 0.0983 (0.0008) | 0.1325 (0.0009) | 0.1708 (0.0036) | |
| All | 0.1144 (0.0003) | 0.1537 (0.0009) | 0.1436 (0.0003) | |
| N. punctata | PNT_LB | 0.2508 (0.0010) | 0.2267 (0.0008) | −0.0557 (0.0017) |
| PNT_SERF | 0.2525 (0.0010) | 0.2305 (0.0007) | −0.0449 (0.0017) | |
| All | 0.2516 (0.0007) | 0.2286 (0.0005) | −0.0502 (0.0012) |
Pairwise relatedness between individuals revealed that average kinship coefficients (k) within each N. lloydii population were higher than within N. punctata populations (Table 5). The pairwise k of individuals within populations was higher than between populations. The highest average pairwise k within a location for N. lloydii was found at Mt Edwards (Table 5), where the two most related N. lloydii individuals were also identified with a kinship coefficient of 0.254 (Appendix 1: Table A4a–f).
| Species | Population | N. lloydii | N. punctata | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Esk | MtCr | LB | Moggill | Kholo | MtEd | LB | SERF | ||
| N. lloydii | Esk | 0.0547 | – | – | |||||
| MtCr | 0.0002 | 0.0306 | – | – | |||||
| LB | 0.0002 | 0.0013 | 0.0472 | – | – | ||||
| Moggill | 0.0000 | 0.0033 | 0.0010 | 0.0566 | – | – | |||
| Kholo | 0.0013 | 0.0072 | 0.0042 | 0.0045 | 0.0366 | – | – | ||
| MtEd | 0.0075 | 0.0015 | 0.0041 | 0.0005 | 0.0067 | 0.1149 | – | – | |
| N. punctata | LB | – | – | – | – | – | – | 0.0231 | |
| SERF | – | – | – | – | – | – | 0.0001 | 0.0214 | |
3.3 Reproductive life history trait analysis
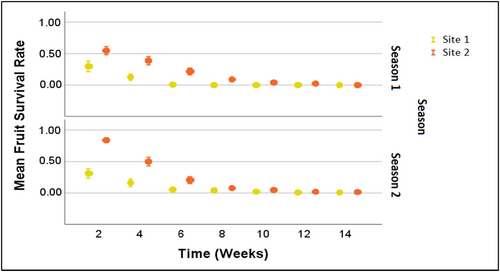
A significant difference in the number of N. lloydii flowers produced in the two seasons (season 1—October 2017 to March 2018; season 2—October 2018 to March 2019) was found (Table 6). However, the flower to fruit conversion ratio for un-bagged branches was not significantly different between the two seasons (p > .05). Not all immature fruits developed into mature fruits bearing seeds. Most of the small fruits at the initial stages remained small or excised (Figure 4). The mean survival rates over time were also not significantly different between the seasons, and the same pattern was observed between the two sites (Appendix 1: Figure A3). Nevertheless, the percentage of developed fruits was significantly higher in season 2, compared to season 1, in both bagged and un-bagged branches (Table 6). While the percentage of fruit removal was lower in season 2 (3.7%) than in season 1 (26.0%), the number of fruits removed in season 2 was higher because the total number of developed fruits was higher than in season 1. Mean rainfall and temperature did not significantly differ between the two seasons (Appendix 1: Table A5). However, a comparison during the first month of the two seasons revealed that mean rainfall and mean maximum temperature were significantly different (Appendix 1: Table A5).
| Branch type | Group | Average | SD | p-Value | |
|---|---|---|---|---|---|
| The average number of florets per branch | Bagged | Season 1 | 64.20 | 50.99 | .0330 |
| Season 2 | 46.38 | 28.22 | |||
| Un-bagged | Season 1 | 56.70 | 45.13 | .0043 | |
| Season 2 | 36.46 | 18.91 | |||
| The average flower to fruit conversion rates | Bagged | Season 1 | 0.77 | 0.29 | .0001 |
| Season 2 | 0.51 | 0.34 | |||
| Un-bagged | Season 1 | 0.78 | 1.38 | .0960 | |
| Season 2 | 0.44 | 0.35 | |||
| The percentage of the developed fruits from the initial fruits | Bagged | Season 1 | 1.32 | 2.64 | .0017 |
| Season 2 | 8.02 | 14.09 | |||
| Un-bagged | Season 1 | 0.96 | 2.59 | .0319 | |
| Season 2 | 4.79 | 11.35 |

During season 1, less than 1% of the initial fruits developed into mature fruits on un-bagged branches (Table 7). Even though slight variations were observed between bagged and unbagged branches, the difference was not significant (Appendix 1: Table A6). Thus, to check the exact fruit removal rates, only developed fruits were considered as they were the ones producing a fleshy mesocarp to attract seed dispersal agents and were viable. As shown in Table 7, the percentage loss of developed fruits was equal or higher for un-bagged compared with bagged branches and this difference was due to fruit removal by frugivores.
| Season | Site | % fruit loss | % removal by frugivores | |
|---|---|---|---|---|
| Bagged | Un-bagged | |||
| Season 1 | Site 1 | 100.0 | 100.0 | 0.0 |
| Site 2 | 39.6 | 91.7 | 52.1 | |
| Season 2 | Site 1 | 92.6 | 100.0 | 7.7 |
| Site 2 | 100.0 | 100.0 | 0.0 | |
The nearest-neighbour analysis in Moggill and LB-Park revealed that at both sites, N. lloydii exhibited a clumped spatial distribution: the index of aggregation (R) was less than 1 (R = .437 and .592 for Moggill and LB-Park, respectively) and the absolute value for z was greater than 1.96 (|z| = 4.673 and 5.100 for Moggill and LB-Park, respectively).
4 DISCUSSION
4.1 Spatial genetic structure and gene flow
Small, fragmented plant populations may exhibit genetic differentiation facilitated by geographical and ecological factors, plant life history and reproductive traits and human impacts (Bezemer et al., 2019; Duminil et al., 2009; Huang et al., 2019; Loveless & Hamrick, 1984). In this study, all known populations of N. lloydii were sampled and distinct geographic clustering was found. In contrast, all the N. microcarpa samples clustered together with overlap of samples from New South Wales and the Lockyer Valley in Queensland, indicating little genetic differentiation. A significant finding was that N. lloydii samples from Mt Edwards in Queensland clearly overlapped with N. microcarpa, indicating close genetic affinity and suggesting that further investigation is required to determine whether they constitute two separate species.
Our analysis of genetic structure indicated moderate (Fst = 0.05–0.15) to high (Fst = 0.15–0.25) genetic differentiation and restricted gene flow between N. lloydii populations separated by less than seven kilometres. Furthermore, the population genetic structure of N. lloydii exhibited a pattern of isolation by distance rather than landscape resistance associated with remnant vegetation cover. Isolation by distance is often found in species with low dispersal ability combined with habitat specificity (Cipollini et al., 2017). While N. lloydii produces small drupes (Guymer, 1987) which may be easily dispersed long distances by frugivores, juvenile plants are rarely encountered, thus suggesting that recruitment by seedling germination is low and may be habitat-specific. Furthermore, population structure can be influenced by plant life-history traits such as the mating system, with self-compatible species likely to experience higher population differentiation via inbreeding (Cipollini et al., 2017; Foxe et al., 2010; St Onge et al., 2011). Interestingly, our surveys of flowering indicate that N. lloydii is self-compatible, but that fruit survival is low. In addition, founder effects at the time of colonisation can also lead to pronounced population structure (Loveless & Hamrick, 1984). Owing to their life history, annual plants typically experience strong founder effects, but high levels of gene flow ameliorate these effects. However, in woody perennials, recovery is slow due to their long-life cycle (Austerlitz et al., 2000). In this instance, founder effects may result in reduced genetic diversity and strong population differentiation, and this may be the case for N. lloydii.
4.2 Genetic diversity and relatedness
The genetic diversity of a given population could vary depending on the plant's life-history characteristics (Hamrick & Godt, 1996), and determining whether a specific level of genetic diversity is low or high can be challenging. Complicating matters further, genetic diversity measures have been calculated using various molecular markers (SSR, ALFP, SNP etc.), making the task more complex. Nevertheless, comparisons of genetic diversity estimated using similar molecular markers (e.g., SNPs), between closely related species, can provide valuable insight. In this study, the genetic diversity (i.e., expected heterozygosity) of the populations of both Notelaea species ranged from 0.13 to 0.23, which was relatively lower than that observed for other Oleaceae species with similar life history traits. Belaj et al. (2012) used SNPs to examine the diversity conserved in the World Olive Germplasm Bank (WOGB) located in Spain and found high genetic diversity (He = 0.442) for Olea europaea L. (Oleaceae), while Lee et al. (2022) conducted a population genomic study of an endangered shrub, Abeliophyllum distichum Nakai (Oleaceae), endemic to South Korea, and reported a mean expected heterozygosity of 0.319, using SNPs makers. In our study, we restricted our sampling of N. punctata populations to where they occurred in sympatry with N. lloydii in SE-QLD because it is recommended in studies of rarity that both rare and common species should be sampled from the same geographical area (Gibson et al., 2008). However, as a result it is possible that the relatively low genetic diversity we observed for N. punctata could be the result of inadequate sampling, as this species has a widespread distribution.
Many studies have shown that rare and geographically restricted species tend to have lower genetic diversity than common, widespread congeners (Cole, 2003; Gibson et al., 2008; Gitzendanner & Soltis, 2000; Hamrick & Godt, 1996). Similarly, we found significantly lower genetic diversity within and among N. lloydii populations compared with the common and widespread (throughout SE-QLD) N. punctata. All populations of N. lloydii exhibited lower levels of observed heterozygosity than expected heterozygosity indicating higher inbreeding. The opposite pattern was found for N. punctata populations; they exhibited significant heterozygosity excess and no evidence of inbreeding. Inbreeding in N. lloydii populations may be the result of small population size and restricted gene flow due to isolation by distance. However, the comparative analysis of genetic diversity between sympatric N. lloydii and N. punctata populations suggests that the reproductive biology of the two species may be different and that mechanisms linked to successful founder events may be important determinants of genetic diversity and inbreeding.
Pairwise kinship coefficients between individuals can be used to determine the degree of relatedness within and between populations and to detect founder effects during colonisation. Biparental inbreeding is known to be an unavoidable consequence in small, isolated populations in which individuals are clustered together (Sweigart et al., 1999). However, in each N. lloydii population, only a few individuals showed a half-sib relationship, and the mean pairwise kinship coefficient was low, indicating that most individuals were unrelated. In general, pairwise kinship coefficients of individuals within a population were greater than between individuals of different populations, indicating some degree of founder effect, which could explain the observed structure and low genetic diversity.
Analysis of the distribution of individual N. lloydii plants at two sites revealed a clumping pattern of plants. In these sites, the individuals appeared to be of similar age, and only a few juvenile plants were found. Also, no germination was observed during preliminary seed germination tests (data not shown), and hence, seed germination and seedling establishment might be limited for N. lloydii. Therefore, the clumped distribution could be due to vegetative regeneration/propagation from lignotubers. However, if this was the cause of clumping, individual plants would be expected to have an identical genetic constitution, which was not the case, as very low genetic relatedness was generally found. This indicates that clumps of N. lloydii most likely arise by germination from a frugivore depositing seeds in a localised area. While some lignotubers were noted in this study (personal observation), these resulted mainly from the destruction of the above-ground parts, usually by fire.
Low genetic diversity and inbreeding in small populations can lead to inbreeding depression (Ellstrand & Elam, 1993; Keller & Waller, 2002) which affects many different components of fitness in plants, such as plant size and growth, resistance to stress, seed yield, germination rate etc. (Charlesworth & Charlesworth, 2017; Frankham et al., 2002; Husband & Schemske, 1996; Keller & Waller, 2002; Naito et al., 2005). Even though theoretical models and empirical observations suggest that the magnitude of inbreeding depression is lower in habitually selfing plants because deleterious recessive alleles are expressed and purged through selection, some highly inbred species can maintain considerable levels of inbreeding depression (Husband & Schemske, 1996; Naito et al., 2005). Given the high level of inbreeding detected among proximate populations of N. lloydii, it is critical to evaluate whether inbreeding depression is impacting on the reproductive life history of the species, so that appropriate conservation measures can be developed.
4.3 Reproductive life history trait analysis
Notelaea lloydii produced flowers and fruits in two consecutive seasons but despite having many flowers, only a few N. lloydii fruits survived to maturity. Two to three days after full opening, some florets were naturally excised from the pedicel, while for most florets the petals wilted and fell off, but the enlarged ovaries remained attached to the pedicels. While these enlarged ovaries indicate early-stage fruit development, surprisingly only about 1% developed into large, dark purple ripened fruits. Most of these enlarged ovaries/immature fruits were either excised 3–4 weeks later or remained as is for the duration of the season, raising doubts as to whether they were successfully pollinated and fertilised or had undergone selective fruit/embryo abortion.
Plant reproductive success is a function of both intrinsic and extrinsic biotic and abiotic factors (Abdala-Roberts et al., 2014), and in our study, local weather conditions clearly impacted the reproductive output of N. lloydii. The percentage of developed fruits was significantly higher in the second season (about 5% on un-bagged branches during Sep 2018—Mar 2019) relative to the first season (about 1% on un-bagged branches during Sep 2017—Mar 2018). Mean rainfall and temperature during the first month of the flowering season (September) was significantly different between seasons—it was dry and hot in 2017 but comparatively wet and cool in 2018. However, regardless of the improved weather conditions in the second season, the percentage of developed fruits was only 5%, indicating high fruit abortion. Early high fruit abortion rates can be due to a range of reasons including lack of pollinators, insect attack or inbreeding depression (Simiqueli et al., 2018). However, in our study the average number of florets per branch, flower to fruit conversion rates and the percentage of developed fruits were not significantly different between bagged and un-bagged branches. Therefore, the impact of pollinator availability and insect attack on N. lloydii was negligible and the high fruit abortion rate may be due to inbreeding depression and/or suboptimal conditions.
Interestingly, regardless of the high number of initial florets, some N. lloydii plants did not produce any mature fruit, indicating that these individuals may undergo high selective fruit abortion. Furthermore, no germination was observed during preliminary seed germination tests (data not shown), and only a few juvenile plants were found at each study site (personal observation). It is possible that certain environmental and/or climatic conditions are required for mass flowering, fruiting and seed germination in this species. For some plants, flowering and seed production can be highly variable between years and/or synchronising within years among individuals within a population, which is known as masting (Koenig et al., 2015). Future field observations are required to identify such processes, if occurring, and any significant changes in the long-term reproductive success of N. lloydii in SE-QLD.
4.4 Conservation implications
The results of our recent molecular systematics study indicate that N. lloydii and N. microcarpa do not show reciprocal monophyly (Manawaduge et al., 2022), but they do exhibit distinguishing growth forms and leaf morphologies (Guymer, 1987). Because leaf size and shape are functionally significant, adaptive traits (Nicotra et al., 2011; Read et al., 2014), even if N. microcarpa and N. lloydii constitute a single species, populations with different leaf form may be worthy of conservation. However, conservation policies do not encourage the listing of infraspecific ranks such as forms, morphs, or subvarieties (IUCN, 2012) under threat categories. It may be more appropriate to apply the concept of management units (Moritz, 1994) for populations that do not show reciprocal monophyly yet exhibit low levels of gene flow and are functionally independent. Results of this study indicate that the populations of N. lloydii are small, inbred and genetically isolated, placing them at risk of localised extinction as a result of stochastic events (genetic, demographic, environmental). It is particularly concerning that two proximate populations (approximately 6.5 km apart) located in protected areas (Moggill and Lloyd Bird Park) and connected by remnant vegetation, exhibit significant, moderate genetic structure. While isolated plants exist outside of protected areas, ongoing habitat disturbance, loss and fragmentation is a major threat in this rapidly urbanising region. Our results indicate that SE-QLD populations represent unique management units that require local conservation management.
5 CONCLUSIONS
This study has identified significant genetic structure and low genetic diversity in the populations of N. lloydii. We conclude that founder effects are the most likely explanation for the low genetic diversity and significant genetic structure observed in SE-QLD and unlike other woody perennials (Austerlitz et al., 2000), N. lloydii seems unsuccessful in maintaining adequate gene flow to overcome such effects. At this stage, it is not clear if such failure is due to lack of pollination and/or seed dispersal. Furthermore, inbreeding depression in both early and late reproductive stages may be occurring. Given ongoing threats associated with urbanisation, we recommend conservation management of SE-QLD populations to prevent localised extinction. We also recommend further investigation of the population genetics of N. lloydii and N. microcarpa across their complete geographical range, combined with ecological studies to identify any functional or life history differences, and to better understand long-term reproductive output.
AUTHOR CONTRIBUTIONS
Chapa G. Manawaduge: Conceptualization (equal); data curation (lead); formal analysis (lead); funding acquisition (equal); investigation (equal); methodology (equal); writing – original draft (lead). James Ryan: Formal analysis (equal); writing – original draft (supporting). Matthew J. Phillips: Conceptualization (equal); investigation (equal); methodology (equal); supervision (equal); writing – review and editing (equal). Susan Fuller: Conceptualization (equal); funding acquisition (equal); investigation (equal); supervision (lead); writing – review and editing (equal).
ACKNOWLEDGEMENTS
We would like to acknowledge Queensland University of Technology and the Royal Society of Queensland Research Fund for providing financial support for this project. We would like to thank the Queensland Government and Brisbane, Ipswich and Logan City Councils for granting permission to collect specimens in their reserves. We are grateful to Prof. Peter Prentis and A/Prof. David Hurwood for their insightful comments on this research and Ms Briana Holgate for her kind assistance in mapping.
APPENDIX 1

| Species | Sample ID | Location/population |
|---|---|---|
| N. lloydii | N_lloydii_01 | Esk |
| N_lloydii_02 | Esk | |
| N_lloydii_03 | Esk | |
| N_lloydii_05 | Esk | |
| N_lloydii_06 | Esk | |
| N_lloydii_07 | Esk | |
| N_lloydii_08 | Esk | |
| N_lloydii_09 | Esk | |
| N_lloydii_10 | Esk | |
| N_lloydii_11 | Esk | |
| N_lloydii_12 | Esk | |
| N_lloydii_13 | Esk | |
| N_lloydii_18 | Esk | |
| N_lloydii_19 | Esk | |
| N_lloydii_20 | Esk | |
| N_lloydii_21 | Esk | |
| N_lloydii_22 | Esk | |
| N_lloydii_23 | Moggill | |
| N_lloydii_24 | Moggill | |
| N_lloydii_25 | Moggill | |
| N_lloydii_27 | Moggill | |
| N_lloydii_28 | Moggill | |
| N_lloydii_29 | Moggill | |
| N_lloydii_30 | Moggill | |
| N_lloydii_31 | Moggill | |
| N_lloydii_32 | Moggill | |
| N_lloydii_33 | Moggill | |
| N_lloydii_34 | Moggill | |
| N_lloydii_35 | Moggill | |
| N_lloydii_36 | Moggill | |
| N_lloydii_45 | Moggill | |
| N_lloydii_46 | Moggill | |
| N_lloydii_47 | Moggill | |
| N_lloydii_50 | Lloyd Bird Park | |
| N_lloydii_51 | Lloyd Bird Park | |
| N_lloydii_52 | Lloyd Bird Park | |
| N_lloydii_53 | Lloyd Bird Park | |
| N_lloydii_54 | Lloyd Bird Park | |
| N_lloydii_57 | Lloyd Bird Park | |
| N_lloydii_58 | Lloyd Bird Park | |
| N_lloydii_59 | Lloyd Bird Park | |
| N_lloydii_60 | Lloyd Bird Park | |
| N_lloydii_61 | Lloyd Bird Park | |
| N_lloydii_74 | Lloyd Bird Park | |
| N_lloydii_75 | Lloyd Bird Park | |
| N_lloydii_76 | Lloyd Bird Park | |
| N_lloydii_77 | Lloyd Bird Park | |
| N_lloydii_78 | Lloyd Bird Park | |
| N_lloydii_79 | Lloyd Bird Park | |
| N_lloydii_80 | Kholo | |
| N_lloydii_81 | Kholo | |
| N_lloydii_82 | Kholo | |
| N_lloydii_83 | Kholo | |
| N_lloydii_84 | Kholo | |
| N_lloydii_85 | Ebbw | |
| N_lloydii_86 | Mt Crosby | |
| N_lloydii_87 | Mt Crosby | |
| N_lloydii_88 | Mt Crosby | |
| N_lloydii_89 | Mt Crosby | |
| N_lloydii_90 | Mt Crosby | |
| N_lloydii_91 | Mt Crosby | |
| N_lloydii_92 | Mt Crosby | |
| N_lloydii_93 | Mt Crosby | |
| N_lloydii_94 | Mt Crosby | |
| N_lloydii_95 | Mt Crosby | |
| N_lloydii_96 | Mt Crosby | |
| N_lloydii_97 | Mt Crosby | |
| N_lloydii_98 | Mt Crosby | |
| N_lloydii_99 | Mt Crosby | |
| N_lloydii_100 | Mt Crosby | |
| N_lloydii_101 | Mt Crosby | |
| N_lloydii_102 | Mt Crosby | |
| N_lloydii_109 | Mt Edwards | |
| N_lloydii_110 | Mt Edwards | |
| N_lloydii_111 | Mt Edwards | |
| N_lloydii_112 | Mt Edwards | |
| N. punctata | N_punctata_101 | Lloyd Bird Park |
| N_punctata_102 | Lloyd Bird Park | |
| N_punctata_103 | Lloyd Bird Park | |
| N_punctata_104 | Lloyd Bird Park | |
| N_punctata_105 | Lloyd Bird Park | |
| N_punctata_106 | Lloyd Bird Park | |
| N_punctata_108 | Lloyd Bird Park | |
| N_punctata_109 | Lloyd Bird Park | |
| N_punctata_110 | Lloyd Bird Park | |
| N_punctata_111 | Lloyd Bird Park | |
| N_punctata_S1 | Samford | |
| N_punctata_S2 | Samford | |
| N_punctata_S4 | Samford | |
| N_punctata_S5 | Samford | |
| N_punctata_S6 | Samford | |
| N_punctata_S7 | Samford | |
| N_punctata_S8 | Samford | |
| N_punctata_S10 | Samford | |
| N_punctata_S11 | Samford | |
| N_punctata_S12 | Samford | |
| N. microcarpa | N_microcarpa_01 | Somerset |
| N_microcarpa_02 | Tablelands | |
| N_microcarpa_03 | Gladstone | |
| N_microcarpa_04 | North Burnett | |
| N_microcarpa_06 | Locker valley | |
| N_microcarpa_07 | Locker valley | |
| N_microcarpa_08 | Locker valley | |
| N_microcarpa_09 | Locker valley | |
| N_microcarpa_10 | Chinchilla | |
| N_microcarpa_11 | Ipswich | |
| N_microcarpa_12 | Locker valley | |
| N_microcarpa_13 | Locker valley | |
| N_microcarpa_14 | Locker valley | |
| N_microcarpa_15 | Chinchilla | |
| N_microcarpa_16 | Locker valley | |
| N_microcarpa_17 | Ipswich | |
| N_microcarpa_18 | Tablelands | |
| N_microcarpa_19 | Tablelands | |
| N_microcarpa_20 | Goondiwindi | |
| N_microcarpa_21 | Central highlands | |
| N_microcarpa_22 | Flinders | |
| N_microcarpa_23 | Murweh | |
| N_microcarpa_24 | Tenterfield (NSW) | |
| N_microcarpa_25 | NSW | |
| N_microcarpa_26 | Central highlands | |
| N_microcarpa_27 | Etheridge | |
| N_microcarpa_28 | Toowoomba | |
| N_microcarpa_29 | Charters towers | |
| N_microcarpa_30 | Balonne |
| LO_LB | LO_SERF | |
|---|---|---|
| LO_LB | 59.19 | |
| LO_SERF | 0.0272 |
| Species | Parameter | df | F | Sig. |
|---|---|---|---|---|
| N. lloydii | H o | 328,507 | 149.290 | .000 |
| H e | 328,206 | 64.590 | .000 | |
| F is | 168,341 | 118.431 | .000 | |
| N. punctata | H o | 112,502 | 1.422 | .233 |
| H e | 112,502 | 13.038 | .000 | |
| F is | 89,430 | 20.005 | .000 | |
| N. lloydii & N. punctata | H o | 441,015 | 42954.536 | .000 |
| H e | 440,714 | 18017.997 | .000 | |
| F is | 257,777 | 18887.046 | .000 |
| (a). Esk | N_lloydii_01 | N_lloydii_02 | N_lloydii_03 | N_lloydii_05 | N_lloydii_06 | N_lloydii_07 | N_lloydii_08 | N_lloydii_09 | N_lloydii_10 | N_lloydii_11 | N_lloydii_12 | N_lloydii_13 | N_lloydii_18 | N_lloydii_19 | N_lloydii_20 | N_lloydii_21 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N_lloydii_02 | 0.044 | |||||||||||||||
| N_lloydii_03 | 0.081 | 0.222 | ||||||||||||||
| N_lloydii_05 | 0.056 | 0.032 | 0.066 | |||||||||||||
| N_lloydii_06 | 0.040 | 0.127 | 0.093 | 0.037 | ||||||||||||
| N_lloydii_07 | 0.049 | 0.055 | 0.058 | 0.099 | 0.059 | |||||||||||
| N_lloydii_08 | 0.027 | 0.070 | 0.046 | 0.033 | 0.050 | 0.035 | ||||||||||
| N_lloydii_09 | 0.076 | 0.028 | 0.038 | 0.108 | 0.057 | 0.041 | 0.030 | |||||||||
| N_lloydii_10 | 0.037 | 0.059 | 0.054 | 0.049 | 0.073 | 0.081 | 0.028 | 0.026 | ||||||||
| N_lloydii_11 | 0.028 | 0.062 | 0.052 | 0.018 | 0.084 | 0.032 | 0.026 | 0.026 | 0.100 | |||||||
| N_lloydii_12 | 0.088 | 0.027 | 0.064 | 0.112 | 0.033 | 0.049 | 0.023 | 0.132 | 0.055 | 0.022 | ||||||
| N_lloydii_13 | 0.042 | 0.094 | 0.059 | 0.023 | 0.091 | 0.066 | 0.021 | 0.025 | 0.233 | 0.171 | 0.026 | |||||
| N_lloydii_18 | 0.010 | 0.059 | 0.033 | 0.005 | 0.046 | 0.012 | 0.020 | 0.013 | 0.021 | 0.033 | 0.013 | 0.025 | ||||
| N_lloydii_19 | 0.029 | 0.044 | 0.059 | 0.056 | 0.030 | 0.069 | 0.028 | 0.029 | 0.085 | 0.036 | 0.054 | 0.075 | 0.010 | |||
| N_lloydii_20 | 0.049 | 0.030 | 0.043 | 0.163 | 0.040 | 0.072 | 0.031 | 0.059 | 0.061 | 0.032 | 0.085 | 0.077 | 0.009 | 0.076 | ||
| N_lloydii_21 | 0.033 | 0.159 | 0.105 | 0.034 | 0.144 | 0.080 | 0.033 | 0.021 | 0.090 | 0.088 | 0.024 | 0.126 | 0.063 | 0.075 | 0.057 | |
| N_lloydii_22 | 0.033 | 0.014 | 0.044 | 0.036 | 0.022 | 0.023 | 0.020 | 0.022 | 0.041 | 0.041 | 0.058 | 0.030 | 0.010 | 0.068 | 0.049 | 0.020 |
| (b). MtCr | N_lloydii_86 | N_lloydii_87 | N_lloydii_88 | N_lloydii_89 | N_lloydii_90 | N_lloydii_91 | N_lloydii_92 | N_lloydii_93 | N_lloydii_94 | N_lloydii_95 | N_lloydii_96 | N_lloydii_97 | N_lloydii_98 | N_lloydii_99 | N_lloydii_100 | N_lloydii_101 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N_lloydii_87 | 0.018 | |||||||||||||||
| N_lloydii_88 | 0.033 | 0.019 | ||||||||||||||
| N_lloydii_89 | 0.027 | 0.007 | 0.118 | |||||||||||||
| N_lloydii_90 | 0.018 | 0.039 | 0.015 | 0.015 | ||||||||||||
| N_lloydii_91 | 0.022 | 0.007 | 0.053 | 0.034 | 0.006 | |||||||||||
| N_lloydii_92 | 0.017 | 0.018 | 0.059 | 0.043 | 0.018 | 0.114 | ||||||||||
| N_lloydii_93 | 0.066 | 0.019 | 0.053 | 0.031 | 0.029 | 0.061 | 0.015 | |||||||||
| N_lloydii_94 | 0.015 | 0.029 | 0.020 | 0.026 | 0.024 | 0.020 | 0.027 | 0.007 | ||||||||
| N_lloydii_95 | 0.017 | 0.018 | 0.021 | 0.011 | 0.014 | 0.007 | 0.023 | 0.009 | 0.019 | |||||||
| N_lloydii_96 | 0.011 | 0.047 | 0.025 | 0.010 | 0.075 | 0.049 | 0.071 | 0.034 | 0.019 | 0.014 | ||||||
| N_lloydii_97 | 0.012 | 0.069 | 0.013 | 0.068 | 0.053 | 0.013 | 0.021 | 0.020 | 0.021 | 0.018 | 0.058 | |||||
| N_lloydii_98 | 0.022 | 0.073 | 0.037 | 0.004 | 0.048 | 0.012 | 0.034 | 0.052 | 0.032 | 0.030 | 0.084 | 0.085 | ||||
| N_lloydii_99 | 0.006 | 0.006 | 0.004 | 0.002 | 0.003 | 0.005 | 0.007 | 0.002 | 0.007 | 0.006 | 0.008 | 0.003 | 0.003 | |||
| N_lloydii_100 | 0.012 | 0.024 | 0.013 | 0.013 | 0.042 | 0.005 | 0.025 | 0.048 | 0.014 | 0.020 | 0.068 | 0.025 | 0.073 | 0.119 | ||
| N_lloydii_101 | 0.014 | 0.019 | 0.035 | 0.108 | 0.020 | 0.024 | 0.021 | 0.010 | 0.025 | 0.022 | 0.018 | 0.140 | 0.019 | 0.003 | 0.009 | |
| N_lloydii_102 | 0.009 | 0.052 | 0.014 | 0.009 | 0.098 | 0.008 | 0.023 | 0.026 | 0.016 | 0.029 | 0.056 | 0.095 | 0.082 | 0.006 | 0.048 | 0.023 |
| (c). Lloyd bird | N_lloydii_50 | N_lloydii_51 | N_lloydii_52 | N_lloydii_53 | N_lloydii_54 | N_lloydii_57 | N_lloydii_58 | N_lloydii_59 | N_lloydii_60 | N_lloydii_61 | N_lloydii_74 | N_lloydii_75 | N_lloydii_76 | N_lloydii_77 | N_lloydii_78 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N_lloydii_51 | 0.120 | ||||||||||||||
| N_lloydii_52 | 0.025 | 0.069 | |||||||||||||
| N_lloydii_53 | 0.024 | 0.035 | 0.018 | ||||||||||||
| N_lloydii_54 | 0.113 | 0.118 | 0.044 | 0.019 | |||||||||||
| N_lloydii_57 | 0.113 | 0.115 | 0.048 | 0.016 | 0.126 | ||||||||||
| N_lloydii_58 | 0.062 | 0.108 | 0.043 | 0.024 | 0.076 | 0.106 | |||||||||
| N_lloydii_59 | 0.053 | 0.075 | 0.030 | 0.055 | 0.046 | 0.042 | 0.094 | ||||||||
| N_lloydii_60 | 0.005 | 0.017 | 0.013 | 0.016 | 0.004 | 0.000 | 0.036 | 0.023 | |||||||
| N_lloydii_61 | 0.032 | 0.064 | 0.046 | 0.023 | 0.034 | 0.025 | 0.071 | 0.134 | 0.038 | ||||||
| N_lloydii_74 | 0.070 | 0.134 | 0.087 | 0.041 | 0.091 | 0.079 | 0.110 | 0.086 | 0.016 | 0.081 | |||||
| N_lloydii_75 | 0.012 | 0.016 | 0.036 | 0.015 | 0.010 | 0.014 | 0.011 | 0.017 | 0.007 | 0.025 | 0.037 | ||||
| N_lloydii_76 | 0.067 | 0.113 | 0.047 | 0.025 | 0.056 | 0.063 | 0.091 | 0.068 | 0.014 | 0.059 | 0.111 | 0.010 | |||
| N_lloydii_77 | 0.064 | 0.088 | 0.037 | 0.023 | 0.058 | 0.056 | 0.101 | 0.065 | 0.020 | 0.069 | 0.109 | 0.012 | 0.093 | ||
| N_lloydii_78 | 0.019 | 0.010 | 0.018 | 0.013 | 0.016 | 0.011 | 0.009 | 0.020 | 0.003 | 0.028 | 0.032 | 0.073 | 0.023 | 0.025 | |
| N_lloydii_79 | 0.006 | 0.027 | 0.028 | 0.008 | 0.007 | 0.010 | 0.034 | 0.046 | 0.011 | 0.029 | 0.036 | 0.079 | 0.021 | 0.027 | 0.082 |
| (d). Moggill | N_lloydii_23 | N_lloydii_24 | N_lloydii_25 | N_lloydii_27 | N_lloydii_28 | N_lloydii_29 | N_lloydii_30 | N_lloydii_31 | N_lloydii_32 | N_lloydii_33 | N_lloydii_34 | N_lloydii_35 | N_lloydii_36 | N_lloydii_45 | N_lloydii_46 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N_lloydii_24 | 0.043 | ||||||||||||||
| N_lloydii_25 | 0.039 | 0.220 | |||||||||||||
| N_lloydii_27 | 0.034 | 0.217 | 0.230 | ||||||||||||
| N_lloydii_28 | 0.013 | 0.132 | 0.211 | 0.124 | |||||||||||
| N_lloydii_29 | 0.036 | 0.133 | 0.229 | 0.156 | 0.106 | ||||||||||
| N_lloydii_30 | 0.041 | 0.045 | 0.086 | 0.050 | 0.031 | 0.074 | |||||||||
| N_lloydii_31 | 0.088 | 0.052 | 0.075 | 0.060 | 0.023 | 0.058 | 0.075 | ||||||||
| N_lloydii_32 | 0.053 | 0.028 | 0.078 | 0.044 | 0.034 | 0.066 | 0.198 | 0.082 | |||||||
| N_lloydii_33 | 0.096 | 0.017 | 0.021 | 0.021 | 0.011 | 0.024 | 0.039 | 0.040 | 0.038 | ||||||
| N_lloydii_34 | 0.027 | 0.061 | 0.123 | 0.060 | 0.051 | 0.073 | 0.078 | 0.075 | 0.086 | 0.041 | |||||
| N_lloydii_35 | 0.048 | 0.035 | 0.048 | 0.028 | 0.020 | 0.034 | 0.069 | 0.069 | 0.046 | 0.053 | 0.095 | ||||
| N_lloydii_36 | 0.048 | 0.030 | 0.042 | 0.021 | 0.022 | 0.034 | 0.035 | 0.029 | 0.039 | 0.063 | 0.067 | 0.053 | |||
| N_lloydii_45 | 0.032 | 0.052 | 0.100 | 0.061 | 0.050 | 0.068 | 0.056 | 0.054 | 0.048 | 0.041 | 0.051 | 0.044 | 0.031 | ||
| N_lloydii_46 | 0.049 | 0.020 | 0.026 | 0.021 | 0.018 | 0.019 | 0.044 | 0.038 | 0.031 | 0.040 | 0.026 | 0.050 | 0.039 | 0.026 | |
| N_lloydii_47 | 0.031 | 0.014 | 0.019 | 0.017 | 0.009 | 0.013 | 0.023 | 0.044 | 0.022 | 0.042 | 0.025 | 0.023 | 0.040 | 0.028 | 0.030 |
| (e). Kholo | N_lloydii_80 | N_lloydii_81 | N_lloydii_82 | N_lloydii_83 |
|---|---|---|---|---|
| N_lloydii_81 | 0.221 | |||
| N_lloydii_82 | 0.004 | 0.009 | ||
| N_lloydii_83 | 0.012 | 0.014 | 0.015 | |
| N_lloydii_84 | 0.025 | 0.040 | 0.010 | 0.015 |
| (f). MtEd | N_lloydii_109 | N_lloydii_110 | N_lloydii_111 |
|---|---|---|---|
| N_lloydii_109 | |||
| N_lloydii_110 | 0.077 | ||
| N_lloydii_111 | 0.069 | 0.115 | |
| N_lloydii_112 | 0.069 | 0.254 | 0.105 |
| Season | Sep to Mar | September | |||||
|---|---|---|---|---|---|---|---|
| Average | SD | p-Value | Average | SD | p-Value | ||
| Mean rainfall (mm) | Season 1 | 111.37 | 87.39 | .0559 | 2.53 | 3.01 | .0358 |
| Season 2 | 65.67 | 60.65 | 16.27 | 7.02 | |||
| Mean max temp (°C) | Season 1 | 29.40 | 1.87 | .3492 | 28.43 | 1.21 | .0480 |
| Season 2 | 30.08 | 2.66 | 25.80 | 1.08 | |||
| Mean min temp (°C) | Season 1 | 17.94 | 3.19 | .8705 | 11.73 | 2.61 | 1.0000 |
| Season 2 | 18.11 | 3.57 | 11.73 | 2.20 | |||
- Source: The Bureau of Meteorology, Australia: http://www.bom.gov.au/.
| Group | Count | Sum | Average | SD | F | p-Value | |
|---|---|---|---|---|---|---|---|
| Mean number of florets per branch | B | 50 | 3210 | 64.20 | 50.99 | 0.6066 | .4379 |
| UB | 50 | 2835 | 56.70 | 45.13 | |||
| Flower to fruits conversion rated | B | 50 | 38.33 | 0.77 | 0.29 | 0.0066 | .9357 |
| UB | 50 | 39.14 | 0.78 | 1.38 | |||
| % developed fruits | B | 48 | 63.49 | 1.32 | 2.64 | 0.4518 | .5032 |
| UB | 44 | 42.05 | 0.96 | 2.59 |
Open Research
OPEN RESEARCH BADGES
This article has earned Open Data and Open Materials badges. Data and materials are available at https://doi.org/10.5061/dryad.hmgqnk9pb.
DATA AVAILABILITY STATEMENT
The data are deposited in DRYAD and can be accessed via the link https://doi.org/10.5061/dryad.hmgqnk9pb.




