Blocking elevated VEGF-A attenuates non-vasculature Fragile X syndrome abnormalities
Abstract
Fragile X syndrome (FXS) is the most common form of inherited mental retardation. In exploring abnormalities associated with the syndrome, we have recently demonstrated abnormal vascular density in a FXS mouse model (Galvan and Galvez, 2012). One of the most prominent regulators of vascular growth is VEGF-A (Vascular Endothelial Growth Factor A), suggesting that FXS is associated with abnormal VEGF-A expression. In addition to its role in vascular regulation, VEGF-A also induces cellular changes such as increasing cell proliferation, and axonal and neurite outgrowth independent of its effects on vasculature. These VEGF-A induced cellular changes are consistent with FXS abnormalities such as increased axonal material, dendritic spine density, and cell proliferation. In support of these findings, the following study demonstrated that FXS mice exhibit increased expression of VEGF-A in brain. These studies suggest that increased VEGF-A expression in FXS is contributing to non-vascular FXS abnormalities. To explore the role of VEGF-A in mediating non-vascular FXS abnormalities, the monoclonal antibody Bevacizumab was used to block free VEGF-A. Bevacizumab treatment was found to decrease FXS Synapsin-1 expression, a presynaptic marker for synapse density, and reduce FXS testicle weight to control levels. Blocking VEGF-A also alleviated FXS abnormalities on novel object recognition, a test of cognitive performance. These findings demonstrate that VEGF-A is elevated in FXS brain and suggest that its expression promotes non-vascular FXS abnormalities. © 2016 Wiley Periodicals, Inc. Develop Neurobiol 77: 14–25, 2017
Introduction
Fragile X Syndrome (FXS) is the leading form of inherited mental retardation and the most common known single gene cause for autism spectrum disorder (Cornish et al., 2008). Although the cause for the syndrome [transcriptional silencing of FMR1, the gene that codes for the Fragile X Mental Retardation Protein (FMRP)] is known (Pieretti et al., 1991; Verkerk et al., 1991), our current understanding of the mechanisms mediating the behavioral and anatomical deficits are not understood. In exploring FXS abnormalities, studies in a mouse model of the syndrome have demonstrated increased neocortical blood vasculature (Galvan and Galvez, 2012). These findings are consistent with reports from FXS patients demonstrating abnormal cerebral blood profusion (Balci et al., 2006; Kabakus et al., 2006) and suggest abnormal vascular development in FXS. One of the most prominent regulators of vascular growth, with increased expression being synonymous with increased vasculature, is VEGF-A (Vasculature Endothelial Growth Factor A) (Neufeld et al., 1999). These studies suggest that VEGF-A expression is elevated in FXS.
In support of this hypothesis, molecular studies have further suggested that VEGF-A expression is elevated in FXS. One of the most prominent theories of FMRP function has suggested that it regulates mGluR (metabotropic glutamate receptor) dependent synthesis of various proteins through mTORC1 (mammalian target of rapamycin) activation (Bear et al., 2004; Sharma et al., 2010). mTORC1 is a complex of proteins that mediate activation of various cellular substrates and exhibits elevated phosphorylation in FXS (Sharma et al., 2010; Hoeffer et al., 2012). Interestingly, mTORC1 has been shown to mediate activation of HIF1α (hypoxia inducible factor 1, alpha), a transcription factor that promotes VEGF-A transcription (Brugarolas et al., 2003; Dodd et al., 2015). Consistent with these findings, blocking mTORC1 in cancer cells decreases VEGF-A expression (Li et al., 2015). These findings suggest that in FXS, an absence of FMRP, resulting in increased mTORC1 phosphorylation, would subsequently phosphorylate HIF1α, resulting in increased VEGF-A production. This mechanism would further suggest that VEGF-A expression is elevated and the most likely cause for increased vasculature in FXS (Galvan and Galvez, 2012).
In addition to its primary role in angiogenesis, recent studies have demonstrated that VEGF-A can alter neuronal properties independent of vasculature. Specifically, studies have shown that VEGF-A can increase neuronal survival and proliferation (Sondell et al., 1999; Matsuzaki et al., 2001; Jin et al., 2002). Furthermore, VEGF-A stimulation of cultured neurons, in the absence of blood vessels, induces axonal outgrowth (Sondell et al., 1999; Sondell et al., 2000) and dendritic/neurite growth (Silverman et al., 1999; Jin et al., 2002). In further support of a neuronal role for VEGF-A, anatomical studies have characterized VEGF-A receptors (VEGFR2/Flk-1) on dorsal root ganglia neurons and HN33 cells (an immortalized hippocampal neuronal cell line), suggesting that VEGF-A can directly alter neuronal properties independent of its role on vasculature (Jin et al., 2000; Sondell et al., 2000; Jin et al., 2002). These studies suggest that in addition to its prominent role in vascular growth, VEGF-A also acts directly on neurons altering various neuronal properties such as cell proliferation, axonal sprouting, and neurite outgrowth.
Interestingly, these VEGF-A induced cellular properties are consistent with many FXS abnormalities. Studies have demonstrated that similar to VEGF-A induced increased cell proliferation (Sondell et al., 1999; Matsuzaki et al., 2001; Jin et al., 2002), FXS has been associated with increased proliferation of Sertoli cells (Slegtenhorst-Eegdeman et al., 1998) and hippocampal neurons (Luo et al., 2010). Furthermore, consistent with VEGF-A induced increased axonal and neurite outgrowth (Silverman et al., 1999; Sondell et al., 1999; Sondell et al., 2000; Jin et al., 2002), excessive axonal growth and increased dendritic material have been observed in a drosophila and mouse model of FXS (Galvez et al., 2003; Antar et al., 2005). Also in line with these finding FXS neurons have been shown to exhibit an abundance of long-thin dendritic spines (Irwin et al., 2002; Galvez and Greenough, 2005; Grossman et al., 2006). Interestingly, two photon FXS studies have further demonstrated that this increase in dendritic spine density is due to an increase in dendritic spine formation, suggesting that an unknown factor is actively stimulating dendritic spine production in FXS (Pan et al., 2010). These findings, along with the fact that VEGF-A stimulates neurite outgrowth (Silverman et al., 1999; Jin et al., 2002), suggests that elevated VEGF-A expression in FXS is stimulating dendritic spine production and possibly other neuronal anatomical abnormalities.
The following study demonstrates that VEGF-A is elevated in FXS brains and suggests that this increase is due to an mTORC1 dependent pathway. To explore the role of VEGF-A in mediating non-vascular FXS abnormalities, VEGF-A was blocked and found to alleviate both behavioral and anatomical FXS abnormalities. These studies provide the first account for the role of VEGF-A in contributing towards FXS abnormalities.
Methods
Experimental procedures were conducted in accordance with and approved by the Illinois Institution of Animal Use and Care Committee at the University of Illinois at Urbana-Champaign. All studies and analyses were further conducted with the researcher blinded to genotype and drug treatment to avoid experimenter bias.
Housing and Care of Animals
Adult (PND 60) male C57/B6 fmr1 knockout (FXS) and wildtype (WT) mice were used. Mice were housed in standard laboratory conditions (12 hr-12 hr light/dark cycle with food and water provided ad libitum).
VEGF-A Expression
For analysis of VEGF-A expression, dissected cortical hemispheres, including cortical white matter and hippocampus (referred to as cortical hemispheres from this point on) from FXS (n = 3) and WT (n = 3) mice were homogenized and protein concentration estimated via bicinchonic acid assay (Thermo Scientific). Protein (40 µg) in a 1:1 loading buffer (475 µL Laemmli + 25 µL βME) ratio was loaded and run on a 4-15% electrophoresis gel (Biorad) at 100 V for 10 min followed by 200 V for 35 min. The separated proteins were then transferred to a nitrocellulose membrane at 100 V for 1 hr, blocked with 5% milk in TBS-T (Tris Buffered Saline with 0.05% Tween 20) and probed with VEGF-A antibody (1:1000; Santa Cruz) overnight at 4°C. The membrane was then washed and incubated in anti-rabbit secondary antibody (1:1000; Cell Signaling Technology) for 2 hr prior to chemiluminescent substrate exposure for 5 min and imaged via a BioRad ChemiDoc Touch Gel Imaging System (BioRad). Once imaged, blots were re-probed for GAPDH (1:1000; Santa Cruz) as a loading control. The relative intensity of VEGF-A was determined by dividing its optical density, determined using Image Lab v5.2.1 (BioRad), by GAPDH. GAPDH has been used in previous FXS studies as an effective loading control (Yu et al., 2013; Pretto et al., 2014; von Leden et al., 2014).
To explore the proposed mechanism for the increased VEGF-A expression and the dependency on mTORC1 activation in FXS, brain slices from FXS (n = 3) mice were treated with rapamycin followed by western blot analysis of VEGF-A expression. For collection of brain slices, mice were transcardially perfused with an ice cold high sucrose slicing solution (206 mM sucrose, 10.0 mM MgCl2, 11.0 mM glucose, 1.25 mM NaH2PO4, 26 mM NaHCO3, 0.5 mM CaCl2, and 2.5 mM KCl at ph 7.4) and brains sectioned via vibrating tissue slicer (300 microns) into a holding chamber with oxygenated artificial cerebrospinal fluid (126 mM NaCl, 2.0 mM MgCl2, 10.0 mM glucose, 1.25 mM NaH2PO4, 26 mM NaHCO3, 2.0 mM CaCl2, and 2.5 mM KCl at ph 7.4). The sections were also cut in half and stored in separate chambers to allow for within animal control of rapamycin treatment. After a 3-4 hour incubation at 37°C in a cell culture incubator maintained at >80% humidity and 5% CO2, half of each brain section was treated with 50 nM rapamycin or equivalent DMSO vehicle for 30 min. After drug treatment, the sections from each animal and hemisphere were pooled, homogenized and examined for VEGF-A expression with western blot detection as described above.
Blocking VEGF-A
To determine the role of VEGF-A in mediating FXS abnormalities, its ability to bind to its receptor was blocked using Bevacizumab (Genentech). Bevacizumab is a monoclonal antibody that binds to free-floating VEGF-A preventing it from binding with its receptor. To determine Bevacizumab's ability to block VEGF-A in brain, WT (n = 6) and FXS (n = 6) mice were given 5 mg/kg Bevacizumab or Saline IP every other day for 10 days. This dose has been shown to attenuate VEGF-induced angiogenesis in mouse brain (Lu et al., 2012; Walker et al., 2012). VEGF-A expression in dissected cortical hemispheres was then examined with western blot analyses. Samples were prepared and processed as described above on a large 10% electrophoresis gel at 150 V for 1 hr followed by 200 V for 4 hrs. The separated proteins were then transferred to a nitrocellulose membrane at 20 V at 100 mA overnight. The membrane was then probed for VEGF-A and GAPDH with western blot detection as described above.
Synapse Expression
To examine the role of VEGF-A in mediating synapse density, mice were given Bevacizumab as outlined above. The day following the last injection, mice were either processed for western blot analyses of VEGF-A as described above or transcardially perfused for immunohistochemical analyses. For western blot analyses dissected cortical hemispheres were examined from WT (n = 6) and FXS (n = 6) mice for Synapsin-1 expression. Synapsin-1 is a synaptic vesicle binding protein whose expression correlates with synapse number (Sudhof et al., 1989; Cesca et al., 2010). Synapsin-1 antibody (Sigma) was probed at 1:1000 dilution, incubated overnight and imaged along with GAPDH to control for loading differences. For immunohistochemical analyses six sections (30 μm) spanning primary visual cortex (V1) (FXS Saline n = 5, FXS Bevacizumab n = 4, WT Saline n = 5, WT Bevacizumab n = 4) and CA1 of hippocampus (FXS Saline n = 4, FXS Bevacizumab n = 5, WT Saline n = 5, WT Bevacizumab n = 5) were examined. V1 was selected because in rodents it exhibits many forms of experience-induced plasticity that were previously believed to only be found in higher processing regions of the cortex. For example V1 neurons exhibit stimulus familiarity (Sawtell et al., 2003; Frenkel et al., 2006; Cooke and Bear, 2010), reward-timing prediction (Shuler and Bear, 2006; Chubykin et al., 2013), and spatiotemporal sequence learning (Gavornik and Bear, 2014). As a result studies have recently utilized plasticity in V1 as an indicator of cognitive health for various neurological disorders (Cooke and Bear, 2012; Gavornik and Bear, 2014). Furthermore, V1 is the brain region where FXS neuronal and vascular abnormalities have been the most extensively examined (Irwin et al., 2002; Galvan and Galvez, 2012) making it a suitable structure for examining FXS abnormalities. CA1 was selected due to its well established link with cognition (Zola-Morgan et al., 1986) and the fact that it exhibits FXS dendritic spine abnormalities (Grossman et al., 2006).
For staining, sections were incubated in Synapsin-1 antibody (1:500; Sigma) overnight at 4°C followed by a 2 hr biotinulated anti-rabbit secondary antibody incubation at room temperature (1:100; Jackson ImmunoResearch). Synapsin-1 expression was visualized through avidin-biotin amplification (Vector) and nickel enhanced DAB detection (0.5 mg/mL DAB, 6.95 mg/mL nickel ammonium sulfate, 0.033 µL/mL 30% hydrogen peroxide). Sections were imaged on an Olympus BX50 microscope with a Zeiss AxioCam ICc1 camera at 20x magnification with AxioVision ver4.8.1. The number of Synapsin-1 positive puncta per area was quantified across all layers in V1 and in the apical dendritic field of CA1 of the hippocampus using the particle analysis tool on ImageJ (http://rsbweb.nih.gov/ij/docs/guide/146-30.html). To accurately determine the density of Synapsin-1 staining in the neuropil the area with blood vessels was removed from the analysis. To determine if the absence of FMRP altered the size of V1 or CA1, volumetric analyses were conducted. Briefly, the total area of the region was determined on adjacent evenly spaced cresyl violet stained sections from each brain by tracing the region on ImageJ. These areas were then multiplied by the distance between each section to create an estimation of the volume between sections. This number was then summed with each volume between each of the sections, to provide an overall estimation of the total volume of V1 and CA1.
Testicle Weight
To elucidate the role of VEGF-A in modulating FXS testicle weight, FXS (n = 10) and WT (n = 5) mice were given Bevacizumab or Saline via the dosing scheme outlined above. The day following the last injection, testicles were removed and post-fixed in 4% paraformaldehyde for 24 hr. FXS males exhibit macroorchidism as a result of increased Sertoli cell proliferation (Slegtenhorst-Eegdeman et al., 1998). After post-fixing, testicles were desiccated for 2 days and weighed. Testicular weight has been shown to be a viable metric to assess macroorchidism in adult FXS mice (Kooy et al., 1996). For statistical analyses each testicle was considered an individual unit.
Behavioral Analyses
To determine the contribution of VEGF-A to FXS behavioral and cognitive deficits, mice were given Bevacizumab or Saline (FXS Saline n = 7, FXS Bevacizumab n = 9, WT Saline n = 7, WT Bevacizumab n = 9) as outlined above and tested for hyperactivity and novel object recognition. Using a modified protocol (Ventura et al., 2004), mice were habituated to the training chamber (16.5”L × 8.5” W × 8” H) with no objects for 10 min on the day following the last injection. Between trials, to decrease the influence of scent marking, the cage was wiped down with 70% ethanol and allowed to dry before starting the next trial. Hyperactivity was assessed during habituation. To assess hyperactivity, video recordings of the mice were obtained and the distance traveled over 10 minutes was measured with Anymaze (v4.98). The following day (Training), mice were exposed to two of the same objects (randomly assigned, two beakers or two plastic egg shells) for 10 min. Percent time exploring the objects (direct whisking or sniffing) was determined using the following calculation: (time exploring the objects/total training time)*100. Preference for a specific side of the training chamber (Left vs. Right) was determined using the following calculation: (time spent on each side/total training time)*100. On the third day (Testing), one of the objects was randomly replaced with a novel object and the time spent interacting with each (direct whisking or sniffing) was recorded for 10 min. Percent interaction time for each object was determined via: (interaction with object/sum of total interaction with both objects)*100. Note 50% delineates equal exploration of both objects. On average mice spent 32.9 sec interacting with the objects. Finally the discrimination index for the novel object was determined via: [(interaction with novel object–interaction with familiar object)/interaction with familiar object]*100. Four mice (1 from each group) spent less than 10 sec interacting with any of the objects during the Testing Stage and were thus removed from the study.
Statistical Analyses
The analysis of VEGF-A expression in WT vs FXS and FXS rapamycin treated vs non-treated were conducted with a paired T-Test with equal variance on SAS (http://www.sas.com/en_us/home.html). All additional statistical analyses were conducted with a 2-way mixed model ANOVA on SAS with either genotype and/or drug as between subject factors. For novel object recognition, object (novel vs. familiar) was treated as a within subject factor.
Results
VEGF-A brain expression in FXS was found to be elevated compared to WT mice. FXS dissected cortical hemispheres were found to have 45.6% more VEGF-A expression than WT mice [Fig. 1(a); t(4)=3.06; p < 0.05]. Note, there was no significant difference in GAPDH (loading control) expression in the western blot analysis or any of the subsequent analyses across any of the groups (data not shown). This increased VEGF-A expression in FXS is consistent with the proposed molecular pathway [Fig. 1(c)]. To further investigate the proposed molecular pathway, FXS brain sections were treated with rapamycin to block mTORC1 activation followed by analysis of VEGF-A expression. This study found that blocking mTORC1 activation for 30 minutes decreased VEGF-A expression in FXS brain 13% [Fig. 1(b); t(4)=3.10; p < 0.05]. These findings, although cannot discount a possible mTORC1 independent pathway contributing towards FXS VEGF-A expression, suggest that the increased VEGF-A expression in FXS is at least partially mediated by mTORC1 activation [Fig. 1(c)].
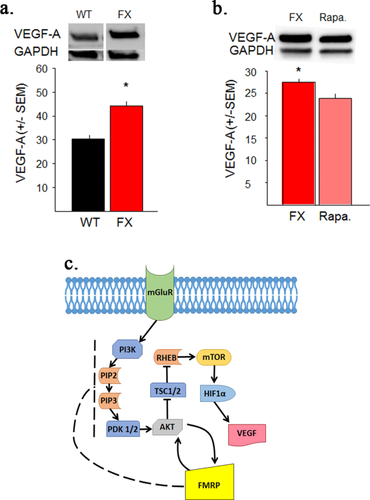
VEGF-A expression is elevated in Fragile X brain. (a) VEGF-A expression in dissected cortical hemispheres. Adult FXS mice have significantly more VEGF-A expression than WT mice. GAPDH was used as a loading control. (b) VEGF-A expression in FXS is mediated by an mTORC1 dependent pathway. Treatment of adjacent coronal hemispheres with Rapamycin to block mTORC1 activity for 30 min significantly decreased VEGF-A expression in FXS brain slices. GAPDH was used as a loading control. Rapa.=Rapamycin c. Schematic of a proposed molecular pathway for VEGF-A production involving the Fragile X Mental Retardation Protein (FMRP), the protein that is absent in FXS. Arrows = activation. Perpendicular line = inhibition. Solid lines = known interactions. Dotted lines = proposed interactions. For a detailed description of the mGluR/FMRP pathway see (Bear et al., 2004; Santoro et al., 2012). *<0.05. [Color figure can be viewed at wileyonlinelibrary.com.]
To explore the role of VEGF-A in mediating FXS abnormalities, VEGF-A's ability to bind to its receptor was blocked with Bevacizumab. Blocking of VEGF-A was found to significantly reduced VEGF-A expression in dissected cortical hemispheres of FXS mice [Fig. 2(a); F(3,11)=12.72; p < 0.05]. These findings demonstrate that Bevacizumab can be used to explore the role of VEGF-A in FXS brain abnormalities.
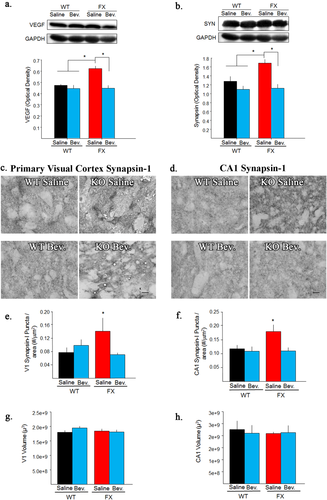
Bevacizumab decreases VEGF-A and Synapsin-1 (SYN) in Fragile X. (a) VEGF-A expression in dissected cortical hemispheres following 10 days of Bevacizumab treatment. Bevacizumab treatment significantly reduced VEGF-A expression in adult FXS mice. GAPDH was used as a loading control. (b) Synapsin-1 expression in treatment groups. Bevacizumab treatment significantly reduced SYN expression in adult FXS mice. GAPDH was used as a loading control. (c) Immunohistochemical expression of Synapsin-1 (dark punctated staining pattern) in primary visual cortex (V1) from treatment groups. Note, FXS saline treated have more dark punctated staining than WT mice and Bevacizumab treatment decreases that amount of dark punctated staining to WT levels. (d) Immunohistochemical expression of Synapsin-1 (dark punctated staining pattern) in CA1 from treatment groups. Note, FXS saline treated have more dark punctated staining than WT mice and Bevacizumab treatment decreases that amount of dark punctated staining to WT levels. (e) Quantification of Synapsin-1 density in V1 following treatment with Bevacizumab. Bevacizumab significantly decreased the density of V1 Synapsin-1 positive puncta in adult FXS mice. (f) Quantification of Synapsin-1 density in CA1 following treatment with Bevacizumab. Bevacizumab significantly reduced the density of CA1 Synapsin-1 positive puncta in adult FXS mice. Note, Bevacizumab did not significantly alter Synapsin-1 expression in WT V1 or CA1. g. Volumetric analysis of V1 following treatment with Bevacizumab. h. Volumetric analysis of CA1 following treatment with Bevacizumab. Bevacizumab did not significantly alter the volume of V1 or CA1. Scale bar =10μm Bevacizumab = Bev. *<0.05. [Color figure can be viewed at wileyonlinelibrary.com.]
One of the most prevalent FXS neuronal abnormality is increased dendritic spine density. To examine synapse properties, we demonstrated with western blot analyses, that Synapsin-1 was significantly increased in FXS dissected cortical hemispheres compared to WT mice [Fig. 2(b); F(3,8)=6.66; p < 0.05]. Subsequent region specific analyses demonstrated that Synapsin-1 was significantly increased in FXS V1 (82%) and CA1 (60%) compared to WT controls. Interestingly, these findings are not consistent with a prior study examining Synapsin staining in FXS (Li et al., 2002). Possible differences could be due to the quantification methods used; however, our findings are consistent with previous FXS anatomical dendritic spine studies (Irwin et al., 2002; Galvez and Greenough, 2005; Grossman et al., 2006; Pan et al., 2010). In addition to replicating the FXS dendritic spine abnormality, our Synapsin-1 analyses demonstrated that Bevacizumab treatment significantly decreased Synapsin-1 density in FXS cortical hemispheres, V1 and CA1 [Fig. 2(b); Cortex F(3,8)=6.66; p < 0.05; Fig. 2e; V1 (F(3,12)=4.10;p < 0.05]; Fig. 2(f); CA1 (F(3,15)=3.86;p < 0.05)]. Note, Bevacizumab did not have a significant effect on Synapsin-1 in WT mice. Furthermore, volumetric analyses of V1 and CA1 demonstrated no significant differences between treatment groups [V1 Fig. 2(g) and CA1 Fig. 2(h)], demonstrating that the observed differences in Synapsin-1 density are not due to Bevacizumab induced changes in the volume of these brain regions. These findings strongly suggest that elevated VEGF-A expression is a contributing factor towards FXS dendritic spine abnormalities.
In addition to neuronal abnormalities, macroorchidism has also been characterized as a prominent abnormality in FXS subjects. Consistent with previous studies (Slegtenhorst-Eegdeman et al., 1998) FXS mice exhibited a significant 20% increase in testicle weight compared to WT controls. However, Bevacizumab treatment decreased FXS testicle weight 11%, making them statistically indistinguishable from WT mice [Fig. 3(a); F(3,26)=6.87;p < 0.05]. Note, Bevacizumab did not have a significant effect on testicle weight in WT mice. These findings strongly suggest that elevated VEGF-A expression also contributes towards FXS testicle abnormalities.
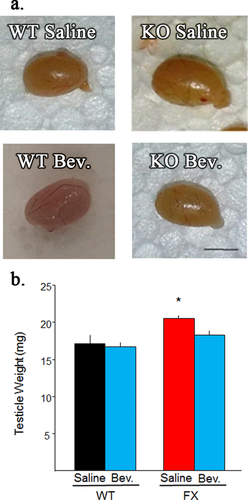
Blocking VEGF-A decreases FXS testicle weight. (a) Representative testicles from treatment groups. Scale bar = 5 mm. (b) Quantification of testicle weight from adult mice treated with Bevacizumab. Bevacizumab significantly reduced testicle weight in FXS mice. Note, Bevacizumab did not significantly alter testicle weight in WT mice. Bevacizumab = Bev. *<0.05. [Color figure can be viewed at wileyonlinelibrary.com.]
Finally, to assess VEGF-A contribution towards FXS behavioral and cognitive abnormalities, mice were given Bevacizumab as outlined above and tested for hyperactivity and novel object recognition. In line with previous findings (Bakker et al., 1994), FXS mice exhibited increased hyperactivity compared to WT mice [Fig. 4(b); t(26)=3.00;p < 0.05]. Bevacizumab did not significantly alter activity levels in either FXS or WT mice. For novel object recognition, WT controls exhibited expected preferential exploration towards the novel vs. familiar object. Likewise, consistent with previous studies, FXS Saline mice did not exhibit preferential exploration towards either object (Ventura et al., 2004; Bhattacharya et al., 2012; Seese et al., 2014). However, Bevacizumab treated FXS, like WT mice, preferentially explored the novel vs. familiar object, suggesting that they remembered which object had been previously explored [Fig. 4(c); Percent Interaction Time (F(1,14)=11.36;p < 0.05), Fig. 4(f); Discrimination Index (F(3,19)=4.68;p < 0.05)].
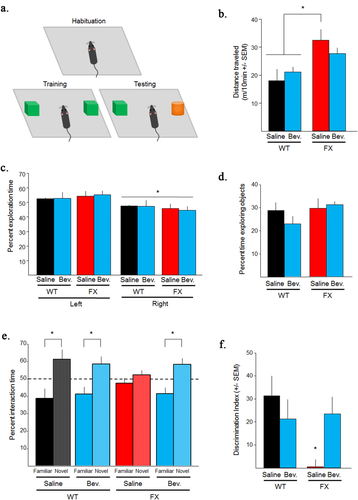
Blocking VEGF-A alleviates FXS novel object recognition (NOR) deficits. (a) NOR training protocol. (b) Amount of movement during habituation. FXS mice had significantly more movement than WT mice. Bevacizumab did not significantly alter the amount of movement for any of the groups. (c) Percent time spent in each side of the training chamber during training. Mice in all groups preferred the right side of the training chamber. This preference was controlled for by randomly placing the novel object on either side of the training chamber during testing. (d) Percent time mice spent exploring objects during training. There were no significant differences across groups. e. Percent time spent interacting with each object during testing. Bevacizumab increased adult FXS preference for the novel object, similar to WT mice. Dotted line represents no preference. (f) Discrimination index for novel object interaction across treatment groups. Bevacizumab alleviated FXS discrimination index deficit. Note, Bevacizumab did not significantly alter any measured behaviors in WT mice. Bevacizumab = Bev. *<0.05. [Color figure can be viewed at wileyonlinelibrary.com.]
As a behavioral control it was further determined that during training mice did not significantly differ in percent time exploring the objects [Fig. 4(d)]. However, all groups exhibited a significant preference for the left side of the training chamber [Fig. 4(c); t(48)=3.20;p < 0.05], with no significant side by group interaction; suggesting that this could not account for any group differences. These findings collectively suggest that blocking VEGF-A can alleviate some FXS cognitive abnormalities.
Discussion
Fragile X Syndrome is the leading form of inherited mental retardation. It is caused by transcriptional silencing of a single gene, FMR1; however, the specific mechanisms by which this genetic mutation causes FXS abnormalities are currently unknown. The current study explored a novel mechanism contributing towards FXS abnormalities, VEGF-A modulation. Historically VEGF-A has been shown to play a prominent role in vascular regulation, with increased expression being synonymous with increased vasculature (Josko et al., 2000). Consistent with these studies we previously demonstrated vascular abnormalities in V1 of adult FXS mice (Galvan and Galvez, 2012). However, in addition to its vascular role, recent studies have demonstrated that VEGF-A can increase axon growth, cell survival and neurite outgrowth, consistent with abnormalities observed in FXS (excessive axonal material, increased neurogenesis, and increased number of immature dendritic spines); suggesting a role for VEGF-A in mediating non-vascular FXS abnormalities (Slegtenhorst-Eegdeman et al., 1998; Silverman et al., 1999; Sondell et al., 1999; Jin et al., 2002; Pan et al., 2004; Pan et al., 2010).
In exploring this mechanism, the current study demonstrated that VEGF-A expression is elevated in the cortex of FXS mice [Fig. 1(a)]. These findings, although novel for FXS and VEGF-A, are consistent with our current molecular understanding of FMRP function. In FXS mTORC1 has been shown to exhibit increased phosphorylation/activation (Sharma et al., 2010). Furthermore, mTORC1 is a critical regulator of VEGF-A, with increased mTORC1 activation resulting in increased VEGF-A expression (Brugarolas et al., 2003; Dodd et al., 2015) [Fig. 1(c)]. Consistent with these findings, we demonstrated that blocking mTORC1 in FXS decreases VEGF-A expression [Fig. 1(b)], suggesting that increased mTORC1 phosphorylation in FXS would result in increased VEGF-A expression. These studies collectively support our current finding of increased VEGF-A expression in FXS and suggest a role for VEGF-A in mediating FXS abnormalities.
To explore the role of VEGF-A in mediating FXS abnormalities, our subsequent studies blocked VEGF-A and demonstrated decreased synapse density in FXS mice [Fig. 2(b–h)]. Note, these studies did not discern the effect of blocking VEGF-A on dendritic spine morphology in FXS. Current studies in the lab are actively exploring this research direction. However, our findings demonstrate that blocking VEGF-A can decrease elevated synapse density in FXS. Synapse abnormalities have been well documented in FXS (Irwin et al., 2002; Galvez and Greenough, 2005; Grossman et al., 2006); with studies suggesting that adult dendritic spine abnormalities are a result of increased dendritic spine proliferation (Pan et al., 2010). These studies have suggested that an unknown factor stimulates dendritic spine proliferation in FXS. Consistent with these findings, VEGF-A stimulation of cultured neurons in the absence of blood vessels increases dendritic growth, axon length and axon density (Silverman et al., 1999; Sondell et al., 1999; Jin et al., 2002). These findings collectively suggest that excessive brain VEGF-A in FXS stimulates dendritic spine proliferation, inducing FXS dendritic spine abnormalities.
Many studies have suggested that FXS neuronal abnormalities such as increased dendritic spine density are an underlying cause for behavioral and cognitive abnormalities. Based upon this rational, we demonstrated that blocking VEGF-A can alleviate FXS cognitive abnormalities using novel object recognition [Fig. 4(e,f)]. When provided with a choice, mice normally explore a novel object over a familiar object. However, FXS mice do not preferentially explore either object, suggesting that they do not remember prior exposure to the familiar object (Busquets-Garcia et al., 2013). Blocking VEGF-A alleviates this behavioral abnormality in FXS. Furthermore, this behavioral effect cannot be explained due to changes in the total number of object interactions or hyperactivity, as blocking VEGF-A did not alter either of these behaviors [Fig. 4(b,d)]. These findings suggest that excessive VEGF-A production, possibly via VEGF-A induced synapse abnormalities, contributes to FXS cognitive abnormalities.
Although the mechanism for VEGF-A induced neuronal growth is still not fully understood, VEGF-A has been shown to predominantly bind to VEGFR1 and VEGFR2. Upon VEGF-A binding to VEGFR1, it activates PLC gamma (phospholipase C) that then phosphorylates DAG (diacylglycerol), which phosphorylates PKC (protein kinase C). PKC then alters various cellular processes including cell proliferation and vasopermeability. In contrast, when VEGF-A binds to VEGFR2 it activates Shc (SH2 domain protein C1) that then phosphorylates RAS (Rat sarcoma), which phosphorylates MAPK. This activation then also alters various cellular processes including gene expression and cell proliferation. More importantly, neuronal studies have suggested that activation of VEGFR2 and the MAPK pathway are primarily responsible for VEGF-A's ability to modulate neuronal processes. For example, VEGF-A stimulation of superior cervical ganglia neurons that predominantly express VEGFR2 and neurophilin-1, induce axonal outgrowth and cell survival (Sondell et al., 1999). Furthermore, inhibiting VEGFR2 or MAPK prevents VEGF-A induced axon growth (Sondell et al., 1999; Sondell et al., 2000). These studies suggest that VEGF-A is mediating its neuronal effects in FXS through VEGFR2 and the MAPK pathway.
Although VEGF-A has been shown to directly alter neuronal properties independent of vascular changes (Sondell et al., 1999), it is possible that the observed attenuation of FXS abnormalities are not due to direct neuronal modulation, but rather due to indirect Bevacizumab induced VEGF-A modulation of vascular properties. Blocking VEGF-A (via Bevacizumab) decreases VEGF-induced angiogenesis in control brains (Walker et al., 2012); however, its effect on already established vascularization in FXS has not been explored. If decreasing VEGF-A expression decreases vascular density in FXS brain, decreased nutrients and other signaling factors being delivered via the blood stream could mediate some or all of the benefits observed. Interestingly, it should be noted that our prior studies demonstrated increased vasculature in aged FXS brains with only mild increases in adults (Galvan and Galvez, 2012), the age group examined in this study. The mild increased vasculature observed in adult FXS brains suggests that abnormalities in vascular density alone could not completely account for the extensive FXS neuronal abnormalities observed at this age. However, this does not preclude VEGF-A from acting directly on neurons. Furthermore, our finding of increased VEGF-A expression in the absence of excessive vascular growth (Galvan and Galvez, 2012) suggests that a compensatory mechanism is reducing vasculature in FXS brains. Current studies in the lab are actively exploring this and other possible mechanisms for the role of VEGF-A in FXS abnormalities. Although further studies will be needed to determine the specific mechanism by which blocking VEGF-A is able to attenuate these FXS abnormalities, the current studies strongly suggest that increased VEGF-A expression plays a role in mediating FXS neuronal abnormalities. Additionally, these studies demonstrate that reducing VEGF-A binding, irrespective of the mechanism, helps alleviate some FXS neuronal abnormalities.
In addition to playing a role in FXS neuronal abnormalities, our studies suggest that excess VEGF-A expression is mediating other FXS abnormalities such as macroorchidism. Studies have shown that FXS is associated with increased testicle weight, due to increased Sertoli cell proliferation (Slegtenhorst-Eegdeman et al., 1998). Consistent with these findings, VEGF-A stimulation increases neuronal survival rate and proliferation of Schwann cells (Xiao et al., 2007). These studies suggest that abnormal VEGF-A expression in FXS is mediating the increased Sertoli cell proliferation. In support of this hypothesis, we demonstrated that blocking VEGF-A in FXS significantly reduced testicle weight [Fig. 3(b)]. These findings further suggest that excessive VEGF-A production in FXS plays a role in mediating both neuronal and non-neuronal FXS abnormalities.
The current studies demonstrate that blocking VEGF-A can alleviate FXS abnormalities in testicle weight, synapse density, and cognition. Although the specific mechanism mediating these effects is still not fully understood, these studies elucidate a potential novel mechanism, VEGF-A modulation, for FXS abnormalities. Furthermore, it is worth noting that the benefits following blocking of VEGF-A were observed in adult FXS, further suggesting that VEGF-A modulation could be used to help alleviate adult FXS abnormalities.
The authors thank Stephanie Ceman, PhD for her intellectual input throughout the course of the project and Daniel A. Llano, MD/PhD for his assistance with the in vitro slice preparation.