Maternal Stress and Maternal Microbiome Manipulations Remodel Offspring Medial Prefrontal Cortex in a Sex-Dependent Manner
Gregory E. Demas and Cara L. Wellman should be considered joint senior authors.
ABSTRACT
Maternal stress and disruptions of the maternal microbiome during development can have profound organizational effects on the brain and behavior of offspring. We have previously demonstrated that these manipulations have marked, sex-dependent effects on aggressive behavior in Siberian hamsters, Phodopus sungorus. Given that the prelimbic cortex is sensitive to stress and may play a role in modulating social behaviors, here we investigated how maternal stress and disruption of the microbiome during pregnancy may affect the development of the prelimbic cortex in offspring. Pregnant hamsters were exposed to either a broad-spectrum antibiotic, social stress, combined treatments, or no manipulation (i.e., control). Adult offspring (PND 107–115) were euthanized, brains were stained using Golgi histology, and apical and basilar dendritic lengths of pyramidal cells in the prelimbic cortex were quantified. Our data indicate that maternal stress and microbiome manipulation have a sex-dependent effect on offspring dendritic morphology. Maternal stress increased apical dendritic length in female but not male offspring relative to controls. However, the combination of maternal stress and maternal antibiotics ameliorated the effect of stress alone. Thus, maternal stress and disruption of the microbiome interact to produce lasting changes in the prefrontal cortex of female offspring. Such changes may contribute to the behavioral effects of these manipulations.
1 Introduction
Prenatal experiences can alter developmental trajectories, producing long-term, and often sex-specific, effects on offspring (Cusick et al. 2022; Duckworth et al. 2015; Jasarevic et al. 2017; Seckl and Meaney 2004). For example, manipulation of the maternal microbiome (i.e., the commensal, symbiotic, and pathogenic microorganisms and their genes and gene products) impacts neurodevelopment of offspring and consequently offspring behavior (e.g., Ahmed et al. 2014; Hartman et al. 2019; Jasarevic and Bale 2019; Tochitani et al. 2016). Likewise, maternal stress also influences both neurodevelopment and behavior of offspring (e.g., de Kloet et al. 2005; Hartman et al. 2019).
These maternal physiological systems do not function in isolation—changes in one system can affect the other (e.g., Jasarevic et al. 2017; Jasarevic et al. 2018; Jasarevic et al. 2015; Joëls et al. 2008). Changes in the hypothalamic–pituitary–adrenal (HPA) neuroendocrine axis, which is responsible for regulating many homeostatic functions (e.g., energy, immune, reproduction), can result in changes in the gut microbiome (e.g., Gur et al. 2015; Jasarevic et al. 2015; Noguera et al. 2018; Wei et al. 2020) that in turn affect behavior (e.g., Partrick et al. 2018). Similarly, alterations of the gut microbiome can influence HPA activity (Tetel et al. 2018) and sensitivity (Sudo et al. 2004) and can also attenuate the impacts of stress (Kuti et al. 2020; Langgartner et al. 2018; Provensi et al. 2019). Maternal stress and the maternal microbiome could therefore affect offspring development independently or in an interactive manner.
In animal models, maternal stress and HPA manipulations alter the development of social and aggressive behaviors. For instance, prenatal dexamethasone administration reduced social play in marmosets (Pryce et al. 2011), and prenatal maternal restraint stress impaired social recognition in rats (Marrocco et al. 2014). Further, in Siberian hamsters, prenatal maternal social stress increased aggressive behavior in female but not in male offspring (Cusick et al. 2022).
The gut microbiome also plays a critical role in the development of brain and behavior (Li et al. 2021; Mazmanian et al. 2005). For example, germ-free (GF) mice—which are raised in sterile conditions and harbor no microbiome—display increased aggression relative to control mice, an effect that is eliminated by restoration of the microbiome at birth (Watanabe et al. 2021). Antibiotic treatment, which depletes the microbiome and alters its composition, also decreases aggression in male and female Siberian hamsters. Although aggression in males returns to pretreatment levels after a recovery period, aggression in females does not recover (Sylvia et al. 2017). As the maternal microbiome is largely inherited by the offspring, maternal treatment with antibiotics has also been shown to affect the social and aggressive behavior of offspring (Cusick et al. 2022; Friswell et al. 2010; Harshaw et al. 2022). Both pre- and postnatal stress alter the composition of the microbiome, suggesting that the HPA axis and the gut–brain axis may share communication pathways (Bailey and Coe 1999; Bailey et al. 2004). Therefore, investigating the interactive effects of stress and microbiome manipulations may help elucidate why certain individuals are at greater risk of developing stress-related conditions.
Siberian hamsters are an excellent species in which to test the effects of the maternal environment on offspring development and social behavior. In this species, investigation and aggression are essential for interactions with conspecifics and for reproduction (Rendon et al. 2017; Munley et al. 2018), and have been well documented in the lab (e.g., Scotti et al. 2007; Sylvia et al. 2017; Munley et al., 2020). Further, antibiotic administration has been successfully used to manipulate the microbiome in male and female Siberian hamsters and produces sex-specific changes in adult social behavior (Sylvia et al. 2017). Importantly, we recently demonstrated that maternal stress and microbiome manipulation in Siberian hamsters (Phodopus sungorus) alter adolescent offspring social behavior in a sex-dependent manner (Cusick et al. 2022). Specifically, maternal social stress via an aggressive encounter with an intruder increased aggression in female but not male offspring. This effect is attenuated by alteration of the maternal microbiome via antibiotic administration.
An important gap in understanding how maternal stress and the microbiome affect offspring behavior is how these manipulations may influence the brain. The prefrontal cortex is a key brain region involved in executive function, impulse control, and emotion regulation, thus modulating a wide range of behaviors, including social behaviors (Baarendse et al. 2013; Friedman and Robbins 2022; Himmler et al. 2014). A variety of chronic stressors produce sex-dependent dendritic remodeling in laboratory rats and mice. For instance, chronic stress reduces apical dendritic length in pyramidal neurons in the medial prefrontal cortex in male but not female laboratory rats and mice (Cook and Wellman 2004; Garrett and Wellman 2009; Moench and Wellman 2017; Woodburn et al. 2021), and these effects may vary with developmental stage and type of stressor (e.g., Breach et al. 2019; Eiland et al. 2012; Farrell et al. 2016; Muhammad et al. 2012).
While less is known about how the gut microbiome can influence prefrontal morphology, recent findings suggest that disruption of the microbiome can affect the development of structures within the brain. For example, GF mice displayed hypermyelinated axons within the prefrontal cortex. Notably, microbial colonization of GF mice postweaning prevented this hypermyelination (Hoban et al., 2016). Further, although dendrites in the amygdala of GF mice were longer than those of conventionally colonized mice, dendrites in the hippocampus of GF mice exhibited atrophy relative to controls (Luczynski et al. 2016).
Based on previous findings from our laboratory and others, we predict that (a) maternal stress will affect dendritic morphology in the prefrontal cortex of females but not males, and (b) manipulation of the maternal microbiota will modulate the effects of stress. To test this hypothesis, we assessed dendritic morphology in the medial prefrontal cortex in adult male and female Siberian hamsters whose mothers had been exposed to either antibiotic treatment, stress, or their combination during pregnancy.
2 Materials and Methods
2.1 Animal Housing
As described previously (Cusick et al. 2022), all hamsters were individually housed in polypropylene cages (28 × 17 × 12 cm) and were maintained at an ambient temperature of 22°C ± 2°C with a relative humidity of 55 ± 5%. All hamsters were maintained in a 16:8 light to dark photoperiod and had ad libitum access to standard hamster chow (Envigo Teklad Global Diets 18% Rodent Diet) and water. To control possible differences in hamster chow, all hamsters were fed from the same batch number throughout the duration of the study. All procedures were performed in accordance with the US National Research Council's Guide for the Care and Use of Laboratory Animals, the US Public Health Service's Policy on Humane Care and Use of Laboratory Animals, and the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the Bloomington Institutional Animal Care and Use Committee (BIACUC 19–023) at Indiana University.
2.2 Maternal Treatment
As described in Cusick et al. (2022), male and female hamsters were paired across 5 days, and male hamsters were removed 24 h after pairing and housed separately. All female hamsters (n = 34) were randomly assigned to receive one of four treatments: antibiotics (“Maternal ABX,” n = 9), stress (“Maternal Stress,” n = 8), both antibiotics and stress (“Maternal ABX + Stress,” n = 9), or Control (n = 8). Female hamsters received treatment for 10 days, beginning 5 days after pairing and continuing until 4 days prior to the expected birth date of the pup litter. All hamsters were weighed daily.
Hamsters assigned to the Maternal ABX treatment group received 0.3 µL per gram body mass of a broad-spectrum antibiotic (enrofloxacin [Baytril, Elanco Animal Health Inc., Greenfield, Indiana] 10% oral solution) via oral administration daily for the duration of the treatment phase. Enrofloxacin is a fluoroquinolone that does not easily cross the blood–brain barrier and functions as an antimicrobial agent by inhibiting DNA synthesis (Alvarez et al. 2008; Ooie et al. 1997a, 1997b; Slate et al. 2014). Antibiotics were administered via a sterile pipette between 12:00 p.m. and 3:00 p.m. ET, a validated procedure in our lab (Sylvia et al. 2018; Sylvia et al. 2017). This antibiotic regimen disrupts the natural composition of the gut microbiome in Siberian hamsters (Sylvia et al. 2017). Furthermore, we have demonstrated that this maternal treatment alters the composition and diversity of the gut microbiome of male and female littermates of the offspring used in the present study (Cusick et al. 2022).
Hamsters assigned to the Maternal Stress treatment group were exposed to a 15-min resident–intruder trial a total of five times, on a randomly generated schedule that resulted in exposure on Days 1, 3, 4, 8, and 10. A resident–intruder trial consisted of placing a nonexperimental, weight-matched, and same-sex hamster (i.e., “intruder”) in the cage of the experimental animal (i.e., “resident”) to induce a stress response. This procedure elevates serum cortisol in the resident (Cusick et al. 2022). The resident's cage remained unchanged beginning 3 days prior to the treatment phase and continuing through the duration of the treatment phase. Resident–intruder trials occurred within the first 3 h of the resident's dark phase and were conducted under dim red light. Hamsters in the Maternal Stress group also received 0.3 µL water per gram of body mass orally via a sterile pipette between 12:00 p.m. and 3:00 p.m. ET to control for any potential effects of handling on the hamsters receiving antibiotics. Hamsters assigned to the Maternal ABX + Stress group received daily antibiotic treatment and were exposed to a resident–intruder trial five times, evenly spaced throughout the treatment phase as described above. Hamsters assigned to the Control group received 0.3 µL of water per gram of body mass orally via a sterile pipette between 12:00 p.m. and 3:00 p.m. ET.
2.3 Offspring Husbandry
Of female hamsters that received maternal treatment, 27 of the 34 produced viable pups, with litter sizes varying from 2 to 10 (mean = 5.25 ± 0.32) and sex ratios varying from 5:1 to 1:9 male to female (mean 3:3). Pups were weaned on PND 21, at which point they were sexed based on anogenital distance and individually housed. Following weaning, 1–3 offspring per litter were randomly selected for behavioral testing and microbiome analysis; results of that study have been published (Cusick et al. 2022). The remaining 1–2 offspring per litter were left unperturbed, other than standard cage changes and cleaning, until adulthood. Because the prefrontal cortex is quite sensitive to stress manipulations, the offspring in the present study did not undergo behavioral testing.
2.4 Golgi Histology and Dendritic Reconstruction
Offspring were overdosed via intraperitoneal injection (0.3 mL) of ketamine (150 mg/kg) and xylazine (30 mg/kg) cocktail in 0.9% saline on PND 107–115. Offspring body mass at perfusion did not vary significantly across groups for either males or females (data not shown). After perfusion with 0.9% saline, brains were removed and processed for Golgi–Cox staining (Wellman 2017). In brief, brains were immersed in Golgi solution for 10 days before being transferred to a 30% sucrose solution for 3 days. Following staining, 220-µm coronal sections were taken via vibratome (Campden Instruments 5100mz-77). Sections were mounted, alkalinized, developed in Dektol (Kodak), fixed in Ilford rapid fixer, dehydrated in a graded series of ethanols, cleared in xylenes, and cover-slipped (Wellman 2017).
Fifty-eight offspring were generated for this study. The brains of two offspring were eliminated due to tissue processing errors; thus, the final sample size was 56 offspring (Control, n = 8 male from 5 litters and 7 female from 7 litters; Maternal ABX, n = 6 male from 6 litters and 7 female from 7 litters; Maternal Stress, n = 7 male from 5 litters and 6 female from 5 litters; Maternal ABX + Stress, n = 8 male from 6 litters and 7 female from 5 litters).
Pyramidal neurons in layer 2–3 of the prelimbic cortex were reconstructed (Figure 1A,B). The prelimbic cortex was chosen because this region of the medial prefrontal cortex has been shown to be sensitive to chronic stress both early in development and in adulthood (e.g., Breach et al. 2019; Eiland et al. 2012; Farrell et al. 2016; Moench and Wellman 2017; Muhammad et al. 2012). It is readily identified by its position on the medial wall of the rostral cortex and its location dorsal to the infralimbic cortex, which is markedly thinner and has fewer, less well-defined layers (Zilles and Wree 1995). Within the prelimbic cortex, layer 2–3 was easily identified in Golgi-stained material based on its characteristic cytoarchitecture. Its position is immediately deep to the cell-poor layer 1 (which also contains the distal dendritic tufts of layer 2–3 pyramidal neurons) and immediately superficial to layer 5; in prelimbic cortex, this boundary is additionally identifiable because of the greater cell packing density and smaller somata of pyramidal cells in layer 2–3 relative to layer 5 (Cajal 1995; Zilles and Wree 1995). Pyramidal neurons were identified by a distinct, single apical dendritic tree extending from the apex of the soma toward the pial surface of the cortex and two or more basilar dendritic trees extending from the base of the soma.
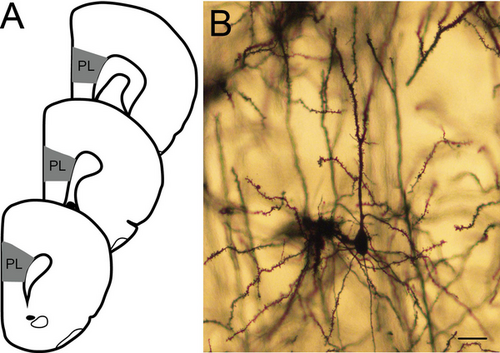
We reconstructed eight pyramidal neurons from each animal, selected from 2–4 evenly spaced sections. To do so, in each section, all pyramidal neurons in layer 2–3 of the prelimbic cortex whose branches were readily discriminated, with a nontruncated apical dendrite and at least one nontruncated basilar dendrite, were identified, and a random number generator was used to select a subset of these neurons for reconstruction. Morphology of the whole apical dendritic tree (including trunk, horizontal, and oblique branches) and all nontruncated basilar dendrite trees was reconstructed in three dimensions at a final magnification of 900× using a computer-based neuron tracing system (Neurolucida, MBF Biosciences; Figure 1A). The experimenter was blind to maternal treatment and sex of the offspring.
2.5 Statistical Analyses
Statistical analyses were conducted in SPSS v 27, and graphs were generated in R v 4.4.2. Because we expected treatments to differentially affect male and female offspring, data were analyzed separately for each sex. To assess how these maternal treatments broadly influenced offspring dendritic morphology, the total length of the apical tree (hereafter referred to as “apical length”), total length of branches per basilar tree (“basilar length per tree”), total number of branches in the apical tree (“apical branch number”), and total number of branches per basilar tree (“basilar branch number per tree”) were compared using two-way ANOVAs (ABX × Stress) within each sex. For all analyses, measures were averaged across neurons within each animal; thus, the unit of analysis for statistics is the individual animal. Significant effects were followed by planned comparisons, which consisted of t-tests performed within the context of the overall ANOVA (Hayes 1994; Maxwell and Delaney 2004). To minimize the number of comparisons and therefore the likelihood of a Type II error, comparisons were limited to Controls versus each experimental condition within each sex.
To more precisely assess how the distribution of dendrites in offspring is altered by these maternal treatments, Sholl analyses were conducted. These analyses sum the length of dendritic material between concentric spheres centered on the cell body of the neuron, which allows localization of changes within the dendritic arbor. Sholl analyses were analyzed using three-way repeated measures ANOVA (ABX × Stress × Radius) within each sex and were followed by planned comparisons as described above.
3 Results
3.1 Apical Dendrites
3.1.1 Branch Number and Length
For males, two-way ANOVA revealed no main effect of maternal ABX (F1, 28 = 1.32, ns), no main effect of maternal stress (F1, 28 = 0.03, ns), and no interactive effect of maternal ABX and maternal stress (F1, 28 = 0.40, ns) on apical branch number (see Table 1). Similarly, there was no significant effect of maternal ABX (F1, 28 = 0.68, ns) or maternal stress (F1, 28 = 0.04, ns) on apical length, and no interactive effect (F1, 28 = 2.69, ns; Figure 2).
| Apical | Basilar | |||
|---|---|---|---|---|
| Treatment | Male | Female | Male | Female |
| Control | 11.73 ± 0.45 | 10.68 ± 0.81 | 4.83 ± 0.18 | 5.11 ± 0.37 |
| Maternal ABX | 13.13 ± 0.36 | 12.52 ± 1.59 | 5.20 ± 0.35 | 4.62 ± 0.38 |
| Maternal Stress | 12.10 ± 0.64 | 12.71 ± 1.11 | 5.47 ± 0.29 | 4.86 ± 0.47 |
| Maternal ABX + Stress | 12.50 ± 1.17 | 12.20 ± 1.29 | 5.08 ± 0.37 | 5.21 ± 0.27 |
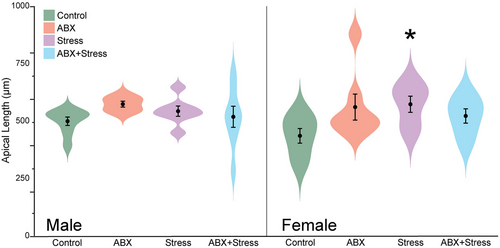
For females, neither maternal ABX (F1, 26 = 0.28, ns), maternal stress (F1, 26 = 0.47, ns), nor their interaction (F1, 28 = 0.89, ns) significantly altered apical branch number (Table 1). Although maternal ABX (F1, 26 = 0.83, ns) and maternal stress alone (F1, 26 = 0.1.47, ns) did not significantly alter apical length, the effect of maternal stress varied significantly with maternal ABX (F1, 26 = 4.68, p = 0.04). Planned comparisons revealed that female offspring with maternal stress exhibited significantly longer apical dendrites than control female offspring (p = 0.014; Figure 2).
3.1.2 Distribution of Dendritic Material
Sholl analyses revealed a more detailed picture of how these maternal treatments affected offspring dendritic morphology (Figure 3). For males, three-way repeated measures ANOVA revealed, as expected, that apical dendritic length varied with distance from the soma (F1, 25 = 146.12, p < 0.001). However, there was no main effect of maternal ABX (F1, 25 = 0.69, ns) or maternal stress (F1, 25 = 0.01, ns) on the distribution of apical dendritic material, and no significant interaction of maternal ABX and stress (F1, 25 = 2.27, ns). This did not vary across the apical arbor (radius × maternal ABX: F13, 25 = 0.55, ns; radius × maternal stress: F13, 25 = 0.63, ns; radius × maternal ABX × maternal stress: F13, 25 = 1.27, ns; Figure 3A).
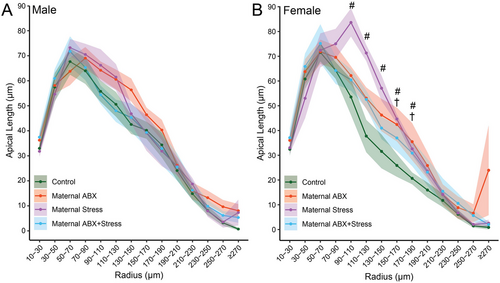
In females, the distribution of dendritic material also varied across the apical arbor, as expected (F1, 23 = 112.52, p < 0.001). Neither maternal ABX nor maternal stress significantly altered apical length (F1, 23 = 0.90 and 1.57, respectively, both ns), and this did not vary significantly across the arbor (radius × maternal ABX: F13, 23 = 1.08, ns; radius × maternal stress: F13, 23 = 2.22, ns). However, the effects of maternal stress on the distribution of apical dendritic material varied with maternal ABX (F1, 23 = 5.03, p = 0.04), and this interactive effect varied across the arbor (radius × maternal ABX × maternal stress: F13, 23 = 2.61, p = 0.002). Female offspring with maternal stress exhibited significantly increased apical dendritic length relative to control female offspring in the range of 110–190 µm (ps < 0.014). Planned comparisons revealed that female offspring with combined maternal ABX and maternal stress exhibited a smaller, more localized increase in apical dendritic material relative to control female offspring, in the range of 170–190 µm from the cell body (ps < 0.019; Figure 3B).
3.2 Basilar Dendrites
3.2.1 Branch Number and Length
For both males and females, two-way ANOVAs revealed no significant effect of maternal ABX (F1, 28 = 0.00 and F1, 26 = 0.04, respectively, both ns) or maternal stress (F1, 28 = 0.72 and F1, 26 = 0.21, respectively, both ns) on basilar branch number per tree. The interactive effect of Maternal ABX and Maternal Stress was not significant for males or females (F1, 28 = 1.52 and F1, 26 = 1.24, respectively, both ns; Table 1).
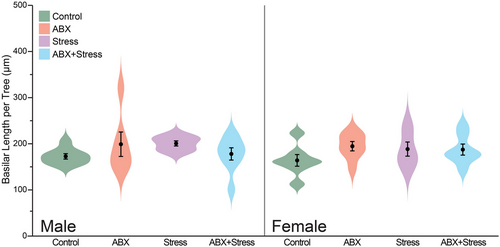
Likewise, there was no significant effect of maternal ABX (F1, 28 = 0.02 and F1, 26 = 1.41, respectively, both ns) or maternal stress (F1, 28 = 0.07 and F1, 26 = 0.47, respectively, both ns) on basilar length per tree. The interactive effect of Maternal ABX and Maternal Stress was not significant for males or females (F1, 28 = 3.16 and F1, 26 = 1.63, respectively, both ns; Figure 4).
3.2.2 Distribution of Dendritic Material
For males, three-way repeated measures ANOVA revealed that basilar dendritic length varied with distance from the soma (F1, 25 = 511.31, p < 0.001). However, there was no main effect of maternal ABX (F1, 25 = 0.86, ns) or maternal stress (F1, 25 = 1.29, ns) on the distribution of basilar dendritic material, and no significant interaction of maternal ABX and stress (F1, 25 = 2.05, ns). This did not vary significantly across the apical arbor (radius × maternal ABX: F13, 25 = 0.85, ns; radius × maternal stress: F13, 25 = 1.88, ns; radius × maternal ABX × maternal stress: F13, 25 = 0.51, ns; Figure 5A).
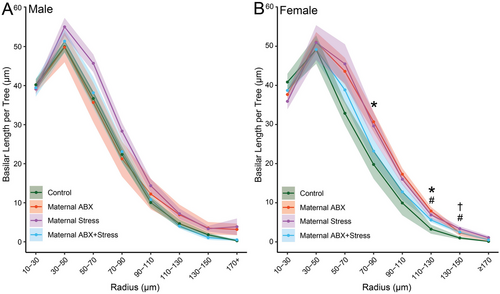
Unsurprisingly, in females, the distribution of dendritic material also varied across the basilar arbor (F1, 23 = 458.87, p < 0.001). Neither maternal ABX nor maternal stress significantly altered basilar length (F1, 23 = 0.36 and 0.22, respectively, both ns), and this did not vary significantly across the arbor (radius × maternal ABX: F13, 23 = 1.08, ns; radius × maternal stress: F13, 23 = 2.22, ns). Although overall the effects of maternal stress on the distribution of basilar dendritic material did not interact with maternal ABX (F1, 23 = 3.83, ns), the interactive effect of maternal ABX and maternal stress did vary across the arbor (radius × maternal ABX × maternal stress: F13, 23 = 5.18, p < 0.001). Planned comparisons revealed that in female offspring, maternal ABX significantly increased basilar dendritic length at 70–110 µm (p < 0.037) and 130 µm from the cell body (p = 0.011). Maternal stress increased basilar dendritic length from 130 to 150 µm from the cell body (p < 0.044), whereas the combined maternal ABX and maternal stress treatment increased basilar dendritic material only at 150 µm from the cell body (p = 0.024; Figure 5B).
4 Discussion
The present study investigated how maternal stress and/or maternal microbiome manipulations affect dendritic morphology in the prelimbic cortex of adult male and female offspring. Specifically, we analyzed pyramidal neurons in layer 2–3 of the prelimbic cortex of male and female offspring and demonstrated that maternal stress increased apical length in female offspring, but not in male offspring. Both maternal antibiotic administration and maternal stress increased basilar dendritic length in a localized fashion in females. Notably, in both apical and basilar dendrites, the combination of maternal stress and maternal antibiotics ameliorated the effect of either stress or antibiotic alone.
4.1 Sex-Dependent Effect of Maternal Stress
Layer 2–3 pyramidal neurons in the prelimbic cortex responded to maternal stress in a sex-dependent manner. Although maternal stress had no effect on apical dendritic length in male offspring, it increased apical length throughout the middle of the arbor in female offspring. Maternal stress produced a more modest but significant effect on basilar dendrites in female but not male offspring. Our findings contrast with a previous report in rats demonstrating subtle reductions in apical but not basilar dendritic material in the prelimbic cortex of male offspring after prenatal maternal stress (Suenaga et al. 2012). Alternatively, others (Muhammad et al. 2012) have found increases in apical and basilar length in the mPFC of male offspring after prenatal maternal stress. However, these reports also observed sex differences, with female offspring showing either no effects of maternal stress on prelimbic dendritic morphology (Suenaga et al. 2012) or a maternal stress-induced increase in basilar but not apical dendritic length (Muhammad et al. 2012). These discrepancies may be explained by differences in the species studied or by qualitative or quantitative differences in the stress manipulations—aggressive encounters in the present study versus repeated restraint (Suenaga et al. 2012) or elevated platform exposure (Muhammad et al. 2012).
We previously found a similar pattern of sex-dependent effects of maternal stress on offspring's aggressive behavior. Juvenile female offspring of stressed dams demonstrated increased aggressive behavior, comparable to aggression displayed by male offspring with no maternal treatment. Meanwhile, there was no effect of maternal stress on male offspring (Cusick et al. 2022). Interesting, these female littermates of offspring in the present study tended to have higher social stress-induced serum corticosterone concentrations relative to female offspring from other groups, whereas serum corticosterone concentrations in male offspring from stressed mothers were not elevated relative to controls (Cusick et al. 2022). Thus, the behavioral and morphological effects could be mediated by a sex-dependent hyperactivation of the HPA axis resulting from maternal stress. For example, basal adrenocorticotropic hormone (ACTH) concentrations were elevated in female rats exposed to maternal stress, while basal ACTH in male rats was unaffected (McCormick et al. 1995). In addition, 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) is found in the placenta of both humans and rodents, where it converts active glucocorticoids, corticosterone and cortisol, into their inactive form, 11-dehydrocorticosterone and cortisone, respectively (Almanzar et al. 2016; Stewart et al. 1995; Waddell et al. 1998). Maternal stress decreases levels of 11β-HSD2, allowing for active glucocorticoids to reach the fetus, potentially modifying the developing HPA axis (Lucassen et al. 2009; Mairesse et al. 2007; Wyrwoll and Holmes 2012). Critically, however, potential sex differences in 11β-HSD2 concentration in offspring exposed to maternal stress have not been thoroughly examined.
4.2 Sex-Dependent Effects of Maternal Antibiotic Treatment
We also found that maternal antibiotic treatment increased basilar dendritic length in female offspring but had no significant effect in male offspring. Currently, little is known about how microbiome manipulations can affect dendritic morphology. Previous findings in other brain regions, however, lend partial support for our sex-dependent findings. For example, male GF mice have longer apical and basilar dendrites in basolateral amygdala pyramidal neurons (Luczynski et al. 2016). Furthermore, adult male mice given antibiotics showed an increased spine density on basolateral amygdala pyramidal neurons, which was not observed in females. The different direction of these sex-dependent effects could reflect either differences in the magnitude of the microbiome manipulation or regional specificity of effects. Nonetheless, such sex-dependent effects may be behaviorally relevant. For instance, females but not males displayed compromised aversive learning and cued recall after antibiotic treatment (Geary et al. 2021). Similarly, in mice, low doses of penicillin during the final week of gestation decreased anxiety-like behavior in adult female but not male offspring. Instead, prenatal penicillin treatment decreased sociability and aggressive displays in adult male offspring. (Champagne-Jorgensen et al. 2020).
4.3 Interaction of Maternal Stress and Antibiotics in Female Offspring
Maternal stress and maternal antibiotics interacted differently in male and female offspring. In male offspring, combined maternal stress and antibiotics did not significantly alter either apical or basilar dendrites. In female offspring, maternal antibiotic treatment attenuated the effect of maternal stress, producing apical dendritic lengths that were intermediate to the Control and Maternal stress groups, resulting in small increases in the middle of the arbor. Furthermore, although the interactive maternal treatment increased the length of distal basilar dendrites in female offspring, no effect on basilar dendrites was observed in male offspring. This sex-dependent interaction parallels previous findings from our lab, in which maternal antibiotic administration attenuated the maternal stress-induced increase in aggression in female offspring (Cusick et al. 2022).
While the lack of an overall effect from the interactive treatment was unexpected, it is nonetheless intriguing given the sex-dependent effects of the stress-only and antibiotics-only maternal treatments. Most of the existing research has examined the effects of the gut–brain axis on the HPA axis or vice versa, but the interactive effect of manipulations of these two systems is understudied. Nonetheless, a few studies are consistent with our finding that antibiotic administration mitigates the effects of stress. For example, in a rat model of stress-induced hypertension, stress treatment significantly elevated blood pressure, as expected. Interestingly, administration of antibiotics to rats undergoing stress significantly attenuated this increase. This attenuation may be mediated by a dampening of HPA axis activation during the stressor, as concentrations of ACTH and corticosterone in stressed rats that received antibiotics were similar to those of control rats (Wu et al. 2020).
In the present study, treatments were administered prenatally. Thus, these maternal manipulations may alter the prenatal environment and thereby affect development during gestation (e.g., Ahmed et al. 2014). Alternatively, prenatal treatments could alter maternal behaviors, which in turn could influence offspring outcomes. Indeed, a large body of research indicates that early life licking and grooming maternal behavior can have an extensive influence on offspring development. For instance, offspring anxiety-like behavior is modulated by maternal grooming, possibly via altered offspring glucocorticoid and/or dopamine receptor expression (Pena et al. 2014; Sequeira-Cordero et al. 2013; Weaver et al. 2004). Cross-fostering studies could reveal whether a similar mechanism mediates the separate or interactive effects of maternal antibiotics and stress.
4.4 Functional Implications
The findings reported above have important functional consequences for brain and behavior relations. Inputs to cortical pyramidal neurons are segregated to different dendritic compartments (Lafourcade et al. 2022; Xu et al. 2012). For instance, layer 2–3 pyramidal neurons preferentially receive input as a single domain clustered around the soma. Layer 5A pyramidal neurons segregate input into basal and apical domains, targeting basilar dendrites and distal regions of the apical dendrites, respectively (Mao et al. 2011). Selective remodeling of specific regions of the dendritic arbor in response to the maternal environment could potentially disrupt the fine tuning of different inputs onto the pyramidal cells (Little and Carter 2012). This would likely alter information processing in these neurons.
Such a change in prefrontal function could alter behaviors mediated by the prefrontal cortex (Wellman et al. 2020). Given that the prefrontal cortex selects and sequences contextually important behaviors (Barbas and Zikopoulos 2007), disruption of this function could contribute to the changes in social and aggressive behaviors that have been observed. Behaviors under relatively direct control of the mPFC can be inherently affected, but other, contextually dependent behaviors likely can be modulated by mPFC and could also be affected. Aggression is one such social behavior likely affected by dysregulated prefrontal signaling to the amygdala (Whittle et al. 2008). Thus, dendritic alterations in the prelimbic cortex could contribute to the increased aggressive behavior our lab has observed from maternal stress treatments in female offspring (Cusick et al. 2022).
Importantly, we examined dendritic morphology in the prelimbic cortex because morphology in this region is quite plastic, demonstrating stress-induced remodeling, often in a sex-dependent manner. While its role in executive function and context-dependent behavioral selection suggests that alterations in its structure and function could contribute to changes in aggression, other brain regions such as the orbitofrontal cortex, lateral septum, and medial amygdala play critical roles in aggressive behavior (e.g., Hashikawa et al. 2016; Rosell and Siever 2015). Thus, it will be important in future studies to examine such critical nodes in the social brain network.
5 Conclusions
Here, we observed sex-dependent effects of maternal stress and microbiome manipulation on dendritic morphology in the prefrontal cortex of adult offspring. Female offspring were more susceptible to maternal stress, and microbiome manipulation attenuated this effect. Thus, maternal stress and disruption of the microbiome interact to produce lasting, sex-dependent changes in the prefrontal cortex of offspring. Such changes may contribute to the long-term behavioral effects of these manipulations. More broadly, these findings provide additional data supporting a critical link between the gut microbiome and the neuroendocrine system in the regulation of social behavior across vertebrate species.
Author Contributions
J.A.C., G.E.D., and C.L.W. designed the experiment. J.A.S. and L.G. collected data. L.G. and C.L.W. analyzed the data. L.G., J.A.C., G.E.D., and C.L.W. wrote and revised the manuscript.
Acknowledgments
This work was supported by a National Institutes of Health (NIH) training grant (T32HD049336) and a National Science Foundation (NSF) grant (IOS-1656414). We thank all members of the Demas Lab, including Kat Munley, Beth Morrison, Jessica Deyoe, Kate Adaniya, and all the undergraduate volunteers who assisted with the study. We also thank the Indiana University Veterinary and LAR staff, including Alicia Meranda, Calan Quate, and Dr. Randalyn Shepherd, for animal care.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.