Developmental Disturbances in Animal Models of Autism Spectrum Disorder
Funding: This work was supported by projects 2/0057/23 of the Grant Agency of the Ministry of Education and Slovak Academy of Sciences (VEGA), Hungarian-Slovak Bilateral Collaboration Grant HAS-SAS-2022-02/HAS—NKM2023-6/2023 and by the Slovak Research and Development Agency projects APVV-21-0189, National Brain Research Program (NAP 3.0) of the Hungarian Academy of Science, and the Thematic Excellence Program 2021 Health Sub-program of the Ministry for Innovation and Technology in Hungary (within the framework of the TKP2021-EGA-16 project of the Pécs University).
ABSTRACT
Although the early signs of autism spectrum disorder (ASD) are widely studied, the significant ambiguity and heterogeneity in symptoms require the comparison of available models, approaches, and the search for common denominators and key indicators. Early ASD symptoms in animal models include impaired somatic development (e.g., delayed eye opening), alterations in primitive motor reflexes, disrupted sensory function as well as communication deficits, such as reduced ultrasonic vocalization. This review aims to summarize early ASD-related symptoms based on studies involving transgenic or neurotoxic rodent models (postnatal days 1–21) and to compare these with human resemblance. The key brain areas (subventricular zone, cortex, hippocampus, cerebellum, etc.) as well as relevant neurotransmitter systems (GABA-glutamate imbalance, developmental GABA shift, serotonin, dopamine, oxytocin [OT], etc.) were identified as potential targets for intervention. OT, although a promising candidate, exemplifies the translational challenges inherent in ASD research. Therefore, it is recommended to monitor a wide range of behavioral signs simultaneously and employ diverse models (e.g., genetic, developmental, environmental, or combination) in preclinical studies to more accurately reflect the complexity of the disorder.
1 Introduction
Autism spectrum disorders (ASD) represent a range of complex neurodevelopmental conditions, characterized by core symptoms in social communication and repetitive behavioral patterns. Autistic symptoms often also include changes in perception in both positive (hyperresponsiveness) and negative (hyporesponsiveness) directions (Jensen and Girirajan 2017; Goncalves and Monteiro 2023; Baranek et al. 2013; Feldman et al. 2022). One critical sensory change of ASD patients is observed in the sense of smell (Sweigert et al. 2020), which requires more attention, as olfaction plays a significant role in neurogenesis and early brain development (Grubb et al. 2008). Furthermore, olfaction significantly influences social interaction and behavior, which are key issues in ASD (Hartig et al. 2021). The prevalence of ASD has been increasing, and current estimates suggest that approximately 1%–2% of children are affected globally (Zeidan et al. 2022). ASD emerges from a complex interplay between genetic susceptibility and environmental influences (Cheroni et al. 2020; Love et al. 2024). Although ASD appears early during development, vast majority of available data are derived from older children as well as from adult animal models. Nevertheless, it would be essential to focus on early developmental alterations.
Although the symptoms of ASD are relatively well characterized, they can vary greatly among individuals. These variations depend on factors such as age, sex, and the severity of the condition (Hyman et al. 2020). For example, sensory stimulation may not lead to hyperactivity in certain age groups of children but can manifest more prominently in preteens (Cakar et al. 2023). Additionally, some studies suggest that females may exhibit different autistic phenotypes compared to males (Van Wijngaarden-Cremers et al. 2014). The most consistently diagnosed qualitative signs of ASD include language delay, avoidance of eye contact, diminished response to one's name, and excessive fear, which may lead to reduced social interaction (Spain et al. 2020; Hirota and King 2023).
Although many human studies rely on neuroimaging techniques, these often lack the resolution needed to fully elucidate underlying neurobiological mechanisms. In addition, behavioral tests used in ASD diagnosis present challenges such as time-consuming evaluations, high cost, limited accuracy, and low confidence in using ASD diagnostic tools due to the lack of know-how (Megerian et al. 2022). These limitations further highlight the need for animal experiments to investigate the mechanisms of ASD. Although animal models cannot fully replicate human ASD symptoms, they offer valuable insights into pathogenesis. Indeed, a growing body of data on ASD mechanism comes from studies using animal models, particularly those focusing on brain development, neurogenesis, and synaptogenesis in the sensory brain areas (Mihalj et al. 2024; Zatkova et al. 2016; Vyas et al. 2021). However, it remains crucial to explore potential parallels between findings in animal models and human developmental processes. Various transgenic mouse models, conditional knockouts (KOs), and chemically induced models provided data on alterations in sensory processing that correlate with changes in social behavior (Kazdoba et al. 2016; Kuruppath et al. 2023). Specific behaviors, such as maternal separation-induced ultrasonic vocalization (MS-USV)—a well-studied symptom associated with anxiety—appear less prominent in certain ASD models suggesting diminished social communication (Wohr 2014). In contrast, other studies have emphasized the importance of developmental milestones delays during early life stages in the context of ASD (Kuo et al. 2022).
This review will focus on the developmental disturbances in animal models of ASD. Early manifestations of ASD are highlighted first in animals and then correlated with human symptoms. As underlying mechanisms, neurodevelopmental problems at certain brain areas and specific neurotransmitter imbalances are summarized in animal models and discussed further as important hubs for therapeutic interventions.
2 Models of Autism
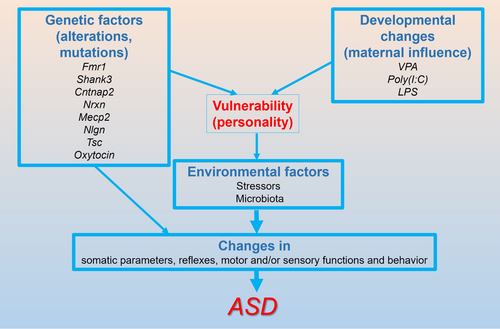
In accordance with the Engels’ biopsychosocial model (Engel 1977) and the three-hit theory (Daskalakis et al. 2013), recent studies suggested that ASD results from a combination of genetic factors, developmental changes, and environmental influences (Cheroni et al. 2020; Laszlo et al. 2023). These factors (Figure 1) likely contribute to structural and functional changes in the brain that underlie the autistic manifestations (Beopoulos et al. 2022; Falougy et al. 2019).
As with the modeling of any disease, including ASD, it is critical that animal models undergo rigorous evaluation based on fundamental validity criteria, of which the following 3 are most often examined: face validity, predictive validity, and construct validity (Willner 1986) (Table 1). ASD models demonstrating strong face validity are characterized by behavioral phenotypes that closely resemble core symptoms of the disorder, including impaired social interactions, deficits in communication, repetitive behaviors with restricted interests, and sensory processing abnormalities. Predictive validity refers to an animal model's ability to accurately predict the therapeutic response to interventions that are effective in humans. However, this aspect of the models remains challenging due to the lack of a universally effective, evidence-based therapy for ASD in humans. A potential example could be oxytocin (OT) administration, which had promising effect on social symptoms in humans (Audunsdottir et al. 2024) as well as has been shown to enhance social behavior in Shank3-deficient mice (Dai et al. 2018) and in valproate (VPA)-exposed rats (Voros et al. 2023). Construct validity refers to the shared underlying biological mechanisms of the models and human ASD. As several genetic alterations as well as environmental factors are known to contribute to ASD development as well as many neurochemical and structural mechanisms that were already implicated, animal models can easily address this criterion. However, it is essential to underscore that significant limitations exist, as many ASD animal models do not fully replicate the complexity and heterogeneity of human ASD.
| ASD animal model | Face validity | Predictive validity | Construct validity |
|---|---|---|---|
| Fmr1 KO mouse (Yang et al. 2022; Pietropaolo et al. 2011) | High (social deficits, repetitive behavior) | Moderate (responds to certain ASD-targeted drugs) | High (genetic relevance to Fragile X Syndrome) |
| Mecp2 KO mouse (Percy 2011; Ornoy et al. 2024) | High (social deficits, repetitive behavior) | (Limited therapeutic response data) | Mutations in the Mecp2 gene are a well-established cause of Rett syndrome, which shares overlapping features with ASD |
| Cntnap2 KO mouse (Peñagarikano et al. 2011; Cording et al. 2024) | Moderate (hyperactivity and epileptic seizures) | (Limited therapeutic response data) | High (genetic association with ASD) |
| Nrxn1α KO mouse (Grayton et al. 2013; Khoja et al. 2023) | Moderate (altered social behavior) | (Limited therapeutic response data) | High (genetic association with ASD) |
| Nlgn3 mutation (Burrows et al. 2015; Wang et al. 2024; Rothwell et al. 2014; Etherton et al. 2011) | High (repetitive behavior, impaired social interactions) | (Risperidone reduced aggression levels) | High (Nlgn3 R451C mutation was first identified in individuals with ASD) |
| Shank3 KO mouse (Jaramillo et al. 2017; Dai et al. 2018) | High (social deficits, repetitive behavior) | Moderate (responsive to oxytocin, risperidone) | High (mutations linked to human ASD cases) |
| Tsc mutation (Kashii et al. 2023) | Moderate (social deficits, abnormal responses to sensory input) | High—mTOR inhibitors—Rapamycin/Everolimus improve social deficit | Tsc mutation is associated with neurological symptoms, including ASD |
| VPA-induced model (Mabunga et al. 2015) | High (social deficits, repetitive behaviors) | Moderate (oxytocin ameliorates some core symptoms) | Moderate (epigenetic influences, but not a direct genetic model) |
| LPS induced model (Okano et al. 2022; Gzieło et al. 2023) | High (repetitive behavior, impaired social interactions) | Administration of anti-inflammatory agents or compounds targeting specific neurotransmitter systems has been shown to ameliorate some ASD-like behaviors | LPS exposure triggers an inflammatory response that mirrors the neuroinflammatory processes observed in individuals with ASD |
- Abbreviations: ASD, autism spectrum disorder; Cntnap2, contactin-associated protein-like 2; Fmr1, fragile X messenger ribonucleoprotein 1; KO, knockout; LPS, lipopolysaccharide; Mecp2, methyl CpG binding protein 2; Nlgn, neuroligin; Nrxn, neurexin; Shank3, SRC homology and multiple ankyrin repeat domains 3; Tsc, tuberous sclerosis complex; VPA, valproate.
Concentrating on the first hit, several genetic—mostly mice—models of ASD exist (see, e.g., Table 2. in Laszlo et al. 2023). According to the Simons Foundation Autism Research Initiative (SFARI) database as of 2025 (https://gene.sfari.org/autdb/Welcome.do), there are currently 1483 genes implicated in ASD. These genes are scored on the basis of the strength of evidence linking them to ASD. It is worth noting that although many genes have been identified, current research suggests that variations in as many as 1000 different genes could affect susceptibility to ASD, though not all these associations have been definitively confirmed (Cirnigliaro et al. 2023; Willsey et al. 2022). As Fragile X syndrome (FRX) is a monogenic cause of ASD and the most inherited form of intellectual disability, it is not surprising that one of the best characterized animal model is the Fmr1 KO mouse (Razak et al. 2020). Another neurodevelopmental disorder sharing common neurobiological basis with ASD is the Rett syndrome, which can be modeled in methyl CpG binding protein 2 (Mecp2) mutant animals (Percy 2011; Ornoy et al. 2024). The contactin-associated protein-like 2 (Cntnap2) is the longest gene in the human genome (Ashley 2016) and encodes cell-adhesion and growth factor repeats. The Cntnap2 KO mouse is also considered an ASD model. However, this genetic disturbance accounts for very few human cases (Alarcón et al. 2008; Brunner et al. 2015; Thabault et al. 2024). Nevertheless, Cntnap2 KO mouse exhibits hyperactivity, deficits in the three-chamber social preference test, and reduced vocalizations in response to the scent of an estrous female (Brunner et al. 2015). Another cell adhesion molecule, Neurexin 1α (Nrxn1), is also important in the vertebrate nervous system, and its mutations are strongly associated with neurodevelopmental disorders such as ASD (Armstrong et al. 2020). Indeed, Nrxn1 KO mice show ASD-like behavioral alterations.
| Changes in ASD | Test | Outcome | Animal model | References |
|---|---|---|---|---|
| Somatic growth | Body length |
↓ ↓ ↓ ↓ |
Nrxn1 KO Nlgn2 KO Arid1b+/− VPA (rat) |
Armstrong et al. (2020) Wohr et al. (2013) Ellegood et al. (2021) |
| Tail deformity | ↑ | VPA | Ruhela et al. (2017), Jiang et al. (2022) | |
| Pinna detachment |
↓ = = = |
Poly(I:C) Shank3 KO VPA LPS |
Lan et al. (2023) Pillerova et al. (2022) Wang et al. (2018) Foley et al. (2014) |
|
| Incisor eruption |
↓ ↓ ↓ = |
Nlgn2 KO Shank3 KO Poly(I:C) LPS |
Wohr et al. (2013) Pillerova et al. (2022) Lan et al. (2023) Foley et al. (2014) |
|
| Ear opening | ↓ | Nrxn1 KO | Armstrong et al. (2020) | |
| Eye opening |
↓ ↓ ↓ ↓ ↓ = |
Nlgn2 KO Shank3 KO ♂ VPA LPS Poly(I:C) VPA |
Wohr et al. (2013) Pillerova et al. (2022) Ruhela et al. (2019), Schneider and Przewlocki (2005), Yang et al. (2016) Foley et al. (2014) Lan et al. (2023) Wang et al. (2018) |
|
| Primitive reflexes | Grasping reflex |
↓ ↓ = |
Nrxn1 KO Nlgn2 KO LPS |
Armstrong et al. (2020) Wohr et al. (2013) Ryabushkina et al. (2024) |
| Righting reflex |
↓ = = = = |
Nrxn1 KO Syn2 KO Shank3 KO Arid1b+/− LPS |
Armstrong et al. (2020) Michetti et al. (2017) Pillerova et al. (2022) Ellegood et al. (2021) Foley et al. (2014) |
|
| Cliff avoidance |
= = |
VPA LPS |
Wang et al. (2018) Ryabushkina et al. (2024) |
|
| Negative geotaxis |
↓ ↓ ↓ = |
Nrxn1 KO ♂ Arid1b+/− VPA LPS |
Armstrong et al. (2020) Ellegood et al. (2021) Ruhela et al. (2019), Schneider and Przewlocki (2005), Wang et al. (2018) |
|
| Pivoting locomotion | ↓ | Arid1b+/− | Ellegood et al. (2021) | |
| Locomotion | Grid walking |
↓ = |
VPA VPA |
Wang et al. (2018) Schneider and Przewlocki (2005) |
| Spontaneous movement (open-field) | ↑ | Nrxn1 KO | Armstrong et al. (2020) | |
| Sensory functions | Acoustic startle reflex |
↓ = |
Shank3 KO Nlgn2 KO |
Pillerova et al. (2022) Wohr et al. (2013) |
| Olfactory discrimination | ↓ | VPA | Schneider and Przewlocki (2005), Yang et al. (2016) | |
| Communications deficit | Ultrasonic vocalization |
↓ ↓ ↓ ↓ ↓ ↓ ↓ ↑ ↑ ↑ ↑ = |
Nrxn1 KO Nlgn2 KO Shank KO 1,2,3 Syn 1,2 Arid1b+/− VPA LPS Fmr1 KO BTBR Tsc1 KO 15q11-13 Poly(I:C) |
Armstrong et al. (2020) Wohr et al. (2013) Wohr (2014), Schmeisser et al. (2012) Michetti et al. (2017) Ellegood et al. (2021) Wohr (2014), Servadio et al. (2016), Tyzio et al. (2014) Kirsten et al. (2012) Tyzio et al. (2014) Scattoni et al. (2008) Tsai et al. (2012) Nakatani et al. (2009) Lan et al. (2023) |
- Note: Arrows indicate the type of change: ↑ represents an increase or prolongation; ↓ represents a decrease, shortening, or delay; = indicates no change.
- Abbreviations: Arid1b, AT-rich interactive domain 1B; ASD, autism spectrum disorder; BTBR, T+Itpr3tf/J mouse; Fmr1, fragile X messenger ribonucleoprotein 1; KO, knockout; LPS, lipopolysaccharide; Nlgn, neuroligin; Nrxn, neurexin; poly(I:C), polyinosinic polycytidylic acid; Shank3, SRC homology and multiple ankyrin repeat domains 3; Syn, synapsin; Tsc, tuberous sclerosis complex; VPA, valproate.
Synapse formation is also disturbed in ASD; therefore, it is not surprising that the lack of a cell adhesion protein on the postsynaptic membrane in the neuroligin (Nlgn) 2 KO mice can be also considered an ASD model (Wohr et al. 2013). In line with this, mutations in the SRC homology 3 and multiple ankyrin repeat domains protein (Shank), which encodes a synaptic scaffolding protein, also lead to social, communicative, repetitive, and sensory processing abnormalities associated with ASD (Bukatova et al. 2021; Rendall et al. 2019; Peca et al. 2011; Yoo et al. 2019; Reichova et al. 2020). Mutations in synapsin (Syn) genes have also been described in subjects with ASD (Michetti et al. 2017). In line with epigenetic problems, chromatin modification is thought to be one of the causal mechanisms underlying neurodevelopmental disorders, including ASD (Ellegood et al. 2021). Haploinsufficiency in AT-rich interactive domain 1B (Arid1b), a gene in the chromatin modification complex, revealed several behavioral abnormalities relevant to ASD. In line with metabolic disturbances, the BTBR T+Itpr3tf/J mouse (BTBR), originally bred for studies on insulin-resistance, diabetes-induced nephropathy, and phenylketonuria, displayed strong and consistent ASD-relevant behaviors (Meyza and Blanchard 2017). Tuberous sclerosis complex (TSC) is a genetic disorder leading to noncancerous (benign) tumor growth in the brain and shows high rates of comorbidity with ASD that results from mutation of either Tsc1 or Tsc2 (Tsai et al. 2012). In line with the previously mentioned tumors, a classical tumor suppressor, phosphatase and tensin homolog (PTEN), was also implicated in ASD (Rademacher and Eickholt 2019). PTEN mutation was characterized by macrocephaly and impairments in social interactions, communication, repetitive behavior, and, occasionally, epilepsy. These findings strengthen the connection between ASD and cell differentiation problems. As one of the core problems of ASD is related to social contact, the known social hormones, OT and vasopressin (AVP), were also implicated, and their KO mice or rats (e.g., OT KO mice or AVP-deficient Brattleboro rat) can be used as animal models (Pobbe et al. 2012; Torok et al. 2022). Besides gene deletions, an increase in copy number variants, including chromosome duplication, especially 15q11–13 duplication, was also associated with ASD (Nakatani et al. 2009). In addition to artificial mutations, ASD-like symptoms (repetitive behavioral phenotypes, large nest-building behavior, and high motor stereotypy) can be detected after preferential breeding, for example, in a subpopulation of deer mice—Peromyscus maniculatus bairdii (Hurter et al. 2023).
Not only rodents but also birds (see Foxp2 gene mutation in zebra finch influencing social interactions) or even fishes (zebra fish: Fmr1 KO; Shank3 KO) are used (Csillag et al. 2022; Wu et al. 2017; Washbourne 2023), but here we focused on rodent models.
In addition to genetic disturbances, early developmental deficits are strongly emphasized in these models (Chung et al. 2022; Harlow et al. 2010). However, disturbances during early development can lead to ASD development not only in genetically predisposed individuals, but also per se. One of the best studied models is the VPA-induced ASD, with 569 PubMed publications from 2020 to 2024 (Reza Naghdi et al. 2024; Voros et al. 2023). VPA is a widely used antiepileptic drug and mood-stabilizer (Loscher 2002; Perucca 2002). Clinical studies have indicated that VPA treatment during pregnancy is teratogenic, and—among other effects—it increases the risk of ASD in offspring (Bromley et al. 2008). Notably, rodents exposed to VPA prenatally display behavioral deficits resembling those observed in ASD patients (Nicolini and Fahnestock 2018), most likely due to epigenetic modifications (Kataoka et al. 2013; Moldrich et al. 2013). Another possible mechanism is that the previously mentioned PTEN tumor suppressor protein level was decreased in the offspring of VPA-treated mice (Yang et al. 2016). Interestingly, although postnatal VPA treatment is also used for modeling ASD, mortality in this postnatal model is higher than in the prenatal model (Elnahas et al. 2021). Despite its strong face and predictive validity (Nicolini and Fahnestock 2018), a study by Swiss researchers published in 2013 drew the attention to the statistical flaw of these studies (in 91%: 31 out of the 34 studies reviewed) using the individual as a level of statistical analysis rather than the litter (Lazic and Essioux 2013). Moreover, although the prenatal exposure to VPA is widely used to study ASD, not all offspring exposed to VPA develop ASD-like symptoms, particularly under conditions involving dietary supplementation (Turpin et al. 2023). Another noxious agent, thiomersal, commonly used in vaccines, was also thoroughly investigated (Chen et al. 2013), but meta-analysis speaks in favor of its safety (Conklin et al. 2021). Nevertheless, intrauterine maternal immune activation (MIA) using polyinosinic polycytidylic acid (poly(I:C)) or lipopolysaccharide (LPS) injection into the dams (poly(I:C) in rat on embryonic day 14) or into neonates (LPS on postnatal day 3 [PND3]) can be also used as models, as maternal infection is considered a risk factor for ASD (Lan et al. 2023; Ryabushkina et al. 2024; Hall et al. 2023).
As ASD manifests early, there is no strict difference between environmental factors during early development and in adulthood, and the latter are considered stressors. Indeed, as the third hit, stressful situations can trigger pathological behavior at all stages of development, and ASD patients are more sensitive to them (de Vaan et al. 2020). Despite the acceptance of the three-hit theory, gene x environmental interaction is more often emphasized than triple combination (Tordjman et al. 2014), the latter used mainly for modeling depression (Gaszner et al. 2022). Nevertheless, even combined usage of genetic disturbances and noxious agents in animal models is rare. However, different noxious agents are sometimes used in combination (e.g., VPA + LPS; LPS + propionic acid, a bacterial product) (Alhamami et al. 2024; Foley et al. 2014). Moreover, an accumulating number of studies are focused on understanding the composition of the microbiota and how changes during early developmental stages may be linked to the pathogenesis of ASD (Liu et al. 2019). Indeed, alterations in the gut microbiota can affect neural, immune, and metabolic pathways, potentially contributing to ASD (Wang et al. 2020; Korteniemi et al. 2023).
It is crucial to emphasize the importance of standardization in testing conditions, housing enrichment, and background strains for understanding variability in behavioral phenotypes of ASD models (Rein et al. 2020; Kabitzke et al. 2020).
3 Early Somatic Alterations and Behavioral Symptoms in Animal Models of ASD
Due to the lack of reliable biological indicators, the diagnosis of ASD relies heavily on identifying specific behavioral symptoms. Consequently, the validation of ASD rodent models primarily involves matching these distinctive behavioral features (Caruso et al. 2020; Wohr et al. 2013). These symptoms can be relatively heterogeneous and vary depending on the model and the specific stage of development (Table 2). Here, we will focus on alterations observable in pups, that is, between birth and weaning at around PND21.
As a first sign, somatic development might be already impaired. Indeed, reduced body length was observed in Nrxn1 KO (Armstrong et al. 2020), Nlgn2 KO (Wohr et al. 2013), and Arid1b+/− (Ellegood et al. 2021) mice pups as well as in a rat intrauterine VPA treatment model (Ruhela et al. 2017; Schneider and Przewlocki 2005). Interestingly, impairment in body weight gain was observable only in adult Cntnap2 KO mice, but not during earlier development (Brunner et al. 2015). In the Arid1b+/− mice, not only the body length and body weight but also the head width were decreased (Ellegood et al. 2021). However, it is important to emphasize sex differences, as both body weight and length were lower in Nrxn1 KO male mice but not in females, compared to their wild-type (WT) counterparts (Armstrong et al. 2020). It can be hypothesized that hypophagia, among other factors, may play a role in contributing to reduced somatic growth (Pal et al. 2022). This condition is not only observed in ASD animal models, but it has also been documented in ASD children with eating disorders (see the following section).
In addition to delayed somatic development, congenital malformations can also be observed in the VPA-induced ASD animal model (Ruhela et al. 2017; Jiang et al. 2022). Among these, tail deformity is the most common, affecting 61.2% of the VPA-treated offspring (Ruhela et al. 2017), which is roughly consistent with our own observations (65%). In addition to tail deformity, dental malformations (3.82%), genital malformations (3.28%), and paw malformations (1.1%) may also occur; however, these are much less frequent (Ruhela et al. 2017).
Moreover, there might be a delay in the pup's developmental milestones (Zelena et al. 2009). The first step is pinna detachment (or ear unfolding), when the curved ear starts straightening, which normally begins at PND1 (in rats). Afterwards, the incisor eruption begins normally on PND7, whereas ear canal opening occurs on PND9 and eye opening on PND12. The ear, but not the eye opening, was delayed in Nrxn1 KO mice pups (Armstrong et al. 2020), whereas eye-opening impairment was detected in Shank3 KO mice (however, only in males) (Pillerova et al. 2022), as well as after prenatal VPA treatment in rats (Ruhela et al. 2019; Schneider and Przewlocki 2005) and mice (Yang et al. 2016). However, in another mice VPA model, pinna detachment and eye opening were not delayed (Wang et al. 2018), and on PND13, there was only a tendency for delayed eye opening in rats (Moldrich et al. 2013). The dose of VPA (600 vs. 500 mg/kg) as well as the treatment timing (embryonic day 13 vs. 10.5) was different in the two mice studies, which might explain the different outcome. Maternal LPS treatment was not able to influence pinna detachment and incisor eruption; however, eye opening was delayed (Foley et al. 2014). In contrast, MIA with poly(I:C) on embryonic day 12 delayed developmental milestones in pinna detachment, incisor eruption, and eye opening (Lan et al. 2023). Additionally, maternal genotype might also influence development, as the ear opening was premature in AVP-deficient pups born from control mother, while delaying when the mother has also AVP deficiency (Zelena et al. 2009).
As a sign of inadequate neurobehavioral development, there may be disturbances in primitive, infantile reflexes (retained primitive reflexes, RPR), which are indicative of sensory and motor problems. The primary function of primitive reflexes is to allow the infant to move and react to their environment, increase the chances of survival, and lead to the maturation of the nervous system, among others (Melillo et al. 2022). Most of these reflexes are present at birth and then become inhibited. RPRs indicate cortico-subcortical neuronal network impairment or neuronal developmental delay. Moreover, recent studies indicate alterations and delays in sensorineural development after movement restriction, emphasizing the significant role of postnatal experiences in influencing neurodevelopment (Dupuis et al. 2024; Khalki et al. 2024). Palmar grasp reflex is present at birth but gradually disappears and in rodents normally not detectable after PND7. When the offspring are placed on its back on a smooth, flat surface, it will make attempts to assume the normal upright position with all four feet on the table, called righting reflex. Besides the surface or self-righting, we can also examine air righting, which is a recovery to the normal prone position when releasing from the height in a supine position (Wang et al. 2018). Cliff aversion or avoidance is when the offspring is placed with its heads and front paws over the edge of a table and the latency to retract their body from the edge is recorded. A similar stimulation is when the animals are placed on a 45° inclined board with their noses pointing down, eliciting 180° rotation called negative geotaxis (Ruhela et al. 2019). Finding the mother, especially the teat, is very important for survival, and this is probably facilitated by the pivoting locomotion, when on a flat surface, the pup is turning around with a minimum of 90° (if 180°, it is called circle transverse) (Ellegood et al. 2021). The most studied parameters in these tests are the PND when the reflex appears/disappears. However, latency to correct implementation can be also measured. In relation to ASD, grasping reflex is impaired in Nrxn1 (Armstrong et al. 2020) and Nlgn2 KO mice pups (Wohr et al. 2013), but not in Shank3 KO (Pillerova et al. 2022). Righting reflex was delayed in Nrxn1 KO on PND4 (Armstrong et al. 2020) and in Syn2 KO on PND6 (Michetti et al. 2017), but not in Shank3 KO (Pillerova et al. 2022) or Arid1b+/− mice (Ellegood et al. 2021) nor after maternal LPS treatment in rats (Foley et al. 2014). Negative geotaxis was delayed in Arid1b+/− mice (Ellegood et al. 2021) as well as after intrauterine VPA treatments in rats (Ruhela et al. 2019) and in mice (Wang et al. 2018). However, some authors found no difference in VPA-treated rats (Schneider and Przewlocki 2005) (in this case, the VPA dose and timing were the same, and Wistar rats were used; thus, it is hard to explain the difference) and after maternal LPS treatment (Foley et al. 2014). Early postnatal immune challenge with poly(I:C) and/or LPS did not influence grasping, cliff avoidance, and negative geotaxis (Ryabushkina et al. 2024).
Not only fine, but also gross motor problems may occur, which can be observed in an open-field test. Hyperactivity with reduced immobility was observed in Nrxn1 KO mice after PND10 (Armstrong et al. 2020). In the same test, repetitive movement can be also studied during early development. However, contrary to expectations, reduced rather than elevated face washing (also known as grooming) was observed in Nrnx1 KO mice without changes in head rising and shaking (Armstrong et al. 2020). If we interpret grooming as a sign of anxiety, which is exaggerated in ASD (Liu et al. 2021), we can explain the outcome. It is worth noting that motor symptoms in ASD animal models might not only stem from anxiety but could also signify other motor deficits and atypical movements due to structural alteration in brain regions implicated in movement regulation, such as the basal ganglia and cerebellum (Wilkes et al. 2024). The grid walking test conducted before weaning revealed that the number of foot faults was significantly higher in Shank3 KO mice pups (Pillerova et al. 2022) as well as in the offspring of intrauterine VPA-treated rats (Ruhela et al. 2019) than in respective controls. However, in another study, on PND21, the motor coordination was not different between control and intrauterine VPA-treated rats (Wohr 2014). Interestingly, the soleus muscle of 2-month-old intrauterine VPA-treated animals contained a lower amount of antioxidative enzymes (King et al. 2024), suggesting a direct VPA effect on muscles. Indeed, some case reports suggested rhabdomyolysis after adult VPA treatment (Kottlors et al. 2001; Sharma et al. 2023). In contrast, VPA might prevent cachexia-induced muscle wasting (Sun et al. 2016). Moreover, although reduced antioxidative level in the ASD model suggests muscle impairment, the rotarod performances of the animals were not altered, as well as no information is available on the early developmental period.
Sensory problems may also occur. To test olfaction, nest-seeking response (also called maternal scent preference or olfactory discrimination) can be examined, which is delayed following prenatal VPA treatment in rats (Schneider and Przewlocki 2005) and mice (Yang et al. 2016).
Startle is a frequently studied parameter that can be influenced by sensory problems as well as by the highly anxious phenotype of the ASD animals. Air puff-induced tactile startle and tone-induced auditory startle can be observed separately (Wohr et al. 2013). There is a significant delay by approximately 1 day in the maturation of the auditory startle response of Shank3 KO mice in both females and males (Pillerova et al. 2022), but not in Nlgn2 KO mice (Wohr et al. 2013).
Rodents may use ultrasound for communication. Although adults exhibit fear vocalization and juveniles emit USV during play, our discussion focuses on MS-USV in pups. Its impairment is a sign of social communication deficit, a fundamental symptom of ASD, and can be observed in many genetic (Nrxn1 KO (Armstrong et al. 2020); Nlgn2 KO (Wohr et al. 2013); Shank1 KO (Sungur et al. 2016); Shank2 KO (Ey et al. 2013; Schmeisser et al. 2012); Shank3 KO (Wohr 2014); Syn1,2 (Michetti et al. 2017); Arid1b+/− (Ellegood et al. 2021)) and environmental/developmental models (VPA in rats (Servadio et al. 2016; Tyzio et al. 2014) and mice (Wohr 2014); intrauterine LPS in rats (Kirsten et al. 2012)). Not only the frequency but also the duration of calls can be impaired (Wohr 2014). After LPS treatment on PND10, a sex-specific MS-USV impairment occurred in male mice only (Santana-Coelho et al. 2024). However, MS-USV is often considered a sign of anxiety as well, which is known to be enhanced in ASD patients. Therefore, it is not surprising that some ASD models exhibit enhanced rather than reduced MS-USV (Fmr1 KO (Tyzio et al. 2014); BTBR mice (Scattoni et al. 2008); Tsc1 KO mice (Tsai et al. 2012); duplication of mouse chromosome 7 mirroring the human chromosome 15q11-13 duplication (Nakatani et al. 2009)). However, despite enhanced call duration, the USV pattern may be unusual in these animals (Scattoni et al. 2008). Moreover, in an MIA model using poly(I:C) on embryonic day 12, no effect on MS-USV was detected on PND7 (Lan et al. 2023).
Given the significant differences in development between rodent models and humans, it is also beneficial to compare the developmental stages in humans with those in pups (Figure 2).
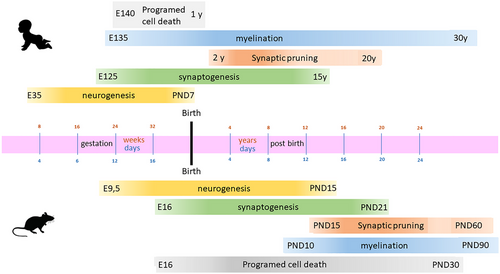
It is clear that animal models and humans share similar brain development processes, including neurogenesis, synaptogenesis, synaptic pruning, myelination, and programmed cell growth. However, the extent and timing of these processes could be markedly different. In humans, disruptions of these processes are linked to neurodevelopmental disorders, leading to behavioral changes and autistic symptomatology.
4 Corresponding Early Behavioral Alterations in Humans
Specifically, in the pediatric population, a diverse range of symptoms are observed, encompassing minor neurological manifestations. Food intake abnormalities, such as selective eating and food refusal, are frequently observed in children with ASD and may represent initial signs of the condition and can contribute to problematic somatic development (Nygren et al. 2021) (Table 3). Indeed, some authors reported delayed growth in ASD children (Levy et al. 2010). These atypical eating behaviors necessitate intervention through customized strategies and nutritional support to ensure the optimal health and developmental progression of children with ASD. Another somatic sign might be the heightened asymmetrical tonicity in neck reactions or a propensity towards opisthotonus, characterized by retroflexion of the head in children (Singhi and Malhi 2023). These symptoms, although mild, are noteworthy because they often precede a higher likelihood of ASD occurrence later (Tényi et al. 2013). As part of problematic development, hearing and visual problems may also occur, similar to delayed ear and eye opening observed in animal models (Singhi and Malhi 2023; Miller et al. 2005).
| Alterations in ASD animal models | Similar human alteration | References of human studies | |
|---|---|---|---|
| Somatic growth | Body length ↓ | Growth delay | Levy et al. (2010) |
| Delayed ear development | Early embryonic development errors often involving internal and external ear malformations | Miller et al. (2005) | |
| Delayed eye opening | Impaired eye contact and ophthalmologic anomalies | Singhi and Malhi (2023), Miller et al. (2005) | |
| Primitive reflexes | Impaired grasping reflex | Normal grasping reflex | Healy et al. (2024) |
| Impaired righting reflex | Delayed psychomotor development—postural control impairment | Melnikov et al. (2023) | |
| Impaired negative geotaxis | Balance and coordination abnormalities | Kaur et al. (2018) | |
| Pivoting locomotion |
Stereotype behavior—joy of spinning Enhanced rooting reflex |
Watt et al. (2008) Healy et al. (2024) |
|
| Locomotion | Impaired grid walking | Impaired timed up and go test (TUG) | Martin-Diaz et al. (2023), Podsiadlo and Richardson (1991) |
| Alterations in spontaneous movement (open-field) | ASD prevent the delayed onset of independent walking typical in intellectual disability | Bishop et al. (2016) | |
| Sensory functions | Impaired olfactory discrimination | Atypical odor threshold | Muratori et al. (2017) |
| Impaired acoustic startle reflex | Alterations in acoustic startle reflex | Takahashi et al. (2016) | |
| Communications deficit | Alterations in ultrasonic vocalization | Impaired verbal communication | American Psychiatric Association (2013) |
- Abbreviations: ASD, autism spectrum disorder; TUG, time up and go test.
Similarly to animals, primitive reflexes are present at birth in humans and then become inhibited within the first few months, with the longest (the grasping reflex) remaining until the end of the first year (Melillo et al. 2022) (Table 3). Some reflexes are retained (RPR) far beyond infancy, whereas others might appear significantly later than expected. Both types can serve as markers for ASD, as reflex disturbances are significantly related to an infant's ability to interact with objects and others (e.g., copying gestures). Postural control impairment (Melnikov et al. 2023) as well as balance and coordination abnormalities (Kaur et al. 2018) were reported in children, similarly to rodents. However, one study found normal grasping reflex in humans (Healy et al. 2024). As part of motor dysfunction, restricted and repetitive behaviors or walking on tiptoes or spinning are highly characteristic for ASD children (Valagussa et al. 2018). However, the joy of spinning might be considered an equivalent of pivoting locomotion (Watt et al. 2008). On the other hand, pivoting locomotion can correspond to the rooting reflex in humans, and this reflex is more prevalent in children with ASD than in typically developing children (Healy et al. 2024). Nevertheless, stereotyped behavior is modeled by enhanced grooming, jumping, or stereotyped sniffing in an anxiogenic, new environment (e.g., open field) in rodents (Crawley 2007). However, it is not that often studied during the early rodent development.
Numerous investigators have noted that delays in the maturation of motor function during early development can foreshadow the development of ASD (Singhi and Malhi 2023; Arabameri and Sotoodeh 2015; Engelhard et al. 2020; Posar and Visconti 2022) (Table 3). Children with ASD often exhibit early impairments in motor development, which can precede the classical social communication deficits associated with ASD. These early motor impairments typically include delayed attainment of motor milestones, such as sitting, standing, and walking, with some stages, like crawling, potentially being bypassed (Bishop et al. 2016; Martin-Diaz et al. 2023).
Sensory processing difficulties are also key symptoms of ASD and may lead to enhanced sensitivity to certain sounds and bright lights. Interestingly, selective eating habits can also be connected to altered sensation as some individuals accept only a narrow range of foods based on color or texture. The odor threshold might be also atypical (Muratori et al. 2017). Impaired acoustic startle is also observable both in rodents ASD models and in ASD patients (Takahashi et al. 2016). This test is based on hearing but also reflects the sensory-motor gating function, which is strongly connected to attention. Joint attention deficit represents a fundamental impairment characterized by an inability to share a focus of interest with another individual. Examples include a lack of pointing to share attention, disinterest in shared activities, and an inability to maintain reciprocal eye contact (Singhi and Malhi 2023; Orekhova and Stroganova 2014). Conversely, individuals may display strong attachments to objects and adhere rigidly to certain routines or rituals, showing resistance to changes in schedules or routes. Furthermore, individuals may exhibit an intense focus on specific topics, accumulating extensive knowledge within these areas. They often retain details that may go unnoticed by neurotypical individuals. Partly due to sensory and attentional problems, a common ASD trait may involve a deficit in mentalization, wherein affected individuals struggle to interpret their surroundings, rendering the world unpredictable to them, unlike neurotypical children who adeptly infer the thoughts of others from subtle cues such as eye contact, facial expressions, and nuanced gestures (Singhi and Malhi 2023).
Although several tests exist to study early motor problems in animals, only MS-USV is considered an early test for social deficits. In contrast, in humans, more attention is given to social disturbances, including aversion of the infant to being held, its minimal interest in human faces, and avoidance of eye contact, or lack of the formation of a secure attachment with the primary caregiver (Singhi and Malhi 2023). As the MS-USV equivalent, cry was also studied in ASD children (Esposito et al. 2013). Not only was its altered pattern confirmed, but cries of ASD children were perceived more negatively from caregivers than of typically developing children, suggesting attachment problems. During later infancy, communication impairment and delayed language acquisition can be also observed (Miller et al. 2021; Pickles et al. 2022). Even when language development commences, it often manifests with linguistic errors and the creation of nonexistent words. Moreover, characteristic features may include echolalia and delayed echolalia, where individuals repetitively echo heard words or phrases, occasionally in inappropriate contexts. Additionally, distinct speech patterns, intonation abnormalities, and pronoun substitution may be observed (Singhi and Malhi 2023).
Besides previously mentioned observations, sleep abnormalities, including difficulties in falling asleep, frequent nocturnal awakenings, and reduced overall sleep duration, are also common in children with ASD and can serve as early indicators of the disorder (Johnson and Zarrinnegar 2021). Indeed, in genetically ASD-prone Shank3 mutant mice, early sleep disturbances were also associated with abnormal ASD-like behavioral symptoms in adulthood (Lord et al. 2022).
5 Structural and Developmental Disturbances in the Central Nervous System Underlying Behavioral Problems
The above-described behavioral abnormalities observed in various ASD models during the early developmental period PND1–21 can be explained by several structural and morphological changes in the whole central nervous system or on specific brain areas. However, there are relatively few studies focused on neuron cell structure and differentiation during the early postnatal stages. Therefore, to explain the pathomechanism, in this section, we also included results from older or adult animals. One of the most frequently studied phenomena in ASD models is the changes in neurogenesis and neuronal cell differentiation (Bukatova et al. 2023).
In adulthood, neurogenesis is maintained in the lateral ventricle subventricular zone (Nogueira et al. 2022). However, in the BTBR metabolic mouse model of ASD, impaired neurogenesis was found on this brain area (Meyza and Blanchard 2017). Moreover, the transcriptome from the subventricular zone of 8-week-old Shank3-deficient mice suggested that epigenetic alteration might lead to increased dormancy of the neuronal stem cells (Kim et al. 2022). Indeed, using cell cultures, numerous studies confirmed the impact of Shank family proteins deficiency on cell fate determination and neuronal differentiation (Liu, Yuan et al. 2022). Paradoxically, some studies found that embryonic Fmr1 KO mice exhibit even accelerated neurogenesis, increased neuronal differentiation, and enhanced accumulation of progenitors in the subventricular zone (Castren et al. 2005; Khalfallah et al. 2017). This accelerated neurogenesis and differentiation may lead to the production of nonfunctional neurons that cannot be properly integrated into the local circuitry, resulting in their elimination and potentially explaining the reduced neuron number observed in the postnatal Fmr1 KO mouse cortex (Lee et al. 2019; Patzlaff et al. 2018).
Another important area of adult neurogenesis is the dentate gyrus region of the hippocampus (Nogueira et al. 2022). A significant reduction in radial glial progenitor cells and immature neuron production was found in the ventral, but not dorsal, dentate gyrus of adult Shank3-deficient mice (Cope et al. 2016). Our studies also indicated alterations in the number of inhibitory neurons on PND5 in Shank3-deficient mice, based on reduced expression of inhibitory GABAergic markers and GABA receptor subunits in various brain regions, including the hippocampus (Bukatova et al. 2021). One study found that Nlgn1 knockdown diminishes the survival rate of adult-generated hippocampal neurons (Schnell et al. 2014).
Cortical areas are also heavily implicated in ASD. An important aspect of ASD is the alterations in the neural circuitry associated with imitation, specifically in the mirror neuron system. Although it seems to be a specific human phenomenon, contagious scratching and yawning have also been recently reported in rodents (Rana et al. 2022); however, it was not studied in ASD models. More is known about changes in specific neuron population. The 8-week-old Fmr1 KO mice had significantly fewer neurons and parvalbumin-positive interneurons, altered cortical lamination patterns, and an increased number of oligodendrocytes (Lee et al. 2019). Downregulation of another cell adhesion molecule, Nrxn1, in the medial prefrontal cortex resulted in decreased expression of cell structure proteins accompanied by reduced length and branch points of neurites in cortical neurons (Wu et al. 2023). Changes in sensory, especially in the auditory cortex, may explain altered processing for speech and language development in humans. Indeed, in PND70 Fmr1 KO mice, functional changes were found in the auditory cortex, which may explain the developmental delay of their sound processing (Wadle et al. 2024). Additionally, abnormal auditory processing and hypersensitive responses were observed in the conditional PTEN KO mice, another model with autistic symptomatology (Croom et al. 2024). Therefore, it is important to investigate the early development of the auditory and frontal cortices in rodent models to better understand atypical language development in humans.
In contrast to hearing problems mentioned above, another ASD model, the Nrxn1 KO mice, had no olfactory deficit in adulthood despite several problems during early development (Armstrong et al. 2020).
Impaired neurogenesis was also observed in the Cntnap2 KO ASD mouse model during PND25 (Lauber et al. 2018). Specifically, the number of parvalbumin-positive neurons was found to be decreased in their striatum. Although not completely understood, the loss of Cntnap2 could lead to striatal neuron hyperexcitability and behavioral inflexibility in mice (Cording et al. 2024). Additionally, the disruption of Nrxn1α in telencephalic excitatory projection neurons, but not in thalamic neurons, resulted in changes in striatal value-modulated neural activity (Alabi et al. 2020). The deletion of Shank3 in striatal inhibitory neurons led to perseverative exploratory behavior in mice (Bey et al. 2018).
In line with motor problems listed before, aberrant cerebellar cortical formation was also detected in Tsc1 KO mice (Tsai et al. 2012) as well as in VPA-treated mouse offspring (Wang et al. 2018). Indeed, decreased cell density and impaired dendritic arborization of the Purkinje neurons with premature migration and excess apoptosis were observed in these animals.
However, controversially, cortical cell cultures from embryonic day 18 showed increased, rather than decreased, spine formation in the VPA mouse model (Yang et al. 2016). Nevertheless, Nrxn1, Nrxn2, and Nrxn3 triple-KO resulted in decreased cerebellar granule cell survival at PND21 (Uemura et al. 2022). A single point mutation in Nlgn3 significantly impacted synapse development and refinement in the cerebellar circuitry from PND10 to 15 (Lai et al. 2021). Overall, individual brain circuits during development can be affected by abnormalities or mutations in adhesion molecules. Such changes can manifest in the cerebellum through altered Purkinje cell synapse formation or in dopaminergic brain areas, potentially leading to stereotypical behavior or decreased motivation for social interaction (as described before).
Although not that much studied, amygdala was also implicated in ASD. In a VPA rat model, hyperactivity of the amygdala was accompanied by enhanced anxiety and abnormally heightened and prolonged fear memory (Markram et al. 2008).
Several authors have affirmed that RPRs reflect anatomical and functional connectivity abnormalities in brain networks in human ASD (Melillo et al. 2022). Indeed, the corpus callosum appears to be the brain area associated with reduced cortical connectivity found in individuals with ASD (Melillo et al. 2022). Magnetic resonance imaging in Syn2 KO mice revealed normal brain volume and morphometry with reduced connectivity (Michetti et al. 2017). In line with the importance of the connectivity, Fmr1 KO mice showed delayed myelination in the brain during early postnatal development (Shi et al. 2019).
These studies highlight that the mechanisms driving autistic symptoms, whether in early development or adulthood, depend on specific brain regions. Importantly, they demonstrate that corticostriatal and cerebellar circuits play a crucial role in these processes, but sensory cortical areas are also implicated. The connections between brain areas are fundamental.
6 Changes in Neurotransmitters and the Role of OT
To understand the pathomechanism of ASD underlying behavioral and motor symptoms, it is important to recognize the neurotransmitter and neuropeptide changes that occur during development due to mutations of specific genes and different noxious agents as ASD models. Here, we mention only some key concepts as detailed description is beyond this review's scope.
The most widely accepted theory is an excitatory-inhibitory imbalance, widely discussed in animal studies. It may have different causes and manifestation in various brain regions and developmental stages and can lead to abnormal neural circuitry development and ultimately to ASD symptoms (Hui et al. 2020; Culotta and Penzes 2020; Carrol 2023). The imbalance might be due to changes in the GABAergic system, as variants in the SLC6A1 genes (Carroll 2023). However, another study found that a missense variant of a glutamate receptor-stabilizing protein also leads to ASD development (Pavinato et al. 2023). An excitatory/inhibitory imbalance was evidenced in BTBR mice (Meyza and Blanchard 2017). Several studies have indicated that an imbalance also occurs in Shank3-deficient mice, specifically in the hippocampus, along with connectivity defects in the medial prefrontal cortex (Lee et al. 2015). Moreover, absence of Nlgn3 resulted in alterations in both GABAergic and glutamatergic synapses resulting in synaptic transmission imbalance and abnormal neurogenesis during postnatal development (Uchigashima et al. 2021).
In line with the imbalance, exposure to VPA might lead to increased levels of GABA (Loscher 2002; Sernagor et al. 2010). This effect is attributed to VPA's inhibition of GABA transaminases, responsible for GABA degradation, and its enhancement of the activity of glutamic acid decarboxylase, an enzyme involved in GABA synthesis (Loscher 2002). Consequently, alterations in GABA levels induced by VPA exposure during critical developmental stages could disturb the formation of neuronal circuits, potentially contributing to the manifestation of autistic behavioral traits (Nicolini and Fahnestock 2018). Another neurotoxic developmental model, MIA also altered transcription factors involved in GABAergic differentiation and migration (Gillespie et al. 2024).
A related concept with a significant developmental component is the GABA excitatory/inhibitory shift during brain maturation. It is well known that GABA inhibits adult neurons, but later studies confirmed that it can excite immature ones due to their initially higher intracellular chloride concentration (Ben-Ari 2015). The GABA shift has been extensively investigated over the past decade and is strongly associated with OT secretion during birth. Several studies have shown that OT secretion is crucial for the proper timing of the GABA shift (Tyzio et al. 2014; Ben-Ari 2015; Leonzino et al. 2016). The OT-GABA relationship was described in more detail elsewhere (Havranek et al. 2024). Here, we would like to draw attention to the fact that OT, especially its receptors, can be found in critical brain cortex and subcortical brain areas, which are important for regulating social behavior and its alteration in ASD (Jurek and Neumann 2018). Thus, it is not surprising that the lack of OT leads to impaired excitatory/inhibitory balance and disturbed brain development resulting in altered social recognition, which can be related to ASD pathogenesis and symptomatology (Lopatina et al. 2018; Penagarikano 2017). Interestingly, an ASD model, the Magel2-deficient mice, had also reduced OT levels (Meziane et al. 2015).
Besides the major excitatory-inhibitory neurotransmitters, monoamines have also been implicated in ASD. Serotonin receptor alteration was found in 15q11-13 duplication mouse model (Nakatani et al. 2009). Moreover, the loss of Nrxn, a cell adhesion molecule, reduced the number of serotonin neurons during the early postnatal development (Cheung et al. 2023). A single prenatal LPS injection in rats resulted in dopamine disturbances in the striatum with ASD-like symptoms (Kirsten et al. 2012). OT might also influence the dopaminergic system as mutation in the synaptic adhesion molecule Nlgn3 resulted in impaired OT signaling in dopaminergic neurons together with an altered behavioral response in the social novelty tests in mice (Hornberg et al. 2020).
7 Potential Interventions During Early Development
Available early intervention strategies for ASD focus on modulating specific behavioral parameters and enhancing social skills, often including various training procedures, such as sensory function enhancement (Weitlauf et al. 2017; Maksimovic et al. 2023). Moreover, some studies stressed that intervention depends on the child's age, the specifics and severity of the ASD diagnosis (Maksimovic et al. 2023). In this line, the extent of language development, whether regressed or advanced, is an important factor in determining therapy (Frazier et al. 2021). Regarding non-pharmacological interventions, various behavioral approaches are utilized in toddlers with ASD, with applied behavior analysis (ABA) being one of the most extensively applied techniques (Eckes et al. 2023). ABA therapy has been shown to significantly improve communication, social skills, and cognitive abilities in young children with autism when started early (Vietze and Lax 2020). However, the evidence base is small, and the durability of the effects is unclear (Weitlauf et al. 2017). Thus, some meta-analyses have raised concerns regarding the effectiveness of these approaches (Daniolou et al. 2022).
Although the previously mentioned social and language training cannot be attributed to rodents, RPRs in animals can resemble human problems. In accordance with this, disturbances of primitive reflexes may be one of the earliest markers of abnormal or delayed cortical maturation in ASD (Melillo et al. 2022). A relatively simple perceptual-motor intervention based on these reflexes can facilitate brain structure development and learning and thus is suitable for therapy. Although it is mainly used for early detection and treatment of infant brain injuries, the practice of elementary sensorimotor patterns (e.g., elementary sitting up, creeping and crawling, see primitive reflexes) based upon Katona neurorehabilitation method might promote normal brain development in general (Hinojosa-Rodriguez et al. 2020).
Pharmacological intervention is also part of managing ASD in children; however, it remains controversial due to significant interindividual variability in clinical response and sensitivity to side effects within the ASD population (Persico et al. 2021). The primary targets are specific symptoms such as anxiety, depression, and self-harm. Medications like antipsychotics, including risperidone and aripiprazole, have been approved for treating irritability associated with ASD, whereas selective serotonin reuptake inhibitors are often prescribed to manage anxiety and depression (Reichova et al. 2018). As epilepsy is often comorbid (Liu, Sun et al. 2022), drugs targeting the GABAergic system are often used, for example, midazolam, a GABAA receptor activator (Hanamoto et al. 2023). Nevertheless, numerous other substances are under investigation in experimental settings, aiming to provide effective treatment for pediatric patients with ASD (Aishworiya et al. 2024; McCracken et al. 2021).
Considerable attention has been directed towards the neuropeptide OT, especially its intranasal administration (Gordon et al. 2013; Heinrichs and Domes 2008; Guastella and Hickie 2016). In recent years, a significant amount of data have been gathered from animal studies demonstrating that OT can help to mitigate ASD symptoms. Studies involving the administration of OT during early development have shown positive effects on social behavior and have also revealed synaptic changes in certain areas of the brain in ASD models like Shank3 (exons 4–9) KO and Fmr1 KO mice, Shank3 KO mice and rats, mice with 16p11.2 deletion, and maternally VPA-treated rats (Reichova et al. 2020; Dai et al. 2018; Harony-Nicolas et al. 2017; Lindenmaier et al. 2022). It is important to note that the design of these studies was variable, with differences in the age and origin of the animals, as well as the characteristics of the transgenic or drug-induced ASD models. Recent studies further confirmed that OT influenced or mediated changes in neuronal morphology, likely affecting neuronal growth and synaptogenesis (Bukatova et al. 2023; Pekarek et al. 2022; Reichova et al. 2021; Wei et al. 2021). On the basis of these observations, several human studies were conducted with promising effects on ASD symptoms (Gordon et al. 2013; Heinrichs and Domes 2008; Guastella and Hickie 2016). However, recent research has raised questions regarding the efficacy of OT (Quintana et al. 2017). Nevertheless, it is evident that OT influences social salience and enhances attention to and perception of social cues (Tillman et al. 2019; Shamay-Tsoory and Abu-Akel 2016).
8 Conclusions and Open Questions
Numerous studies on early behavioral signs of ASD clearly confirmed somatic, motor, sensory, and communication deficits both in rodents and humans. In animal models of ASD, apart from studies on USV, there is limited evidence of social deficits during early development, mainly due to methodological and technical difficulties. The sex dependency of ASD lacks support from animal models.
It is important to note that this review has limitations, especially when directly comparing animal and human data, as direct parallels, such as eye opening in ASD models and human ophthalmologic anomalies, are not feasible. These challenges arise from the inherent properties of animal models and specific aspects of human development. However, we consider it important to draw some kind of parallel between the differences observed in animal models and human symptoms (see Table 3), which are necessary for mechanism research and the identification of new therapeutic targets. Furthermore, this review did not focus on the core symptoms of ASD such as social or repetitive behavior but aimed to discuss early developmental stages.
The current therapy focuses on behavioral training, and the pharmacotherapy is mainly supportive. The examination of implicated brain areas (subventricular zone, cortex, hippocampus, cerebellum, etc.) and neurotransmitters (GABA, glutamate, serotonin, dopamine, OT, etc.) may help in the identification of new therapeutic targets. However, the example of OT, despite being a promising option, highlights translational difficulties. The specific conditions, dosing, and treatment route should be carefully considered. Therefore, during preclinical studies, it is necessary to monitor multiple behavioral signs simultaneously and utilize diverse (e.g., genetic, developmental, and environmental) presumably combined models. More emphasis should be given to individual differences already in rodents (e.g., subgroups based upon neurotoxin susceptibility).
Author Contributions
All authors contributed to the writing and editing of the manuscript and approved the final version for submission.
Acknowledgments
This work was supported by projects 2/0057/23 of the Grant Agency of the Ministry of Education and Slovak Academy of Sciences (VEGA), Hungarian-Slovak Bilateral Collaboration Grant HAS-SAS-2022-02/HAS—NKM2023-6/2023 and by the Slovak Research and Development Agency projects APVV-21-0189, National Brain Research Program (NAP 3.0) of the Hungarian Academy of Science, and the Thematic Excellence Program 2021 Health Sub-program of the Ministry for Innovation and Technology in Hungary (within the framework of the TKP2021-EGA-16 project of the Pécs University).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this manuscript as no datasets were generated or analyzed during the current study.