Characterization of Neural Stem Cells of the Human Embryonic Fetus Across Regions of the Central Nervous System and Through Standard Gestation Period Assessments
Funding: The research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (RS-2023-00243835).
ABSTRACT
In this study, the characteristics of human embryonic fetuses aborted due to ectopic pregnancy were preliminarily evaluated on the basis of gestational age (GA), crown-rump - length (CRL), and their carnegie stage (CS). Further, we attempted to establish the human neural stem cells (NSCs) and neural progenitor cells (NSCs/NPCs) by separating them into corresponding areas of the brain and spinal cord (SC), as much as was visually distinguishable, considering that the distribution of neurons differs across different brain areas. We successfully isolated and cultured NSCs/NPCs from regional brain and SC tissues of human embryonic fetuses. The isolated NSCs/NPCs not only exhibited the ability to self-proliferate but also had the potential to differentiate into neurons, motor neurons, and glial cells. We confirmed that the isolated Sox2 and Nestin expression in the NSCs/NPCs showed strong neural stemness and further verified how the expression markers, MAP-2, β-tubulin III(TuJ1), ChAT, HB9, GFAP, and Olig-2, could be harnessed while differentiating to neurons, motor neurons, and glial cells. NSCs with varying GA, CS, and CRLs were found to be capable of producing all neural lineages of neurons, astrocytes, and oligodendrocytes. We could completely differentiate them to neurons, motor neurons, and oligodendrocytes, except for a difference noted with astrocyte differentiation. This study provides vital experimental data for cell and gene therapy.
1 Introduction
Stem cell therapy is characterized by the regeneration or differentiation of damaged or essential tissues in patients, transplanting externally needed cells to promote growth factors for greater tissue health, and consequently aiding the replacement with new, healthy, and fully functional cells (Hoang et al. 2022). Amongst the various types of stem cells, human embryonic stem cells (ESCs) are derived from the inner cell mass before the first 2 weeks of development (Müller and O'Rahilly 1994; Trujillo et al. 2009). ESCs possess the advantages of being pluripotent and differentiating to all embryonic cell types, self-renewal, and ability to repair structures (Elkabetz and Studer 2008). However, research using ESCs continue to pose ethical problems, given that they are derived from the early stage of human life through the means of a woman's body. Moreover, the probability of somatic cell cloning of embryos is high, which could potentially lead to ethical dilemma regarding human cloning (Lo and Parham 2009).
Human mesenchymal stem cells (MSCs), on the other hand, can be collected from human bone marrow, umbilical cord, adipose tissue, amniotic fluid, and peripheral blood, and do not present the same ethical problems. In addition, being multipotent, they can be differentiated into various cell types such as osteoblasts, chondrocytes, myoblasts, and adipocytes. Yet, the limitations of human MSCs pose the challenge of limited proliferation and differentiation capabilities, especially as the mechanism underlying their immune control has not been fully identified (Lo Surdo and Bauer 2012; Marion and Mao 2006).
Induced pluripotent stem cells (iPSCs) are produced by reprogramming somatic cells to a state similar to ESCs. Interestingly, iPSCs have the characteristics of ESC with the advantage of being able to differentiate into a tertiary reproductive layer while being sourced from non-embryonic means. However, the higher the patient's age, the higher the possibility of mutations in the patient's adult cell, which might adversely affect the cell's function and lead to nuclear reprogramming. Therefore, the efficiency of iPSCs may be low, and they are highly susceptible to cancer (Thanaskody et al. 2022).
Although the many benefits of ESCs derived from human embryonic fetal cells are well-known, their ethical implications are also widely discussed by the public and media, making their ethical commercialization and clinical applications close to impossible. Embryonic fetal cells are derived from embryonic fetal tissues, with their superior proliferative and differentiation capabilities. ESCs reportedly have higher pluripotent potential and lower immunogenicity than other stem cells. Therefore, if stem cell lines derived from embryonic fetal tissues can be developed, there are infinite possibilities for treating various incurable diseases (O'Donoghue and Fisk 2004; Rosner et al. 2023).
Neural stem cells (NSCs) are defined as self-renewing, multipotent cells that can proliferate without limitation to produce progeny that can differentiate into neurons and glial cells (Gage 2000; Price and Williams 2001; Suzuki et al. 2021; Temple 2001). In modern medicine, NSC therapy is reportedly effective for the treatment of various intractable neurological diseases, such as Huntington's disease (HD), amyotrophic lateral sclerosis (ALS), spinal cord injury (SCI), Alzheimer's disease (AD), multiple sclerosis (MS), and stroke, among others that do not have treatments (Nie et al. 2023; Suzuki et al. 2021; Zhao et al. 2021). NSCs arise from the embryonic stage and are present in the central nervous system (CNS). NSCs have been identified in the subventricular zone (SVZ) and dentate gyrus of the hippocampus in the adult human CNS (Okano 2002; Takagi 2016; Taupin 2006; Thanaskody et al. 2022). Isolation of NSCs from various regions has been described, including the forebrain, midbrain, hindbrain, and spinal cord (SC), during the first trimester of human embryonic fetal development. In addition, these findings suggest that NSCs isolated from other regions of the human fetal CNS have radical differences, such as in their proliferative and differentiation capacity and their immunophenotype, suggesting a regional specification that may be regulated through the inherent activation of key transcription factors or through exposure to various molecular patterns, such as growth factors (Barami et al. 2001; Fan et al. 2014; Kim et al. 2006; Ostenfeld et al. 2002; Palmer et al. 1999; Suzuki et al. 2021; Wu et al. 2002).
The only way to obtain the CNS of human embryonic fetal for research is if after 3 months of pregnancy, life-threatening complications such as ectopic pregnancy follow, necessitating abortion on the basis of the doctor's judgment, and the mother subsequently voluntarily resorts to donation, which helps the humanitarian purpose of science. In general, the concept of fetal age is separate from pregnancy. Pregnancy age measures the progression of pregnancy in weeks, whereas fetal age indicates the actual age of the growing baby. Information on the fetal age is conveyed to mothers by obstetricians and gynecologists, usually by measuring the fetal crown-rump - length (CRL) after ultrasound sound scanning to finally predict the average value (Papageorghiou et al. 2014). Thus, the gestational age (GA), defined in weeks, is the duration of pregnancy before birth, on the basis of the reasoning described in a previous study (Yamada et al. 2010).
As previously mentioned, distinguishing each stage of the human fetus development process is challenging due to its small, incomplete, and undifferentiated nature. During the first trimester (1st to 13th weeks of pregnancy), the fetus measures an average of 0.5–5.4 cm, evolving from a fertilized egg to a moving one with distinguishable eyes, ears, and active organs (Gaillard et al. 2014). Estimating the precise age of the fetus in the early stages of development is difficult, as the size of an embryo or fetus is heterogeneous, being heavily influenced by race, heredity causes, and the environment (Workalemahu et al. 2018; Wu 2018). Therefore, the possible solution to explain embryo maturity in an internationally standardized way might be through the carnegie stage (CS), which was devised by embryonic scientists on the basis of the external characteristics of the embryo. The CS is currently a concept that can potentially classify human occurrence objectively (O'Rahilly and Müller 2010), as it divides the period of the first 8 weeks after modification into a standardized system of 23 stages, each of which is determined by appearance characteristics, longest (or head) length, known age, or estimated age (Harkness and Baird 1997; O'Rahilly and Müller 2010; Papageorghiou et al. 2014; Wu 2014; Zhai et al. 2022).
We hypothesized that NSCs derived from the various regions of the human embryonic fetal CNS during the first trimester possess distinct functional potentials. In this study, we attempted to investigate the proliferative and differentiation ability of NSCs by isolating them from various CNS regions on the basis of early CS in the first trimester. Through the investigation of regional NSCs derived from diverse donors, we aimed to elucidate potential variations or similarities in the proliferation and differentiation potentials of regional NSCs during human embryonic fetal development. Various other researchers have established that NSCs and neural progenitor cells (NSCs/NPCs) contribute to functional regeneration in cases of CNS damage, despite major endogenous capabilities (such as cytokines and specific signaling pathways) being associated with CNS plasticity through direct or indirect mechanisms (Fan et al. 2014; Kim et al. 2006; Rushing and Ihrie 2016). Transplantation of NSCs/NPCs has shown immunomodulatory and neurotrophic activity in treating CNS damage, highlighting their immense potential for therapies involving optimal functioning of neuronal cells for neurological diseases.
2 Materials and Methods
2.1 Procedures of Human Ectopic Pregnancy Samples
The donated ectopic pregnancy samples and maternal blood and urine samples were obtained according to the guidelines of the Committee of Medical Ethics of CHA Bundang Medical Center of CHA University (IRB Permit No. 2021-07-008) and CHA Gangnam Medical Center (IRB Permit No. 2021-03-031). The sample tissues were obtained from the obstetrics and gynecology departments of CHA Bundang Medical Center and CHA Gangnam Medical Center, and the investigation was conducted at the CHA Advanced Research Institute. Immediately after abortion owing to ectopic pregnancy at the CHA Medical Center in CHA University, the embryonic fetuses were transferred to a sterile sample container and moved at a temperature of 2–4°C. The storage temperature until the fetal matter was delivered (from the conception) was stabilized in an icebox, and we recorded the temperature in real-time (LogEt-8 KBLE, Elitech).
2.2 Donor Eligibility Screening
The mother's blood and urine samples obtained at fetal autopsy were sent to Eurofins Scientific US to test for hepatitis B core total antibody, hepatitis B surface antigen, hepatitis C antibody, HIV-1 and -2 Plus O, HTLV-I and -II antibody, Neisseria gonorrhoeae-Panther Platform, Syphilis Screening-Nontreponemal, Ultrio Elite-HBV/HCV/HIV-1/2, Zika NAT, and CMV total antibody.
2.3 Micro-Dissection of Human Embryonic Fetal Samples
Most ectopic pregnancies occurred in the mother's fallopian tubes. The fallopian tissues of mothers who consented to voluntary donations before surgery were collected after surgical separation. The sample container with the ectopic pregnancy tissue was opened in a sterilized tissue culture bench, and the fallopian tube was carefully removed and placed in a 100 mm dish (Corning) containing fresh Dulbecco's phosphate-buffered saline (DPBS) (Invitrogen) and gentamycin (Gibco) to remove the blood and impurities. Using a zoom stereoscopic microscope (SZX7, Olympus) and a microscopic dissecting set (Fine Science Tools), the fetal embryonic tissue was found in the fallopian tubes and were carefully micro-dissected again. In this study, three human embryonic fetal brains and SCs were separated (named FCB-01, FCB-02, and FCB-03), and the CS description of the embryonic fetuses helped distinguish the key embryological structural developments. The brain region of FCB-01 was divided into five parts (B0, B1, B2, B3, and B4), and the SC remained as one part. FCB-02 was dissected as two parts of the brain (BB and BC) and the right side of the SC. FCB-03 was separated into one part of the brain (B) and the SC (Table 1).
| Fetal sample | Gestational week | Crown-hip length (mm) | Carnegie stage | Sex |
|---|---|---|---|---|
| FCB-01 | 5 | 5 | 14 | Male |
| FCB-02 | 6 | 8.3 | 15 | Male |
| FCB-03 | 7 | 13 | 17 | Female |
2.4 Isolation of NSCs/NPCs From the Brain and SC Region of Embryonic Fetuses
Brain and SC tissues of the human embryonic fetuses were freshly dissected. The dissected tissue pieces were incubated in 0.1 mg/mL papain solution (MP Biomedicals) and 10 µg/mL DNase I (Roche) for 30 min at 37°C, after which gentamicin (Gibco) was added, and the tissue was incubated for a further 30 min at 37°C. The samples were centrifuged at 1500 rpm for 5 min. The tissues were resuspended in Dulbecco's modified Eagle's medium/Ham's F12 (DMEM/F12) (Gibco) Glutamax-I (Invitrogen) and triturated to extract the NSCs/NPCs.
2.5 Expansion and Proliferation of Human Embryonic Fetal Brain and SC-Derived NSCs/NPCs
Human fetal embryonic brain and SC derived NSCs/NPCs were isolated and plated onto poly-l-ornithine (PLO) (15 µg/mL, Sigma) and laminin-coated (10 µg/mL, Stemcell Technology) plates (Corning) with NSC media containing 3 mL Dulbecco's modified Eagle's medium/Ham's F12 (DMEM/F12) (Gibco) Glutamax-I (Gibco) with 2% B27 supplement Minus AO (Invitrogen), primocin (50 mg/mL, Invitrogen), tocopherol (1 mg/mL, Sigma), bFGF (50 µg/mL, PeproTech), and EGF (500 µg/mL). Briefly, all NSCs/NPCs were seeded at a density of 2 × 105 cells/6 well plates. The cultures were maintained in a humidified incubator at 37°C and 5% CO2 in air, and the media were replenished every 4 days. Upon reaching approximately 90%–95% confluency of growth, the cells were enzymatically detached by adding 1 mL of 0.05% accuses solution (PAA Laboratories) at 37°C for 10–20 min and counted in the hemocytometer (Merck) by the trypan blue stain (Gibco) exclusion method.
To analyze the fold increase in cell numbers, Accutase (Sigma)-treated cells were counted at each passage using a hemocytometer, and the total cell doublings were calculated (starting from passage 0–15). The cells were passaged to new PLO-coated plate on the basis of the appropriate cell number counted after Accutase treatment.
2.6 Flow Cytometry Analysis
The undifferentiated human embryonic fetal brain and SC-derived NSCs/NPCs were resuspended in PBS (Sigma) and fixed in 4% paraformaldehyde (PFA) (Sigma, USA) for 15 min at 25°C. After fixation, the cells were permeabilized with 1 mL of Perm Buffer 3 (BD Biosciences, USA) for 30 min at 4°C. The permeabilized cells were washed with stain buffer (BD Biosciences, USA) and incubated with mouse anti-nestin conjugated with Alexa Fluor 647 (BD Biosciences, USA) and mouse anti-Sox2 conjugated with Alexa Fluor 488 (BD Biosciences, USA) antibodies for 20 min in the dark, washed twice, and resuspended with stain buffer. To eliminate non-specific binding, Alexa Fluor 647 Mouse IgG1 κ isotype Control (BD Biosciences, USA) and Alexa Fluor 488 Mouse IgG2α Isotype Control (BD Biosciences, USA) were used as isotype controls. The isotype controls were prepared in the same manner. After resuspension in PBS, the cells were analyzed by fluorescence-activated cell sorting (FACS) using a CytoFLEX flow cytometer (Beckman Coulter, CA, USA), and the data were analyzed using the FlowJo software (FlowJo LLC, USA). The samples were collected at the seventh passage.
2.7 RNA Isolation and Complementary DNA (cDNA) Preparation
Total RNA was extracted using the TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to standard protocols. For the synthesis of first-strand cDNA, total RNA obtained from the cells and AccuPower Rocket Script RT PreMix kit (Bioneer, South Korea) was used with oligo dT (Bioneer, South Korea) to synthesize cDNA under the following conditions: 10 min at 37°C, 60 min at 50°C, denaturation for 5 s at 95°C for 40 cycles, extension for 10 min at 72°C, and finally maintained at 4°C.
2.8 Reverse Transcription Polymerase Chain Reaction (RT-PCR)
The cDNA was synthesized; 1 µg of it was transferred into AccuPower PyroHotStart Taq PCR PreMix tubes (Bioneer, South Korea), and the reaction was performed under the following conditions: pre-denaturation for 5 min at 95°C, denaturation for 30 s at 95°C, annealing for 30 s at 55°C, and extension for 45 s at 72°C for 35–40 cycles. final extension for 10 min at 72°C, and maintained at 4°C (C1000 Touch Thermal Cycle, Bio-RAD). The primer sequences are listed in Table 2.
| Gene | Primer | Product size (bp) |
|---|---|---|
| SOX2 | Forward 5′- GACGCTCATGAAGAAGGATA-3′ | 432 |
| Reverse 5′ GAGTGGGAGGAAGAGGTAA-3′ | ||
| Nestin | Forward 5′-GGTAGAGAAAGAAACAGCCA-3′ | 354 |
| Reverse 5′- GTGTCCTCTTTTCCTGTAGG-3′ | ||
| GAPDH | Forward 5′-GGCTGCTTTTAACTCTGGTA-3′ | 322 |
| Reverse 5′- CTTGAGGCTGTTGTCATACT-3′ |
2.9 Real-Time Polymerase Chain Reaction
CNS tissue region-specific and neurogenic genes were evaluated by real-time PCR (RT-PCR). The commercial RT-PCR master mix kits included AccuPower RocketScript RT PreMix & Master Mix (Bioneer). Thermal cycle conditions were 95°C for 2 min, then 40 cycles at 95°C for 10 s and 60°C for 30 s. Amplification was monitored using an ABI ViiA 7 Real-Time PCR System (Applied Biosystems). Results were normalized against the housekeeping gene GAPDH, and relative gene expression was analyzed by the 2−Δ Ct method, where Δ Ct = Cttarget − Ct normalizer. RT-PCR was performed using TaqMan Gene Expression Assays (FOXG1 Hs01850784_s1, EMX2 Hs00244574_m1, OTX2 Hs00222238_m1, EN1 Hs00154977_m1, EN2 Hs00171321_m1, GBX2 Hs00230965_m1, HOXB4 Hs01573270_m1, NKX6.1 Hs00232355_m1, HOXA4 Hs01573270_m1, and GAPDH Hs99999905_m1; Thermo Fisher Scientific). RT-PCR was performed in three independent experiments, and duplicate determinations were conducted for each sample.
2.10 Karyotype Analysis
G-banding karyotype analysis of heterogeneous human embryonic fetal CNS was performed by GenDix, Inc. (Seoul, Korea), after several (the seventh) passages.
2.11 Immunocytochemistry Analysis
Human embryonic fetal brain- and SC-derived NSCs/NPCs were plated on cell culture slides (SPF, South Korea). They were washed with PBS and fixed with 4% PFA (Sigma, USA). NSCs/NPCs were incubated for 1 h in blocking solution (1% BSA with 0.25% Triton X-100) and then washed with PBS. NSCs/NPCs were incubated overnight at 4°C with primary antibodies. The copyright holder for the preprint PBS was incubated with secondary antibodies for 2 h at 4°C. Nuclei were stained with DAPI (Sigma, USA). Finally, NSCs/NPCs were washed with PBS and observed under a fluorescence microscope (Olympus, Japan). The list of the antibodies used are enlisted in Table 3.
| Name of antibody | Company | Dilution |
|---|---|---|
| Anti-Nestin | Merck Millipore | 1:1000 |
| Anti-Sox2 | Cell signaling | 1:500 |
| Anti-TUJ1 | Biolegend | 1:1000 |
| Anti-MAP2 | Invitrogen | 1:1000 |
| Anti-GFAP | 1:700 | |
| Anti-Olig2 | 1:700 | |
| Anti-ChAT | 1:1000 | |
| Anti-HB9 | DSHB | 1:1000 |
| Mouse anti-Rabbit FITC | Invitrogen | 1:1000 |
| Rabbit anti-Mouse FITC | 1:1000 | |
| Goat anti-Mouse Alexa Fluor 594 | Abcam | 1:1000 |
| Goat anti-Mouse Alexa Fluor 488 | Invitrogen | 1:1000 |
| Goat anti-Rat Alexa Fluor 488 | 1:500 | |
| Goat anti-Rat Alexa Fluor 594 | 1:500 |
2.12 Differentiation of Human Embryonic Fetal Brain and SC Derived NSCs/NPCs to Neurons and Glial Cells
The differentiation potential of human embryonic fetal brain- and SC-derived NSCs/NPCs to neuronal/glial-restricted progenitor cells and motor neurons was evaluated in a specific differentiation medium after five passages, according to the manufacturer's instructions. Human embryonic fetal brain- and SC-derived NSCs/NPCs are neuronal/glial-restricted progenitor cells that differentiate for 7 days according to a modified protocol (Fan et al. 2014; Papageorghiou et al. 2014; Yamada et al. 2010). All NSCs/NPCs were plated onto PLO with laminin coated culture plate at a density of 20,000 cells/cm2, maintained in a humidified incubator at 37°C and 5% CO2 in air, and were replenished every 3 days. Neuronal differentiation medium (NDM) contained 2% B27 supplement (Gibco, USA), 2 mM GlutaMax I supplement, and 1% antibiotics (Thermo Scientific, USA) in neurobasal medium (Gibco, USA). Glial differentiation medium (GDM) contained 2% B27 supplement (Gibco, USA), 2 mM GlutaMax I supplement, 30 ng/mL T3 (Sigma, USA), and 1% antibiotic (Thermo, USA) in Neurobasal medium (Gibco, USA). Finally, NSCs/NPCs were cultured up to a confluency of 90% in DMEM/F12 media supplemented with 2 mM GlutaMax I, 20 ng/mL bFGF, and EGF for motor neuron differentiation. Thereon, NDM was used with 0.1 µM retinoic acid (Sigma, USA) for 7 days and NDM with 0.5 µM purmorphamine (Sigma, USA) for 21 days.
2.13 Statistical Analysis
All pooled data are presented as mean ± SEM. Statistical tests and the number of biological triplicates relevant for individual experiments are stated in Section 3 as well as in the figure legends.
3 Results
3.1 Dissection of Human Embryonic Fetus in Fallopian Tubes
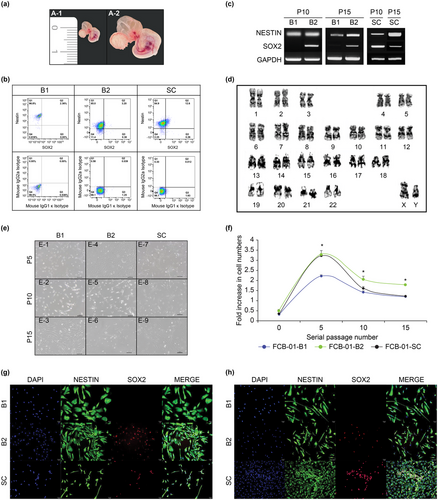
The FCB-01 embryonic fetus, which was isolated from the fallopian tube (uterine tube) during the ectopic pregnancy, had a CRL of 5 mm (Figure 1a. A-1). The FCB-01 embryonic fetus was located inside the amniotic cavity and was connected to the placenta through the umbilical cord, and was within the yolk sac and allantois. FCB-01 had characteristic signs of CS 14 at gestational week 5 (GW 5), with the brain, nasal placode, somite, ocular primordium, branchial arches, cardiac prominence, hepatic capsule, bud of the upper extremity, and bud of the lower extremity structures (Figure 1a. A-2). The CNS of the FCB-01 embryonic fetus was divided into the following anatomical regions: prosencephalon/mesencephalon, mesencephalon/rhombencephalon (B1 and B2), and the SC.
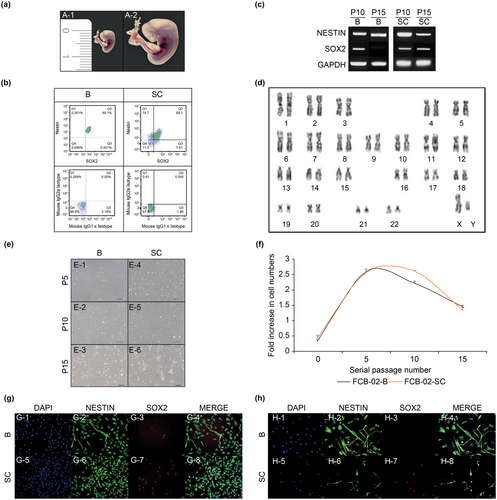
The FCB-02 embryonic fetus was estimated to be CS 15 and GW 6, as the brain, heart, liver, hand plates on the upper limb, lower limb buds, and the somite with a CRL of 8.3 mm could be identified, along with retinal pigments and a foot plate (Figure 2a. A-1, A-2). The CNS of the FCB-02 embryonic fetus was separated into the following anatomical regions: B and SC.
The CRL of the FCB-03 embryonic fetus was stacked on the chorionic villi (Figure 3a. A-1). After carefully removing of the umbilical cord and the amniotic fluid shell, FCB-03 was deposited in the villi, and a viable embryonic FCB-03 fetus was observed (Figure 2a. A-2). The heart, liver, finger ray, retinal pigment, tail bud, footplate, umbilical cord, somite, and brain are shown in Figure 3a, A-3. The FCB-03 was expected to be CS 17 and GW 7, given the various morphological characteristics of human embryonic fetuses CS reported in previous studies (O'Rahilly and Müller 2010; Ostenfeld et al. 2002; Wu 2014). The first trimester human CNS was visually identified as similar (as possible) to the following anatomical regions: telencephalon (Tel), diencephalon (Die), mesencephalon (Mes), metencephalon (Met), myelencephalon (Mye) (B0, B1, B2, B3, and B4), and the SC.

3.2 FACS Analysis of NSCs and NPCs in the Developing Human Embryonic Fetal CNS
We conducted FACS to determine the extent to which the FCB-01, 02, and 03 cell lines maintained the properties of NSCs/NPCs, with several distinct surface markers containing selected surface antigens. FCB-01, 02, and 03 cells were enriched for Sox2 and nestin as unsorted NSCs and NPCs markers at passage 5 by FACS.
FACS analysis revealed that the percentage of cells expressing Sox2 in FCB-01-B1 was 2.36% and 99.16% of the cells expressed nestin, whereas 2.36% of the cells expressed both Sox2 and nestin. In the FCB-01-B2 cells, 88.2% of the cells were nestin-positive, 3.58% were Sox2-containing cells, and 3.20% were nestin and Sox2-positive cells. In FCB-01-SC, 12.86% of the cells expressed Sox2, 97.5% expressed nestin, and 12.6% expressed both Sox2 and nestin (Figure 1b).
In FCB-02-B, 99.1% of the cells expressed nestin and Sox2 simultaneously, 99.55% expressed Sox2, and 99.46% expressed nestin. In FCB-02-SC, 73.91% cells expressed Sox2, 68.1% expressed both Sox2 and nestin, and 82.8% expressed nestin (Figure 2b).
The percentage of cells expressing Sox2 in FCB-03-B0 was 97.78%; 96.6% expressed Sox2 and nestin, and 97.74% expressed nestin. In FCB-03-B1, 98.52% cells expressed Sox2, 94% expressed both Sox2 and nestin, and 94.7% cells expressed nestin. In FCB-03-B2, 99.55% of the cells expressed Sox2, 99.46% expressed nestin, and 99.1% of cells expressed both Sox2 and nestin.
In FCB-03-B3, 93.38% of the cells expressed Sox2, 86.4% expressed both Sox2 and nestin, and 88.61% expressed nestin. In FCB-03-B4, 67.53% of the cells expressed Sox2, 65.9% expressed both Sox2 and nestin, and 93% expressed nestin. FCB-03-SC included 99.4% nestin-positive cells, 22.5% Sox2-positive cells, and 22.5% nestin- and Sox2-positive cells. (Figure 3b).
To eliminate the non-specific binding of cells, mouse IgG1 κ isotype and mouse IgG2a isotype were used as control. The isotype controls were prepared simultaneously using the same method for all the samples (Figures 1, 2, and 3b).
The results indicated that FCB-01-B1/B2/SC were mostly NPCs, whereas FCB-02-B/SC were mostly NSCs/NPCs. Furthermore, FCB-03-B0/B1/B2/B3/B4 were mostly NSCs/NPCs, whereas FCB-03-SC were mostly NPCs.
3.3 RT-PCR Analysis of Nestin/SOX2 Expression and Karyotyping of Human Embryonic Fetal NSCs/NPCs
To further define the relationship between NESTIN and SOX2 expression in the human embryonic CNS, we investigated the mRNA levels of FCB-01/02/03-NSCs/NPCs cell lines. The expressions of NSCs/NPC genes (SOX2 and NESTIN) were detected using their respective RT-PCR primers.
Both prosencephalon/mesencephalon-and mesencephalon/rhombencephalon-derived NSCs/NPCs (B1 and B2) separated from the brain of FCB-01 expressed NESTIN in passages 10 and 15, whereas SOX2 were expressed only in B2 at passages 10 and 15 (Figure 1c). In the SC separated from FCB-01, NESTIN and SOX2 expression was confirmed at passages 10 and 15 (Figure 1c).
In the B region of FCB-02, NESTIN was expressed in both passages 10 and 15, whereas SOX2 was expressed only in passage 10 (Figure 2c). We found that in the SC separated from FCB-02, both NESTIN and SOX2 were expressed at passages 10 and 15 (Figure 2c).
Through RT-PCR, nestin was expressed in passages 10 and 15 of Tel, Die, Mes, Met, and Mye (B0, B1, B2, B3, and B4) cells separated from the brain of FCB-03, whereas SOX2 was only expressed in B1, B3, and B4 (Figure 3c). NSCs/NPCs isolated from the SC of FCB-01 expressed NESTIN and SOX2 weakly in passages 10 and 15 (Figure 3c).
Karyotype (cytogenetic) analysis was performed to confirm the stability of chromosomes in the NSCs/NPCs of FCB-01/02/03 cells at passage 15. Cytogenetic analysis of NSCs/NPCs at passage 15 revealed normal 46 XY (FCB-01) cells (Figure 1d) and 46 XY (FCB-02) (Figure 2d) and 46 XX (FCB-03) (Figure 3d) Karyotype analysis revealed that all the analyzed metaphases were free of any discernable cytogenetic abnormalities.
3.4 Proliferation and Morphological Changes of NSCs/NPCs
To examine the long-term growth kinetics of primary human embryonic fetal NSC/NPC cultures, cumulative population doubling was measured, and cell morphology was observed with respect to the passage number in each NSC/NPC cell line from FCB-01, FCB-02, and FCB-03.
In the early passages of cultivation (passages 0–5), the NSCs/NPCs showed a spindle-shaped and fibroblast-like appearance; however, NSCs/NPCs gradually changed their morphology with continued serial culture. At the intermediate stage, some NSCs/NPCs were irregular and showed intercellular connections that became increasingly larger (passages 5–10). In the later phase (passages 10–15), more NSCs/NPCs appeared uneven and flattened in the cell body, and more inclusions were also found in the cytoplasm.
FCB-01-B1 showed morphological features similar to those of neurons or neuroglial cells from early culture to passage 10, and they were more similar to neuronal cells at passage 15 (Figure S1b). FCB-01-B2 began to demonstrate some cells growing as if spreading to the bottom of the plate from passage 5, and the dendrites were characterized by a thin and long extension at passages 10 and 15; the number of cells did not seem to increase (Figure 1e). However, in FCB-01-B1 and B2, the cell morphology changed, and the number of NSCs/NPCs was significantly reduced, which was also confirmed by a fold increase in the cell number at passage 15 (Figure 1f).
FCB-02-B and FCB-02-SC also took the shape of neuronal cells, extending axons or dendrites around the cell body from the initial culture to passage 15 (Figuer 2e). However, FCB-02-B and SC also changed the morphological shape of the cells, and the overall number of cells was greatly reduced, which was confirmed by the fold increase in the cell number at passage 15 (Figure 2f).
Representative morphological results of NSCs/NPCs derived from the brain (B0, B1, B2, B3, and B4) and SC at passages 5, 10, and 15 from FCB-03 are presented. FCB-03-B0 cells showed numerous branches characteristic of neuronal cells, from normal somatic cells to passage 5 (Figure 3e). However, B0 of FCB-01 maintained the same cell shape from passages 1 to 5, whereas the soma increased from passage 6, and axons and dendrites were clearly visible (Figure S1a). FCB-03-B0 began to resemble neurons and neuroglial cells at passage 10, and this was maintained up to passage 15. FCB-03-B0 showed a pattern of spreading and growing on the bottom of the plate as it approached passage 15, but the number of cells was confirmed to decrease as the passages continued.
FCB-03-B3 also showed a network of numerous branches characterized by common neurons and neuroglial cells from early cultures, and it was confirmed that from passages 10 to 15, they changed to shapes similar to neurons and neuronal cells. In addition, it was confirmed that the number of cells of FCB-03-B1, FCB-01-B3, and FCB-03-SC were significantly higher at passage 15 than at passage 10.
Fold increase in the number of NSCs/NPCs from the brain and SC of FCB-01, 02, and 03 of three different donors and various GWs were confirmed to grow steadily over the observation period until passage 15. We analyzed the number of cells by marking them as a fold change in cell number, as the size of the same tissue site is different due to colonization, even if it was the same tissue site as of FCB-01, 02, and 03.
The cell proliferation rates of FCB-01-B1, FCB-01-B2, and FCB-01-SC continued to increase from passages 0 to 5. In a later passage, at passage 5, the confluency of the FCB-01-B2 culture was twice as much as FCB-01-B1 and -SC. From passage 10 to 15, FCB-01-B1 and -SC showed a tendency to decrease, whereas FCB-01-B2 comparatively maintained an approximately 2-fold increase in the cell number even in passage 15 (Figure 1f). FCB-02-B continued to increase the number of NSCs/NPCs from passage 1 to 6, and FCB-02-SC from passage 7 to 8, but then decreased (Figure 2f). Both FCB-03-B0/B1/B2/B3/B4 and FCB-03-SC were confirmed to have more cells from passages 1 to 15, which was different from the FCB-02 and FCB-03 cell lines (Figure 3f).
3.5 The NSCs and NPCs of Various Markers by Immunofluorescence Staining
To confirm whether the FCB-01-B1/B2/SC, FCB-02-B/SC, and FCB-03-B0, B1, B2, B3, B4, and SC cell lines had characteristics of NSCs or NPCs, immunofluorescence staining was performed using estin and Sox2 at passages 10 and 15. We conducted fluorescent staining for TuJ1 and MAP2 to determine the neuronal cell characteristics of each embryonic fetal cell line (FCB-01, FCB-02, and FCB-03).
Immunostaining was performed on FCB-01-B1, B2, and SC using nestin antibodies at passage 10 (Figure 1g. G-2, G-6, G-10); Sox2 was only expressed in FCB-01-B2 cells and SC (Figure 1g. G7, G-11), whereas FCB-01-B1 cells did not show Sox2 expression (Figure 1g. G-3). FCB-01-B1, B2, and SC all expressed nestin at passage 15 (Figure 1h. H-2, H-6, H-10), but Sox2 was only expressed in FCB-01-B2 and SC cells (Figure 1h. H-7, H-11). In addition, FCB-01-SC were found to have a significantly higher number of NSCs/NPCs expressing nestin and Sox2 at passage 15 than at passage 10 (Figure 1h. H-12).
In FCB-02-B and SC, nestin and Sox2 were found to be expressed at passages 10 and 15, respectively. It was confirmed that FCB-02-B and SC expressed nestin at passages 10 and 15 (Figure 2g. G-2, G-6, H-2, H-6), but Sox2 was not expressed in FCB-02-B (Figure 2h. H-3).
Finally, we confirmed that nestin was expressed at both passages 10 (Figure 3g. G-2, G-6, G-10, G-14, G-18, G-22) and 15 in FCB-03-B0, B1, B2, B3, B4, SC (Figure 3h. H-2, H-6, H-10, H-14, H-18, H-22), and Sox2 was expressed at passage 10 (Figure 3g. G-7, G-15, G-19), except for in B0 and B2 (Figure 3g. G-3, G-11). At passage 15, Sox2 was well-expressed in FCB-03-B1, B3, B4, and SC (Figure 3h. H-7, H-15, H-19, H-23), and FCB-03-B0, B2 were not expressed for Sox2 (Figure 3h. H-3, H-11).
3.6 Induction of Neuron-Restricted Progenitor Cells and Motor Neuron Differentiation in Human Embryonic Fetal Brain and SC-Derived NSCs/NPCs
FCB-01-B2, FCB-01-SC, and FCB-02-B, along with FCB-02-SC, FCB-03-B1, and FCB-03-SC, were the strongest in their groups; therefore, we considered them suitable for confirming their unique differentiation ability and conducted the following neuronal differentiation experiments.
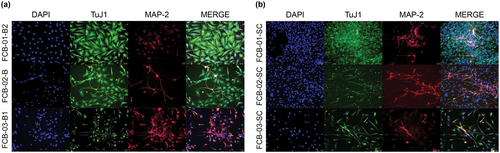
FCB-01-B2, FCB-02-B, and FCB-03-B1 were confirmed to very strongly express TuJ1 and MAP2 after neuronal differentiation upon immunofluorescent staining, and the spreading of the axons and dendrites was clearly confirmed (Figure 4a). Immunofluorescent staining for FCB-01-SC, FCB-02-SC, and FCB-03-SC with their respective markers, TuJ1 and MAP2, after neuronal differentiation confirmed the spread of axons and dendrites similar to the brain-derived cells (Figure 4b).
To confirm the differentiation ability of motor neurons, we proceeded according to the differentiation conditions and confirmed the results using immunofluorescence staining.
FCB-01-B2, FCB-02-B, and FCB-03-B1 all showed typical motor neurons, and it was confirmed that the markers ChAT and HB9 were also strongly expressed (Figure 5a). FCB-01-SC, FCB-02-SC, and FCB-03-SC also induced motor neuron differentiation in the same manner, and when confirmed by fluorescent immunostaining, they were also found to be well expressed (Figure 5b).
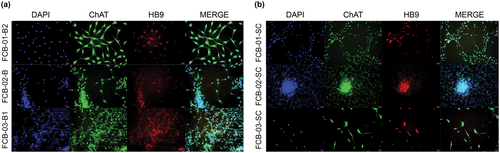
3.7 Differentiation of Glial-Restricted Progenitor Cells of Human Fetal Embryonic Brain and SC-Derived NSCs and NPCs
To determine whether NSCs/NPCs isolated from the CNS of the embryonic fetus had the ability to differentiate into glial-restricted progenitor cells, we differentiated them under glial progenitor cell conditions.
When confirmed through immunofluorescent staining on the astrocyte marker, GFAP, FCB-01-B2 was found to not differentiate into astrocytes, whereas the differentiation to astrocytes in both FCB-02-B and FCB-03-B1 were confirmed. Immunofluorescence staining was performed using Olig-2, a marker of oligodendrocytes, and we confirmed that Olig-2 was very strongly expressed in FCB-01-B2, FCB-02-B, and FCB-03-B1. When checking the merged results, it was confirmed that GFAP and Olig-2 were slightly co-localized in the case of FCB-02-B but hardly co-localized in FCB-03-B1 (Figure 6a).
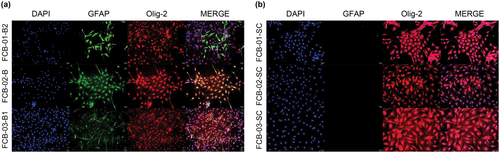
After FCB-01-SC, FCB-02-SC, and FCB-03-SC differentiated into glial-restricted progenitor cells, we confirmed it by immunofluorescence staining. Neither FCB-01-SC nor FCB-03-SC expressed GFAP; only FCB-02-SC expressed it. However, it was confirmed that Olig-2 was strongly expressed in FCB-01-SC, FCB-02-SC, and FCB-03-SC. When all of these results were verified by the “Merge” function, it was confirmed that GFAP and Olig-2 were slightly co-localized in FCB-02-SC (Figure 6b).
3.8 Regional-Specific Heterogeneity Verification of Human Embryonic Fetal CNS-Derived NSCs/NPCs
RT-PCR was performed for each region-specific gene to determine whether there were any differences in the cells of CNS tissue origin for FCB-01/02/03-NSCs/NPCs.
These genes were identified in the same manner as those in the FCB-01 embryonic fetal brain-derived NSCs/NPCs. When the amount of mRNA expression was checked using the marker of each site with FCB-01-brain-derived NSCs/NPCs, FOXG1, EMX2, OTX2, EN1, and EN2 were found to have higher expression in B1 than B2, and GBX2 and HOXAB4 had higher expressions in B2 than B1 (Figure 7b). As a result, we confirmed that FCB-01-B1 appears to be the tissue-derived NSCs/NPCs that is closer to the rostral region of the brain and that B2 is the region corresponding to almost the caudal region of the brain-derived NSCs/NPCs. (Figure 7a). When examining NSCs/NPCs isolated from the entire FCB-02 brain, we confirmed that the rostral and caudal markers of the brain were evenly expressed at high amounts (Figure 7b). The FCB-03 embryonic fetal brain was divided into five sites. FCB-03-B0 and B1 had higher expression of FOXG1 and EMX2 than the other FCB-03-NSCs/NPCs lines; FCB-03-B2 had higher expression of OTX2, EN1, and EN2 genes than the other FCB-03-NSCs/NPCs lines; and finally, FCB-03-B3 and B4 were found to have higher expression of GBX2 and HOXAB4 than the other FCB-03 cell lines (Figure 7b).
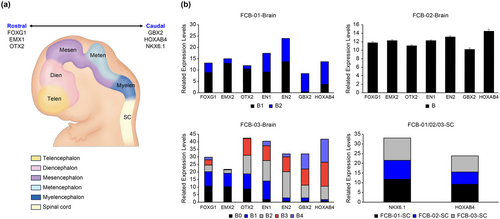
In the case of NSCs/NPCs obtained from the SC of FCB-01/02/03, it was confirmed that both NKX6.1 and HOXAB4 were highly expressed (Figure 7b).
4 Discussion
In the early stages of pregnancy, ultrasound is most commonly used to predict the age of the fetus or the pregnancy cycle of the mother on the basis of the CRL of the fetus. In the first trimester, measuring the fetal CRL helps estimate the GW, whereas after the first trimester, the fetal head (BPD and HD), body (AC), and limb (FL) measurements are used to evaluate the GA. However, within 3 months of pregnancy, this method has potential errors. This is because CRL measurements are used to determine fetal age in pregnancies with unknown menstrual dates or pregnancies with CRL measurements greater than ±7 days; thus, it may be accurate to determine fetal age using menstrual dates (MacGregor and Sabbagha 2008; Ouyang et al. 2020) if CRL and menstrual days are within the normal measurement error range. For a more accurate judgment, it is thought that using a combination of fetal parameters such as BPD, HC, AC, and FL in the ultrasound evaluation of fetal GA in the first trimester would be more accurate than CRL or GA alone (Jakal et al. 2007; MacGregor and Sabbagha 2008). As mentioned earlier, human NSCs/NPCs are mostly present only in the brain and SC of embryonic fetuses, and for the purpose of developing therapies for neurological disorders, analyzing the characteristics of NSCs/NPCs more closely according to the age and tissue origin of human embryonic fetuses is necessary.
We examined FCB-01 (GW 5, CS15), FCB-02 (GW 6, CS15), and FCB-03 (GW 7, CS 17) embryonic fetal brain and SC derived NSCs/NPCs to check for their characteristics by area of fetal age, sex, and region of CNS tissue. We attempted to divide each part of the brain as much as possible using a dissection microscope, and the SC was used to separate the whole brain. In this study, we examined the cultures of NSCs/NPCs derived from the B and SC of FCB-01, FCB-02, and FCB-03 up to passage 15. Although the results were interestingly cultured under the same culture conditions, the form of NSCs/NPCs differed in the same passage, and this pattern was the same for FCB-02, and 03 in NSCs/NPCs derived from the B and SC.
However, these reasons are likely to be a natural outcome. In this study, the nervous system cells inherent in each cause of the tissue type must exist naturally and heterogeneously because they are not specifically markers isolated after tissue separation, according to anatomical findings (Fuentealba et al. 2015; Rushing and Ihrie 2016). NSCs and NPCs separated from the brain and SC of embryonic fetal tissue that we isolated would form heterogeneous cell groups.
We conducted various experiments to confirm whether the cells isolated from embryonic fetal-derived CNS tissues are NSCs/NPCs and had neural stemness across passages. NSCs/NPCs of FCB-01, FCB-02, FCB-03-brain, and SC expressed nestin in passages 5, 10, and 15. However, NSCs/NPCs isolated from the brains of FCB-01-B1 at passage 10/15 did not express SOX2. NSCs/NPCs isolated from FCB-02-B did not express SOX2 at passage 15. In addition, NSCs/NPCs isolated from FCB-03-B0/B2 did not express SOX2 at passage 10 or 15. The transcription factor, SOX2 known as the “NSC stemness” factor, is required for the maintenance of embryonic and NSCs. These NSCs must be functionally capable of differentiating into neurons and glial cells (Mercurio et al. 2019). In the early developmental stages of NSC, SOX2 and NESTIN are co-expressed, but in the later stages of NSCs, only nestin is predominantly expressed. SOX2 is expressed only until the early stages of NSCs and not expressed thereafter (Brazel et al. 2005; Farioli-Vecchioli et al. 2014). According to the results of the fold increase in cell numbers, for FCB-01-B1, the time when the value that went to 3.5 began to decrease to 1.5 was at the tenth passage number, and it remained similar until passage 15. In the case of FCB-02-B/SC, the fold increase in cell number also declined from passage 10. However, in all cases of FCB-03, the value of the fold increase in cell numbers showed an upward trend until passage 15; at the end of passage 15, the value of B0/B2 was the lowest, near 1.5, and that of B1/B3/SC was the highest, near 2.5.
Thus, we confirmed that the presence or absence of Sox2 expression in NSCs/NPSCs was highly related to the maintenance of self-renewal in NSCs. We confirmed the number of neurons in our NSC/NPC-undifferentiated cell line and confirmed that FCB-01-B1 and FCB-03-B2 did not express TuJ1, a marker of immature neurons, in passage 15, and that only the mature neuron marker, MAP-2, was clearly expressed, which is associated with the presence or absence of Sox2 expression during the early developmental stage of ESCs.
Nestin-positive NSCs/NPCs may differentiate into a variety of CNS cells, including neurons and glial cells (Llorente et al. 2022). We selected FCB-01-B2, FCB-02-B, FCB-03-B1, and FCB-01/02/03-SCs, which are the cell lines with the highest fold increase in cell number at passage 5, to confirm their capacity to differentiate into neurons, motor neurons, and glial-restricted progenitor cells, and demonstrated results of much intrigue. FCB-01-B2, FCB-02-B, FCB-03-B1, and FCB-01/02/03-SCs confirmed that TuJ1/MAP-2 and ChAT/HB9 maintained their differentiation capacities very well after neuronal and motor neuron differentiation. In addition, the results of the differentiation of the glial-restricted progenitor cells showed that only FCB-01/02/03-SCs did not express GFAP, an astrocyte marker, and the oligodendrocyte marker, Olig-2, were clearly expressed. The above results suggest that the cell population and its characteristics differ depending on the individual and body part of the embryonic fetus.
However, when the NSC/NPC lines of FCB-01, FCB-02, and FCB-03 were checked overall, the cell line FCB-03, which had the highest CS and CRL, showed the possibility of immature NSCs by expressing Sox2 the most, even at passage 15. Additionally, the cell growth rate continued to increase until passage 15, and it was predicted that this would be the best cell line when developed for cell and gene therapy compared to FCB-01 and FCB-02.
Mammalian CNS development necessitates cell proliferation and differentiation into various types of NSCs/NPCs. In the developing CNS, NSCs/NPCs are managed by a firmly regulated sequence of signals that oversee the proliferation and differentiation of tissue types, unlike neuronal cell types (neurons, astrocytes, and oligodendrocytes) that eventually inhabit the mature brain and SC (Christie and Turnley 2012; Wen et al. 2009). NSCs are self-renewing multipotent cells that generate neurons, astrocytes, and oligodendrocytes. NPCS have a restricted life expectancy, less self-renewal ability, and may be multipotent or unipotent (Llorente et al. 2022;Ottoboni et al. 2020). Heterogeneity of NSCs and NPCs mainly exist in the ventricular zone (VZ) and SVZ at the human embryonic fetal stages (Gilbert et al. 2022; Rushing and Ihrie 2016).
The morphology of human NSCs and NPCs changes over time in culture and region of the CNS. The embryonic fetal brain develops difficulties due to the expansion of neural tubes called vesicles. The primary stage has three regions (forebrain, midbrain, and hindbrain), whereas the secondary vesicle stage has five regions (telencephalon, diencephalon, mesencephalon, metencephalon, and myelencephalon) (Chhetri and Das 2024). The telencephalon and diencephalon (prosencephalon) develop into the forebrain during embryonic fetal brain development. The forebrain contains glutamatergic neurons, cortical astrocytes, GABAergic neurons, striatal astrocytes, cholinergic neurons, forebrain astrocytes, and oligodendrocytes (Ma et al. 2022; Tao and Zhang 2016).
The transcription factor forkhead box G1, FOXG1, is expressed in the developing CNS and plays a critical role in forebrain development, forebrain NPCs induction, and formation of forebrain neuron. The ectopic expression of FOXG1 is selectively over-proliferated in the anterior brain-forming telencephalon and mesencephalon and not in other brain regions (Hettige and Ernst 2019; Hou et al. 2020; Walshe and Mason 2003). FOXG1 gene expression can occur in the forebrain (Horiuchi et al. 2017). The transcription factor, FOXG1, is essential for the normal development of the telencephalon and dorsal diencephalon and is known to have a great influence on some of the outgrowths of the mesencephalon (Ahlgren et al. 2003; Hettige and Ernst 2019; Hou et al. 2020; Liu et al. 2018; Magni et al. 2022; Wong et al. 2019). Additionally, multiple signals are required for the correct specification of the telencephalon, including bone morphogenetic proteins (BMPs), wingless/integrated proteins (WNTs), extracellular signal fibroblast growth factor 8 (FGF8), and sonic hedgehog (SHH) (Chandwani et al. 2019; Furuta et al. 1997; Hettige and Ernst 2019; Lee et al. 2000; Rotheneichner et al. 2014; Walshe and Mason 2003). EMX2, a homeobox gene 2, is expressed in the dorsal telencephalon, including the olfactory bulbs, and dorsal and ventral diencephalon. EMX2 expression is also localized in the hippocampus (Wigle and Eisenstat 2008; Zeng et al. 2023). In humans, the orthodenticle homeobox 2 (OTX2) gene is expressed in the diencephalon, mesencephalon, and cerebellum and is crucial for the development of these brain regions (Larsen et al. 2010; Zeng et al. 2023). OTX2 is known to develop in the caudal forebrain and midbrain and is involved in the differentiation of young neurons in the cortical layer or in the requirement for rostral CNS specification (Georgala et al. 2011; Larsen et al. 2010).
Paired Box 6 (PAX6) was robustly expressed in the proliferative neuroepithelia of the VZ in the forebrain and hindbrain, and the transcription factor, PAX6, has been reported to specify NPC fates during development (Georgala et al. 2011; Kim et al. 2018; Matsunaga et al. 2000). In the embryonic fetal brain, PAX6 expression was detected in the forebrain (telencephalon and diencephalon), midbrain (mesencephalon), and hindbrain (metencephalon and myelencephalon). In the early stage of neural development, PAX6 is expressed in the prosencephalon, whereas homeobox transcription factor engrailed 1 (EN1) and paired box 2 (PAX2) are expressed in the mesencephalon (Matsunaga et al. 2000).
EN1, EN2, PAX2, and PAX5, encoded, respectively, by the homeobox genes EN1 and EN2 and the paired box genes PAX2 and PAX5, are transiently expressed in the midbrain and hindbrain junctions of the embryo. Targeted gene inactivation revealed a critical role for EN1 in the early specification of the entire midbrain region, whereas the function of EN2 is restricted to cerebellar patterning. Herein, we present evidence that the cooperation of PAX2 and PAX5 is indispensable for the function of the organizing center at the midbrain and hindbrain junctions (Chhetri and Das 2024; Gilbert et al. 2022; Harada et al. 2016; Urbánek et al. 1997).
In addition, the diencephalon and midbrain boundaries are determined by the repressive activity of PAX6 and EN1, and the mid-and hindbrain boundaries are determined by the repressive activity of OTX2 and GBX2, which are expressed from the early stage of development, covering the rostral to the midbrain and hindbrain, respectively (Harada et al. 2016; Nolte and Krumlauf 2000–2013; Tailor et al. 2013; Zeng et al. 2023).
GBX2, NKX 6.1 and HOXB4 are well known as regional markers of caudal hindbrain and SC in human (Magni et al. 2022; Tao and Zhang 2016). Homeobox B4 (HOXB4) is a member of the antennapedia (Antp) homeobox family and encodes a nuclear protein with a homeobox DNA-binding domain, which is expressed in the nervous system of vertebrates (Li et al. 2016; Shu et al. 2015). HOXB4 is also a regional marker of the developing hindbrain and the SC in human embryos. Expression of NKX 6.1 in NSCs/NPCs during ventral SC development in human and NKX6.1 is both expressed in the developing CNS, where they are involved in specifying neuronal progenitor identity (Li et al. 2016).
Our findings also confirmed the expression of PAX6 in FCB-01 (B1 and B2), and FCB-02-B, FCB-03 (B0, B1, B2, B3, and B4); however, it was ambiguous to conclude a specific region alone, as it was generally expressed in the early stages of brain development.
To confirm the correlation between the different characteristics of embryonic fetal NSCs/NPCs and individual and regional differences, we identified more detailed anatomical locations of the isolated embryonic fetal NSCs/NPCs by RT-PCR using each brain and SC region marker gene. According to the RT-PCR results of the FCB-01 embryonic fetal brain tissue-derived NSCs/NPCs, the B1 cell line highly expressed FOXG1, EMX2, and OTX2, and it was inferred that it was derived from the vicinity of the telencephalon and diencephalon tissues. B2 showed high expression of EN1, EN2, GBX2, and HOXAB4, and it was confirmed that cells derived from the vicinity of the mesencephalon, metencephalon, and myelencephalon tissues were more strongly expressed. FCB-02 fetuses were separated into the brain and SC due to their low main age and CRL, and the whole brain-derived NSCs/NPCs showed almost equal expression of the genes forming the tissues of telencephalon, diencephalon, mesencephalon, metencephalon, and myelencephalon. Furthermore, NKX6.1 and HOXBS4 were well expressed in whole SC-derived NSCs/NPCs. Finally, upon dividing the FCB-03 embryonic fetus brain tissue into five parts, the B0 and B1 cell lines were found to have strong expression of FOXG1 and EMX2; the B2 cell lines had high expression of OTX2, EN1, and EN2; and B3 and B4 had strong expression of GBX2 and HOXAB4. Consequently, it was confirmed that there was a clear difference in expression of each marker depending on the CNS tissue site.
Thus, numerous NSCs and NPCs were also present in our FCB-01, 02, and 03 brain and SC organizations. The origin and classification of neural NSCs/NPCs have long been the subject of intense research, and efforts to classify NSCs/NPCs by location, function, and expression have already demonstrated that these cells are present as heterogeneous pools in both embryonic and adult brains (Gilbert et al. 2022; Rushing and Ihrie 2016; Tao and Zhang 2016). The heterogeneity of NSCs/NPCs revealed that postnatal NSCs/NPCs are produced during embryonic development and share common percussors with cells of the cortex, striatum, and septum. The regional classification of these precursors is established during embryonic development, and the full plasticity of NSCs/NPCs is likely to be limited in a very short time (Fuentealba et al. 2015; O'Donoghue and Fisk 2004).
Fan et al. (2020) had derived embryonic brain samples from GW 7, GW 8, and GW 9, but the tissues were too small to be separate into regions. Single-cell transcriptomic analysis revealed that NSCs/NPCs were logical excitatory neurons and excitatory neuron-like cells in the pons, whereas the cortical is said to be composed of interneurons, astrocytes, oligodendrocyte lineage cells, and immune cells (such as microglia, macrophages, and T cells), endothelial cells, and blood cells (Fan et al. 2020). Additionally, in the GW 20 fetal brain, astrocytes, neurons, and the early development of immature oligodendrocytes were found to be mainly distributed in the cortical regions and pons (Adams and Morshead 2018; Rushing and Ihrie 2016).
Although the present literature reports much progress in the characterization of adult brain-derived NSCs, the mechanistic systems and behaviors related to adult NSCs are still ongoing, and studies on the characteristics and systems of NSCs in the embryonic brain remain insufficient. A closer examination of the site-specific function and key features of NSCs in the embryonic brain will not only reveal the mechanisms of cell programming and determinants of cell fate but would also provide great potential for guiding treatments for many diseases with neurological phenotypes. The proliferation potential of adult brain-derived NSCs is very limited, but the proliferation potential of embryonic brain- and SC-derived NSCs is very high; therefore, further branching and research is crucial. Furthermore, solving the multiple layers of embryonic fetal NSCs heterogeneity and their functional outcomes in the future will have a significant impact on realizing the normal brain function, plasticity, and repair in adults too. Finally, this would also help develop novel cell and gene therapy modalities. In addition, analysis of the characteristics of NSCs by race, GW, sex, and family history of embryonic fetuses and a comprehensive understanding of heterogeneity are required, which will allow us to provide insights into the cellular and molecular regulation of neural development and neurogenesis, which will help develop new strategies to promote neural regeneration and neural repair (Kapfhamer et al. 2018). Among the limitations of this study, only voluntarily donated fetuses were studied, as characterizing birth fetuses without issues such as ectopic pregnancy is not possible. Therefore, fetal age was predicted by referring to CRL, human fetal development stage, and the CS. Two of the three embryonic fetuses (FCB-02 and FCB-03) almost matched the CRL and CS, but one (FCB-01) was slightly smaller than the CRL, and it differed in its developmental stage and CS. The most likely reason is that most ectopic pregnancy embryonic fetuses do not develop properly; other factors include race, maternal health, genetic effects, and in vitro fertilization (Brand et al. 2018; Kapfhamer et al. 2018; Dubinsky et al. 1996; Huang et al. 2014). Further, finding specific donors of embryonic fetuses in the fallopian tubes and thereon successfully separating the brain and SC tissues of the fetuses remains an immense challenge. Under Korean medical law, receiving a donation of a fetus up 6–7 weeks of age is not possible, as methotrexate is prescribed to the mother when the ectopic pregnancy sac is less than 3–4 cm in size. However, with more than 9 general hospitals within South Korea and overseeing 91 medical institutions in 7 countries around the world, our institution has the resources to avail fetal NSCs/NPCs donations derived from ectopic pregnancies for future research. Further, our findings are to be reinforced with in-depth data and systematic reviews.
On the basis of this study, we will continue our investigations on the NSCs/NPCs derived from various fetal age-specific subdivided brain and SC tissues using various methods. The accumulated results and observing similarities across the heterogenous factors (such as ethnicity) can be harnessed to develop treatments for intractable CNS diseases. We hope that this cornerstone study will encourage additional national projects to fund human fetal CNS-derived NSC/NPC-based treatments for CNS diseases.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (RS-2023-00243835).
Ethics Statement
The fallopian tissues from mothers who agreed to voluntary donations before surgery were obtained after surgical separation. The donated ectopic pregnancy samples and maternal blood and urine samples were obtained following the guidelines of the Committee of Medical Ethics of CHA Bundang Medical Center of CHA University (IRB Permit No. 2021-07-008) and CHA Gangnam Medical Center (IRB Permit No. 2021-03-031).
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data supporting the findings of this study are available within the article and its Supporting Information section.