Daily ovarian hormone exposure and loss of control eating in adolescent girls: A registered report
Abstract
Objective
The daily biobehavioral factors that precipitate loss of control eating (LOCE) in adolescent girls are not well known. Ovarian hormone levels are key biological factors associated with the etiology of eating disorders in adolescent girls. Yet, models on how daily ovarian hormone exposure predicts LOCE in adolescent girls are underdeveloped. The goal of this study is to examine the daily patterns and mechanisms of ovarian hormone levels on LOCE across the menstrual cycle in adolescent girls and the mediating roles of food-related reward anticipation and response inhibition. Ecological momentary assessment (EMA) paired with daily hormonal sampling will be used to examine (1) daily associations between within-person hormones and LOCE, and (2) the mediating role of within-person food-related reward anticipation and response inhibition.
Methods
Normally cycling adolescent girls who have reached menarche will provide daily saliva samples for hormone analysis and complete EMA for 35 days. During EMA, girls will report LOCE and will complete task-based and self-report measures of food-related response inhibition and reward anticipation.
Discussion
This work has implications for the development of new real-world biobehavioral models of LOCE in adolescent girls, which will guide theory improvements and treatment for LOCE. Results will provide preliminary evidence for treatment targets for novel interventions for adolescent girls—for example, a response inhibition intervention.
Public Significance
Adolescent eating disorders are severe mental health conditions, often marked by loss of control eating. Estrogen and progesterone play a role in the development and persistence of loss of control eating. The current study will examine how daily exposure to estrogen and progesterone predicts loss of control eating in adolescent girls and identify possible daily mechanisms linking estrogen and progesterone exposure and loss of control eating.
Binge spectrum eating disorders (BSEDs) are significant public health problems associated with increased mortality and psychiatric morbidity (Mason & Smith, 2022; Smink et al., 2012). As BSEDs typically have poor treatment outcomes (Linardon, 2018; Linardon & Wade, 2018), prevention is a key priority for BSEDs research, particularly among adolescent girls. Prevalence of BSEDs among adolescent girls is 2.1% for bulimia nervosa and 6% for binge-eating disorder (Glazer et al., 2019) and are more common among girls compared to boys, with estimates ranging from 2:1 to 8:1 increased prevalence in females compared to males (Klump, 2013; Klump et al., 2017).
Disordered-eating symptoms are more common than full syndrome BSEDs and may help identify early indicators of BSEDs. Loss of control eating (LOCE) is a type of disordered eating symptom that involves a subjective sense of feeling one cannot stop eating and predicts risk for full threshold BSEDs (Tanofsky-Kraff et al., 2008; Tanofsky-Kraff et al., 2020). While LOCE typically emerges across adolescence and is an early manifestation of BSED psychopathology, predominant theoretical models of LOCE (e.g., affect regulation) have not been consistently supported in adolescents (Mason et al., 2020; Tanofsky-Kraff et al., 2020). Adolescence is a unique developmental period in which there are new and increasing autonomy for eating behaviors (Ziegler, 2022); dramatic changes in brain and hormone functioning that exclusively occur during this timeframe (Blakemore & Choudhury, 2006; Sisk & Zehr, 2005); and increased sensitivity to rewards (Galvan, 2013). Because adolescence is a common period for LOCE onset (Stice et al., 1998), LOCE should be studied in the context of the unique brain and hormonal changes characteristic of the period when these behaviors often onset.
Ovarian hormones (e.g., progesterone, estrogen) are putative risk factors for BSEDs and disordered eating (Klump et al., 2017; Lester et al., 2003). Cycle-based studies have demonstrated postovulatory (when progesterone and estrogen peak) peaks in binge eating and emotional eating (Klump & Di Dio, 2022). Studies utilizing daily saliva sampling demonstrate that binge and emotional eating increased when both estrogen and progesterone were higher-than-usual (e.g., estrogen × progesterone interaction; Klump et al., 2013; Klump et al., 2014; Racine et al., 2013). As estrogen and progesterone are highest postovulation, studies of direct measurement of estrogen and progesterone align with cycle-based research showing postovulatory increases in eating behaviors, and estrogen and progesterone associations with binge-eating behaviors have been demonstrated in both population and clinical samples (Klump & Di Dio, 2022). Thus, estrogen and progesterone may act in concert to influence dysregulated eating (Culbert et al., 2016), and it is critical to study these processes in girls with LOCE. Additionally, it is important to investigate mechanisms connecting daily estrogen and progesterone exposure and LOCE in adolescent girls, including response inhibition and reward anticipation.
Estrogen and progesterone are thought to influence response inhibition (i.e., the ability to suppress or interrupt automatic responses) through interactions with dopaminergic modulation of prefrontal regions, which are important for self-control (Amin et al., 2006). As with other research, many of the studies focused on estrogen and progesterone influence on response inhibition have utilized a phase-based approach and have produced mixed findings (Le et al., 2020). However, if estrogen and progesterone interact to influence response inhibition then one would expect discrepancies in findings of the associations of estrogen and outcomes depending on the level of progesterone, which varies between phases. In addition, research on estrogen and progesterone and response inhibition has typically studied general response inhibition as opposed to food-specific response inhibition, which is particularly salient to LOCE (Smith et al., 2018; Smith et al., 2020). Given the relevance of food-related response inhibition with greater LOCE, daily hormones may lead to lower response inhibition, which in turn increases the likelihood of LOCE.
Estrogen and progesterone may also influence reward processes through the interaction with the dopaminergic system (Ma et al., 2020). One study found lower capacity to wait for reward during the early follicular phase (low estrogen, low progesterone) compared to late follicular phase (high estrogen, low progesterone; Diekhof, 2015). However, this prior work is limited by a focus on the follicular phase of the menstrual cycle, which is characterized by low estrogen and is not suited for studying estrogen and progesterone interactions. This is an important gap as it is theorized that progesterone may exert an inhibitory effect on estrogen on eating behavior (Ma et al., 2020). Other lines of research have found that exogenous increase of estrogen and progesterone during the menopausal transition increases reward responsivity (Thomas et al., 2014). However, exogenous hormone administration may be different that endogenous hormone effects. Given positive associations between and food-related reward anticipation (i.e., a person's “wanting” [or craving] for food]) with greater LOCE (Davis et al., 2009; Espel-Huynh et al., 2018; Finlayson & Dalton, 2012; Seymour et al., 2015; Vigil et al., 2016), daily estrogen and progesterone levels may lead to higher food-related reward anticipation, which in turn increases likelihood of LOCE.
There is a critical need for the examination of within-person, daily relationships between estrogen and progesterone and LOCE, as this research will allow for insight into the maintenance of LOCE and is lacking in adolescents (Mason et al., 2020). Research on estrogen and progesterone, food-related response inhibition, and reward anticipation, particularly in adolescent girls, have historically relied on laboratory-based assessments, which precludes understanding of relationships with LOCE in a free-living environment. There are compelling data indicating that estrogen and progesterone, response inhibition, and reward are not static and vary considerably from moment-to-moment (Fehring et al., 2006; Powell et al., 2017; Smith et al., 2020), necessitating examination of relationships between these variables at the within-person level. Problems of lab-based measures of hormones are amplified in adolescent girls as cycle variability is often greater during the first years following menarche (Shirtcliff et al., 2009).
This study will test a novel within-person biobehavioral model of LOCE in adolescent girls (see Figure 1). This model focuses on within-person relationships of daily ovarian hormone exposure on LOCE mediated through food-related response inhibition and reward anticipation. Daily hormone assessment across the menstrual cycle and ecological momentary assessment (EMA) will be used. EMA involves individuals completing multiple daily reports of cognitions, feelings, and behaviors and will allow for examination of a conceptual model of day-level hormone exposure, food-related reward anticipation and response inhibition, and LOCE.
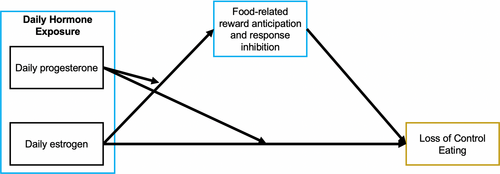
The first aim is to replicate the Klump et al. (2013) study to characterize the association between within-person daily hormones (i.e., that day's hormone level relative to that individual's average hormone level) and LOCE severity in a sample of adolescent girls aged 14–18 with current LOCE. It is hypothesized that estrogen and progesterone will interact such that LOCE severity will be greater on days when estrogen and progesterone are both lower than usual or when estrogen and progesterone are both higher than usual. The second aim is to examine a moderated mediation model of daily hormone exposure and food-related response inhibition and reward anticipation in predicting LOCE severity. It is hypothesized that girls will have lower food-related response inhibition and higher reward anticipation (operationalized as degree of wanting or craving for food as well as being about to eat a craved food) on days when estrogen and progesterone are both higher than usual, and in turn, lower food-related response inhibition, higher wanting and craving, and being about to eat a craved food will mediate the association between daily hormone exposure and LOCE severity. The protocol was preregistered on the Open Science Framework (Mason et al., 2022).
1 METHOD
1.1 Participants
Power analyses were conducted for a multilevel mediation model using Monte Carlo simulations in Mplus Version 8.1 software. Simulations assumed 35 EMA days, with a 78% compliance rate for EMA, α = .05, an intraclass correlation coefficient of .50, and assumed a small within-subject daily effect of r = .13 for model paths. Simulations were based upon 5000 replications. For a given hypothesis, the sample size was varied until the null hypothesis of no effect was rejected on at least 80% of the replications (i.e., .80 power). A final sample of N = 45 adolescent girls would provide sufficient power.
Inclusion criteria are: (1) 14–18 years old; (2) at least 1-year post-menarche; (3) current regular menstrual cycle lasting 24–35 days; (4) ≥2 LOCE episodes in the past month at baseline; (5) read and speak English; and (6) have a smart device compatible with the study apps and willing to download and use the study apps on their smart device. Exclusion criteria are: (1) diagnosis of anorexia nervosa or atypical anorexia nervosa; (2) recent changes in eating disorder treatment or psychiatric medications in the past 4 weeks; (3) enrolled in special education programs; (4) pregnancy or breastfeeding in the past 6 months (including currently); (5) past three-month use of birth control except for barrier and fertility awareness methods; and (6) having a condition that impacts menstrual cycle function (e.g., PCOS).
Exclusions were chosen to reduce factors that have strong impacts on hormones as well as recent changes in eating. Recruitment efforts will include clinical and community sites in Los Angeles (LA); this includes referrals through clinics at the University of Southern California and LA County eating disorder programs and flyers within the local community in East LA. We expect high diversity in race and ethnicity given the demographics of LA (United States Census Bureau, 2021). We will monitor recruitment, and, if diversity is lacking, we will make special effort to oversample racial and ethnic minority girls by recruiting in areas with high racial/ethnic minority representation.
1.2 Procedure
Interested participants will complete a brief screening measure and will be scheduled for a study visit if potentially eligible. At the study visit, girls and their caregivers (for participants <18 years old) will complete informed consent, baseline measures (i.e., clinical interviews, self-report surveys, and anthropometric assessments), and receive instructions for completing EMA surveys and daily saliva samples. Enrolled study participants will be instructed to complete 35 days of saliva collection upon awakening, which will be returned upon study completion, and daily EMA.
Participants will be instructed to collect a saliva sample via passive drool immediately upon awakening and will log each sample collected. Participants will store saliva in their freezer until EMA completion and will be provided instructions for using ice packs/coolers when returning. Samples will be inspected for thawing upon return and then stored at −20°C until shipped to the lab on dry ice for analysis. Samples that are determined to be unusable for quality reasons (i.e., arrived thawed, significant missing data) will not be analyzed. Participants that do not return saliva samples or with unusable samples will be replaced and the final sample of 45 will be based on those with complete and analyzed saliva samples. Daily home collection and storage procedures have been used in previous studies with excellent participant adherence and sample quality (Baker et al., 2021; Edler et al., 2007; Klump et al., 2008). Within-person estrogen and progesterone will be calculated by subtracting the person mean from the daily value, then dividing the result by the person's standard deviation (person-standardized daily hormone level) (Eisenlohr-Moul et al., 2018; Klump et al., 2013; Martel et al., 2017).
EMA will be completed using the Lifedata application (www.lifedatacorp.com). Participants will use their own smartphone to complete semi-randomly-prompted EMA surveys, four times each day during non-school time on weekdays (morning wake-up time; 3:30 p.m.–8 p.m.) and seven times each day on weekend days (9 a.m.–9 p.m.). Girls will not complete random surveys on weekdays while at school to prevent classroom interruptions. Excluding school hours is a common practice in EMA research on youth in order not to interrupt learning (Mason et al., 2020). During the baseline visit, participants will receive information regarding the EMA protocol and training in the completion of EMA on the smartphone. Participants will be asked to proceed with their daily routines as normal and will be instructed not to complete entries at times when they feel they are not able or when safety is a concern. However, participants will be encouraged to complete the entry as soon as possible when able. Up to two reminder prompts will be sent within 30 min of the initial prompt, after which time the survey will close. Participants will receive monetary compensation for their effort and monetary compliance bonuses will be provided. Monetary compensation and compliance bonuses have been used successfully in previous EMA studies (Mason et al., 2020). Study staff will communicate with participants approximately twice during each EMA week to receive feedback regarding their compliance and address technical issues and concerns.
2 MEASURES
2.1 Baseline measures
2.1.1 Anthropometric assessments
Height and weight will be measured in duplicate using an electronically calibrated digital scale and professional stadiometer. Body mass index (BMI; kg/m2) and CDC age and gender-specific BMI z-scores (z-BMI) will be determined using EpiInfo 2005, Version 3.2 (CDC).
2.1.2 BSEDs symptom interview
The Eating Disorder Examination (EDE; Fairburn et al., 2008) is a semi-structured interview that assesses eating disorder symptoms (including LOCE) over the past 28 days and provides diagnostic information for BSEDs. Selected modules from the EDE will be used to assess eligibility criteria as well as for descriptive purposes and will be administered by a licensed clinical psychologist with EDE training. During this interview, adolescents will receive training on the definition of LOCE.
2.1.3 Baseline covariates
Girls will complete measures of demographics, menstrual history (including menarche onset), pubertal development (Pubertal Development Scale; Petersen et al., 1988), internalizing symptoms, (Revised Children's Anxiety and Depression Scale; Chorpita et al., 2000), sleep (Pittsburgh Sleep Quality Index; Monk et al., 1994), attention deficit hyperactivity disorder (ADHD; Current Symptoms Scale; Barkley & Murphy, 2006), and disordered eating (Eating Disorder Examination Questionnaire; Fairburn & Beglin, 1994).
2.2 EMA measures
Exact self-report EMA items are presented in Table 1.
| Construct | Items | Response scale |
|---|---|---|
| Reward anticipation | (1) RIGHT NOW, how much are you craving food? (2) RIGHT NOW, how much do you want food? (3) Are you about to eat a food you are craving in the next 30 min? |
1 (Not at all) to 10 (Very strongly) Yes/no |
| Eating | Did you eat something in the past 2 h? | Yes/no |
| Loss of control eating | While you were eating, (1) did you feel a sense of loss of control? (2) did you feel that you could not stop eating once you had started? (3) did you feel like you could not resist eating? (4) did you feel like a car without brakes, you just kept eating and eating? |
1 (No not at all) to 5 (Yes, extremely) |
| Menstrual cycle | Today, have you experienced any menstrual | Spotting (i.e., bleeding outside normal menstrual period) Bleeding None |
| Negative affect | Please rate your CURRENT mood. (1) Distressed (2) Upset (3) Guilty (4) Scared (5) Hostile (6) Irritable (7) Ashamed (8) Nervous (9) Jittery (10) Afraid (11) Bored |
1 (Not all) to 5 (Extremely) |
| Hunger | Right now, how hungry are you? | 1 (Not at all) to 5 (Extremely) |
| Avoidance food craving | Right now, to what extent are you avoiding eating foods you are craving? | 1 (Not at all) to 5 (Extremely) |
| Eating disorder behaviors | Since your last recording, have you done any of the following to influence your weight or body (Check all that apply)? I vomited. I used laxatives for weight control. I exercised excessively. I skipped a meal I avoided eating |
Yes/no If yes: How long ago did you <behavior>? 0–15 min ago 16–30 min ago 31–45 min ago 46–60 min ago 61–90 min ago 91 min ago to 2 h ago |
2.2.1 Food-related response inhibition
Daily response inhibition will be assessed by a Go/No-go task adapted for mobile devices (Smith et al., 2020). Like Smith et al. (2020), the task will be given mid-day to reduce burden; mid-day represents the time when LOCE becomes more likely. The task will be administered via the Inquist 6.0 application for Android and iOS smartphones. In this task, a neutral non-food or palatable food image will be presented in a randomized order on the screen for 500 ms, followed by a fixation cross. For Go trials, participants will be instructed to respond as fast as possible to categorize the images that have either a red border or a blue border; participants will be instructed to hold the phone horizontally with both hands and use their thumbs to respond to on-screen stimuli. Participants will respond using a colored button (red or blue) displayed on the left or right side of the screen. During No-go trials, a green dot above the image will signal to participants to inhibit responses to stimuli. To establish prepotent responding, a 4:1 Go to No-go trial ratio will be used. Each task administration includes 100 trials (approximately 3 min to complete). The outcome measures will be the number of food-related and neutral commission errors during the task (i.e., failure to inhibit a response in the presence of food or neutral images), with greater commission errors reflecting poorer response inhibition.
2.2.2 Food wanting and craving
Girls will be asked about their degree of food wanting as well as craving (Alabduljader et al., 2018; Goldschmidt et al., 2018). Girls will also be given a behavioral measure that asks if they are about to eat a food they are craving.
2.2.3 LOCE
Girls will be instructed to indicate if the ate anything in the past 2 h. If they did eat, four items will assess degree of LOCE. Items will be averaged to create a composite score at each eating episode, which has shown adequate psychometric properties in previous research (Goldschmidt et al., 2018).
2.2.4 Menstrual cycle
Girls will report on menstrual bleeding (i.e., none, spotting, bleeding). Using this daily data, we will calculate a menstrual cycle day and phase for each day according to the self-reported first day of menstrual bleeding. The day of menstrual onset is “day 1” (foreword count) and the day before menstrual onset is “day-1” (backward count). In line with recommendations, a combination of forward and backward count is used when assigning menstrual phase, and we will use hormone levels to verify cycle phase. Given this, accurate menstrual phase classification can be done regardless of when participants start the study (i.e., participants are able to start on any day of their menstrual cycle). Similar to prior research (Klump et al., 2013), 5-day rolling averages will be calculated to diminish the influence of hormone-release pulsatility and smooth the pattern of hormone variability. For example, hormones on Day 6 will be computed as the average of that subjects hormones on Days 4–8, inclusive. At least three data points are needed for each average. We will calculate within-person raw hormone levels and rolling hormone levels.
2.2.5 EMA covariates
Current negative affect, hunger, avoidance food craving, and other ED symptoms (including compensatory behaviors and restriction) will also be assessed.
2.3 Planned analyses
Descriptive statistics will be examined for all demographics, Go/No-go commission errors, and EMA measures. Variable transformations prior to subsequent analyses will be determined based on distribution and outlier diagnostics. Several missing data techniques will be evaluated for missing data. Covariates (e.g., recruitment source [community vs. clinical], age, menarche onset, race, anthropometrics, depression, sleep, ADHD, EMA covariates, and menstrual cycle) will be screened and included in analyses if related to dependent variables. In addition, correlates of EMA compliance will be examined. Preliminary analyses will involve plotting the distribution of raw and rolling-average of hormones in histograms. General linear mixed models will be conducted to test whether (1) estrogen and progesterone levels differ between phases, and (2) associations of within-subject estrogen levels with within-subject progesterone levels. We will also investigate whether phase independently is associated with outcomes using multilevel models with phase as the primary predictor and controlling for all covariates.
All hypotheses will be tested in multilevel or multilevel structural equation models (observations nested within days nested within girls) with SPSS and Mplus. Model fit indices (−2 LL) and intraclass correlation coefficients will dictate the inclusion/exclusion of autoregressive error structures and random slopes. Aim 1 will be tested with a multilevel model with today's estrogen and progesterone (within), and the interactions of estrogen and progesterone (within) predicting LOCE. Aim 2 will be tested with three-level multilevel moderated-mediation structural equation models, which allow for modeling both within- and between-subject associations between variables across three levels (i.e., prompt, day, and person; Bauer et al., 2006). This protocol generates confidence intervals for the size of the conditional indirect effect. Mediation is supported when the CI does not include zero. Menarche onset will be included as a between-subject a priori covariate. Analyses will be run using within-person raw values of estrogen and progesterone as well as within-person 5-day rolling averages of estrogen and progesterone.
3 CONCLUSIONS
The proposed conceptual model is particularly innovative because it studies the impact of daily hormones on LOCE in adolescent girls. In addition, testing of the conceptual model will provide new evidence for potential mechanisms (i.e., food-related response inhibition and reward) underlying these associations in daily life. Therefore, this study has the potential to improve models of LOCE in adolescent girls by identifying biobehavioral etiological and maintenance mechanisms that have not been previously addressed in theories of LOCE.
This research is the first known study of mechanisms of daily ovarian hormone exposure and LOCE in girls and will inform novel within-day interventions for LOCE, which are urgently needed given the significant within-day variability in LOCE and associated mechanisms. Findings of daily hormone effects would suggest tailoring interventions to be delivered during cycle phase when these mechanisms are more likely to cause LOCE. Next steps include using experimental approaches, such as micro-randomized trials, to intervene upon these risk factors in girls' natural environment. For example, interventions that target inhibitory control and reward, such as inhibitory control training (i.e., Chami et al., 2022) or regulation of cue training (Boutelle et al., 2022), could be utilized at various phases of the menstrual cycle to enhance efficacy.
AUTHOR CONTRIBUTIONS
Raina Pang: Conceptualization; funding acquisition; methodology; writing – original draft. Jeremy C Morales: Writing – review and editing. Kathryn Smith: Methodology; writing – review and editing. Stuart Benjamin Murray: Writing – review and editing. Genevieve Dunton: Writing – review and editing. Tyler Mason: Conceptualization; funding acquisition; methodology; writing – original draft.
FUNDING INFORMATION
This work was funded by the National Institute of Mental Health (R21 MH 126334, mPI Mason & Pang).
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to disclose.
Open Research
OPEN RESEARCH BADGES
This article has earned an Open Data badge for making publicly available the digitally-shareable data necessary to reproduce the reported results. The data is available at [[insert provided URL from Open Research Disclosure Form]].
DATA AVAILABILITY STATEMENT
There is no associated data with this submission.




