The shape of plasma glucose concentration curve during OGTT predicts future risk of type 2 diabetes
Abstract
Background
The aim of the study is to assess the relationship between the shape of plasma glucose concentration during the OGTT and future risk for T2DM.
Methods
2445 non-diabetic subjects from the Botnia study received an OGTT at baseline and after 7–8 years of follow-up.
Results
NGT and IFG subjects who returned their plasma glucose concentration following an ingested glucose load below FPG within 60 min had increased insulin sensitivity, greater insulin secretion and lower risk for future T2DM compared to NGT and IFG subjects whose post-load plasma glucose concentration required 120 min or longer to return their plasma glucose level to FPG level. IGT subjects who had a lower plasma glucose concentration at 1-h compared to 2-h during OGTT had greater insulin sensitivity, better beta cell function and lower risk for future T2DM.
Conclusions
These data suggest that the shape of glucose curve can be utilized to assess future risk for T2DM. Copyright © 2010 John Wiley & Sons, Ltd.
Introduction
Subjects with IFG and/or IGT have an increased risk for future development of T2DM 1 and have been referred to as “prediabetic” 2. Longitudinal prospective epidemiological studies have demonstrated that only ∼50–60% of subjects with “prediabetes” convert to T2DM over the subsequent 5–10 years 2-5. On the other hand, ∼30–40% of subjects who develop T2DM have NGT at baseline 2-5, indicating that the risk for future T2DM is not similar among all subjects in the same glucose tolerance category.
We previously have shown that subjects with IFG differ from subjects with IGT, not only with respect to their FPG and 2-h PG values but also in the shape of their oral glucose tolerance curves 6. Subjects with IFG have an initial excessive increase in plasma glucose concentration that peaks at 1 h compared to subjects with IGT, but the plasma glucose concentration returns to normal or near normal values after 2 h. Subjects with IGT have a more gradual initial increase in plasma glucose concentration compared to IFG subjects. However, their plasma glucose concentration continues to rise after 60 min and at 2 h remains markedly increased compared to IFG subjects 6. Using the euglycaemic insulin and hyperglycaemic clamp techniques, we demonstrated that differences in insulin resistance in skeletal muscle and liver, as well as different patterns of impaired insulin secretion explain the differences in the shape of plasma glucose curves following glucose ingestion in IFG and IGT 6, 7. Other studies which have assessed the metabolic determinants of the shape of plasma glucose curve during the OGTT have reported that subjects with a monophasic OGTT (gradual continuous rise in plasma glucose) have increased insulin resistance and decreased beta cell function compared to subjects with biphasic OGTT (initial rise and subsequent decline in plasma glucose) 8-10. Subjects with NGT are more likely to have a biphasic OGTT, while subjects with IGT are more likely to have a monophasic OGTT 8, 9. Consistent with these observations, we have demonstrated that Mexican American NGT subjects who return their plasma glucose concentration to their FPG level faster during the OGTT have greater insulin sensitivity and better beta cell function compared to NGT subjects who return their plasma glucose concentration to the FPG level slower 11. Further, NGT subjects with slow return of their PG concentration to fasting levels have significantly greater risk for future T2DM compared to subjects with a more rapid return 11. These observations indicate that the risk for future T2DM is related to the shape of plasma glucose concentration curve during the OGTT. In this study, we investigate the relationship between the shape of plasma glucose curve during the OGTT and the risk for future type 2 diabetes amongst various glucose tolerance groups in a Caucasian population.
Research design and methods
Study population
The participants of this study were subjects who participated in the Botnia study 12, were free of diabetes at baseline, had their plasma glucose and insulin concentrations measured during an OGTT and had a repeat OGTT after 7–8 years. Subjects were classified into various categories of glucose tolerance based upon their fasting and 2-h plasma glucose concentrations during the OGTT, according to the ADA criteria 2.
Study design
All subjects received a 75 g OGTT following a 12-h overnight fast. Plasma glucose and serum insulin concentrations were measured at 0, 30, 60 and 120 min. Plasma glucose was measured with glucose oxidase method using a Beckman Glucose Analyzer (Beckman Instruments, Fullerton, CA). Serum insulin was measured in duplicate by radioimmunoassay (Pharmacia, Sweden). The diagnosis of diabetes was based on ADA criteria: 2-h plasma glucose ≥ 200 mg/dL or FPG ≥ 126 mg/dL.
We divided subjects with NGT and IFG into four groups (I–IV), based upon the time (30, 60 or 120 min or never) at which their plasma glucose concentration during the OGTT declined below the fasting glucose concentration (Figure 1). Groups I, II and III included subjects whose plasma glucose concentration fell below the FPG at 30, 60 and 120 min, respectively; group IV included subjects whose plasma glucose never fell below the FPG at any time during OGTT. Because IGT subjects (per definition) do not return their plasma glucose concentration to the fasting level, subjects with IGT and combined glucose intolerance (CGI) were divided into two groups (A and B), based upon the relationship between the plasma glucose concentration at 60 and 120 min. Group A included subjects with a plasma concentration glucose at 60 min that was less than the plasma glucose concentration at 120 min (biphasic OGTT). Group B included subjects with a plasma glucose at 60 min that was greater than the plasma glucose concentration at 120 min (monophasic OGTT).
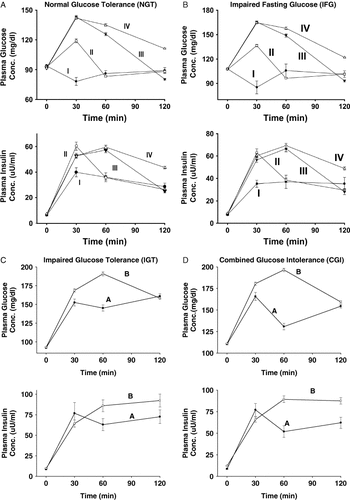
Plasma glucose and insulin concentrations in subjects with normal glucose tolerance (NGT) (A), impaired fasting glucose (IFG) (B), impaired glucose tolerance (IGT) (C) and combined glucose intolerance (CGI) (D)
Calculations
Areas under the glucose and insulin curves (AUCs) were calculated by the trapezoid rule. The homeostasis model assessment of insulin resistance (HOMA-IR) 13 and Matsuda index of insulin sensitivity 14 were calculated as previously reported. The insulinogenic index was calculated by dividing the increment in serum insulin by the increment in plasma glucose during the 0–30 and 0–120 min time periods of the OGTT. The insulin secretion/insulin resistance (disposition) index was calculated as ΔI/ΔG× Matsuda Index.
Statistical methods
Statistical analyses were performed with the SPSS statistical software package. Data are presented as mean ± S.E.M. The means were compared with one-way ANOVA. Categorical variables were tested with Chi square test. Statistical significance was set at P < 0.05.
Results
Table 1 presents the anthropometric, laboratory and clinical characteristics of the study population. Of the 2445 study participants, 1110 had NGT, 949 had IFG, 122 had IGT and 260 had CGI (IGT + IFG) at baseline. A total of 124 subjects (5.1%) developed T2DM over the 7–8 year follow-up period. The conversion rate to T2DM was 2.4%, 5.1%, 11.5% and 13.5% for NGT, IFG, IGT and CGI subjects, respectively.
| NGT | IFG | IGT | CGI | |
|---|---|---|---|---|
| Number | 1110 | 950 | 123 | 260 |
| Sex (F/M) | 661/449 | 428/522 | 72/51 | 155/105 |
| Age (years) | 45 ± 1 | 46 ± 1 | 50 ± 2 | 52 ± 1 |
| BMI (kg/m2) | 24.1 ± 0.7 | 23.5 ± 0.3 | 24.9 ± 0.2 | 25.6 ± 0.2 |
| Waist (cm) | 84.5 ± 0.4 | 88.4 ± 0.4 | 90.1 ± 1.1 | 92.7 ± 0.8 |
| FPG (mg/dL) | 92 ± 1 | 108 ± 1 | 93 ± 1 | 110 ± 1 |
| 2-h PG (mg/dL) | 99 ± 1 | 109 ± 1 | 158 ± 2 | 158 ± 1 |
| T Chol (mM) | 5.4 ± 0.03 | 5.6 ± 0.04 | 5.6 ± 0.09 | 5.8 ± 0.07 |
| FPI (uU/mL) | 6.4 ± 0.1 | 8.3 ± 0.2 | 9.4 ± 0.5 | 11.5 ± 0.5 |
| 2-h PI (uU/mL) | 26 ± 1 | 38 ± 1 | 88 ± 7 | 83 ± 3 |
| HDL (mM) | 1.42 ± 0.01 | 1.37 ± 0.03 | 1.33 ± 0.03 | 1.29 ± 0.02 |
| Triglycerides (mM) | 1.15 ± 0.02 | 1.31 ± 0.03 | 1.51 ± 0.08 | 1.65 ± 0.06 |
| BP (mm Hg) | 125/77 | 129/78 | 132/81 | 139/83 |
| % With MS | 8.1 | 30.5 | 22.8 | 55.4 |
| # Converted to diabetes | 27 | 48 | 14 | 35 |
- FPG = fasting plasma glucose; 2-h PG = plasma glucose concentration at 2 h; T-Chol = total cholesterol; FPI = fasting plasma insulin concentration; 2-h PI = plasma insulin concentration at 2 h; BP = blood pressure; MS = metabolic syndrome.
In NGT subjects, the incidence rate for the development of T2DM was 0% in group I and increased progressively to 1.8%, 2.1% and 2.9% in groups II, III and IV, respectively (P < 0.001) (Table 2). The incidence rate for developing T2DM in subjects with IFG was 0% in group I and increased to 1.5%, 6.8% and 5.2% in groups II, III and IV, respectively (P < 0.0001). In IGT subjects (7.4 vs 12.6%) and CGI (6.5 vs 15.1%), the incidence of T2DM in group A was about half that in group B.
| Group | N | Matsuda Index | HOMA-IR | ΔG (mg/dL) | ΔI (uU/mL) | ΔI0–30/ ΔG0–30 | ΔI0–30/ ΔG0–30× Matsuda | ΔI0–120/ ΔG0–120 | ΔI0–120/ ΔG0–120× Matsuda | % with MS | DM (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal glucose tolerant (NGT) | |||||||||||
| I | 38 | 9.2 ± 0.7 | 1, 34 + 0, 08 | − 0.9 ± 0.2 | 51 + 4 | 30 ± 6 | — | 206 ± 17 | — | 0 | 0 |
| II | 167 | 8 ± 0.3 | 1, 39 + 0, 04 | 0.3 ± 0.1 | 60 ± 3 | 25 ± 4 | 63 ± 8 | 140 ± 6 | 434 ± 52 | 3 | 1.8 |
| III | 283 | 7.1 ± 0.2 | 1, 45 + 0, 04 | 2.5 ± 0.1 | 70 ± 3 | 14 ± 1 | 22 ± 2 | 83 ± 2 | 139 ± 19 | 8.9 | 2.1 |
| IV | 622 | 5.8 ± 0.1 | 1, 64 + 0, 04 | 3.8 ± 0.1 | 79 ± 2 | 12 ± 0.3 | 20 ± 1 | 62 ± 1 | 104 ± 6 | 9.7 | 2.9 |
| Impaired fasting glucose (IFG) | |||||||||||
| I | 18 | 7.1 ± 0.7 | 2.0 + 0, 2 | − 0.7 ± 0.2 | 51 ± 6 | 32 ± 7 | — | 204 ± 34 | — | 17.7 | 0 |
| II | 137 | 5.9 ± 0.2 | 1.9 + 0, 1 | 0.3 ± 0.1 | 62 ± 3 | 20 ± 1 | 53 ± 7 | 108 ± 5 | 269 ± 30 | 16.9 | 1.5 |
| III | 326 | 5.1 ± 0.1 | 2.0 + 0, 1 | 2.9 ± 0.1 | 77 ± 2 | 14 ± 1 | 21 ± 2 | 64 ± 2 | 96 ± 9 | 28.9 | 6.6 |
| IV | 459 | 4.4 ± 0.1 | 2, 3 + 0, 1 | 4.1 ± 0.1 | 90 ± 3 | 13 ± 1 | 19 ± 1 | 48 ± 9 | 72 ± 4 | 36.8 | 5.3 |
| Impaired glucose tolerance (IGT) | |||||||||||
| A | 27 | 4.4 ± 0.5 | 1, 88 + 0, 24 | 5.8 ± 0.3 | 105 ± 15 | 12 ± 2 | 20 ± 3 | 41 ± 4 | 64 ± 7 | 15.4 | 7.4 |
| B | 96 | 4 ± 0.2 | 2, 24 + 0, 15 | 8.0 ± 0.2 | 126 ± 10 | 12 ± 1 | 13 ± 4 | 35 ± 1 | 41 ± 2 | 25.3 | 12.6 |
| Combined glucose Intolerance (CGI) | |||||||||||
| A | 47 | 4.2 ± 0.3 | 2, 25 + 0, 18 | 3.6 ± 0.3 | 93 ± 11 | 14 ± 1 | 22 ± 2 | 52 ± 4 | 75 ± 7 | 40.4 | 6.4 |
| B | 213 | 3 ± 0.1 | 3, 38 + 0, 17 | 6.8 ± 0.2 | 121 ± 5 | 13 ± 1 | 14 ± 1 | 31 ± 1 | 32 ± 2 | 60.0 | 15.1 |
In subjects with NGT and IFG, the incremental areas under the insulin and glucose curves increased progressively from groups I to IV. In contrast, the ratio of ΔI(AUC)/ΔG(AUC) decreased progressively. Insulin resistance, calculated by HOMA-IR, increased progressively from groups I through IV and insulin sensitivity, calculated by the Matsuda index, declined progressively from groups I through IV (Table 2). ΔI0–30/G0–30, a measure of early phase insulin secretion, declined progressively from groups I to IV. The insulin secretion/insulin resistance (disposition) index (both ΔI0–30/ΔG0–30× Matsuda and ΔI0–120/ΔG0–120× Matsuda) progressively decreased from groups I through IV.
To evaluate the importance of the shape of plasma glucose curve during the OGTT compared to the absolute FPG and 2-h PG concentrations in predicting the future risk for T2DM, we combined subjects with 2-h PG < 140 mg/dL (subjects with NGT and isolated IFG) and divided them into four groups based upon the time at which the plasma glucose concentration during the OGTT returned to or below the fasting level (30, 60, 120 min or never). Table 3 demonstrates that subjects whose 2-h PG returned to or below the FPG after 60 min or never had ∼3.5-fold increased risk for T2DM despite comparable FPG and 2-h PG concentrations.
| Group | I + II | III | IV | ANOVA |
|---|---|---|---|---|
| Number | 364 | 586 | 1048 | |
| FPG (mg/dL) | 99 ± 1 | 100 ± 1 | 98 ± 1 | NS |
| 2-h PG (mg/dL) | 96 ± 1 | 86 ± 1 | 116 ± 1 | P < 0.0001 |
| Matsuda index | 7.3 ± 0.2 | 6.2 ± 0.1 | 5.2 ± 0.1 | P < 0.0001 |
| Insulinogenic index | 2.1 ± 0.3 | 1.2 ± 0.1 | 1.1 ± 0.1 | P < 0.0001 |
| ΔI0–120 (µU/mL) | 59 ± 2 | 74 ± 2 | 84 ± 2 | P < 0.0001 |
| ΔG0–120 (mg/dL) | 3 ± 1 | 48 ± 1 | 70 ± 1 | P < 0.0001 |
| ΔI0–120/ΔG0–120 | 5.9 ± 1.3 | 2.8 ± 0.3 | 1.4 ± 0.1 | P < 0.0001 |
| %Conversion to T2DM | 1.1 | 3.75 | 3.8 | |
| Odds ratio (95% CI) | 1 | 3.5 (1.2–10.2) | 3.54 (1.3–3.6) |
- Group III represents subjects whose 2-h PG returned to or below the FPG at 120 min. Group IV represents subjects whose 2-h PG never returned to their FPG level.
In subjects with IGT, the incremental area under the glucose and insulin curves was greater in group B compared to group A in both IGT and CGI. However, the ratio of these areas was similar in both groups. Because subjects in group B have increased insulin resistance compared to subjects in group A, the insulin secretion/insulin resistance (disposition) index was significantly reduced in group B compared to group A.
Discussion
In this study, we demonstrate that the risk of future T2DM is related, not only to the FPG and 2-h PG concentrations but also to the shape of the plasma glucose curve during the OGTT. Both group A and group B subjects with IGT and CGI had similar FPG and 2-h PG concentrations. However, the risk for future T2DM in group B was double than that in group A. This marked increase in the risk for future T2DM in group B was explained by the increased severity of insulin resistance (Matsuda index and HOMA-IR) and greater decrease in beta cell function in subjects in group B compared to group A. Thus, stratifying subjects with IGT based upon the shape of their plasma glucose curve identifies a subgroup of IGT subjects (group B) who are at very high risk for future T2DM. Based upon the recent ADA recommendation 15, pharmacological therapy, in addition to lifestyle change, should be considered in this group of IGT subjects to reduce their future risk for T2DM.
We previously demonstrated that Mexican American NGT subjects can be stratified for risk of progression to T2DM based on the relationship between the FPG concentration and post-load (OGTT) plasma glucose concentration 11. The more quickly the plasma glucose concentration returns to or below the fasting glucose level following glucose ingestion, the lower is the risk for future T2DM. Moreover, stratification based on this criterion identifies physiologically distinct groups with abnormalities in insulin sensitivity and insulin secretion that are characteristic of individuals with T2DM 16-19. In this study we confirm these previous observations in a Caucasian population and extend them to demonstrate that a similar relationship between the time required for the plasma glucose concentration to return to the FPG concentration during OGTT and the risk for future T2DM also characterizes subjects with isolated IFG. Although, in general, subjects with IFG have an increased risk for future T2DM compared to NGT subjects, IFG individuals who return their plasma glucose concentration to or below the FPG concentration at 30 and 60 min (groups I and II) have low risk for future T2DM. However, the future risk for T2DM markedly increases in IFG subjects who return their fasting plasma glucose to fasting level at 120 min or never return (Figure 2). If groups I and II are analyzed collectively and compared to groups III and IV, both groups have comparable FPG (107 and 108, respectively) and 2-h PG (100 and 110, respectively). However, subjects in groups III and IV have a more than four fold increase (5.9%) in the risk for future T2DM compared to subjects in groups I and II (1.4%, P < 0.0001). Furthermore, the risk for future T2DM in IFG subjects in groups I and II was comparable to NGT subjects in groups I and II, even though they had an elevated FPG concentration. Moreover, when subjects with isolated IFG and NGT are grouped together, those whose 2-h PG returns to or below the FPG level at 120 min (group 3 in Table 3) have a FPG that is comparable to that in subjects whose 2-h PG returns to or below the FPG level within 60 min (groups I + II in Table 3), and a lower 2-h PG concentration (86 compared to 100 mg/dL). However, they have a 3.5-fold increase in the future risk for T2DM. This further emphasizes the importance of the shape of plasma glucose curve in determining the risk for future T2DM.

The 7–8 year incidence of T2DM in subjects with NGT or IFG stratified based upon the relationship between fasting and 2-h plasma glucose concentration (groups I–IV) (upper panel) and the ratio between the incremental area under the curve of plasma insulin to plasma glucose concentration curves during the OGTT in the same subjects (lower panel)
Insulin sensitivity, measured with Matsuda index, and insulin secretion, measured with ΔI0–120/ΔG0–120, progressively decreased as subjects progressed from group I to IV (Figure 2). This observation is consistent with a previous study in which we demonstrated that the slope of the decrease in plasma concentration during the OGTT is highly dependent on insulin resistance in skeletal muscle and beta cell function 20. A slower rate of decrease in plasma glucose concentration is indicative of increased insulin resistance in skeletal muscle and/or impaired beta cell function, both of which are strong risk factors for the development of T2DM 21, 22. Thus, stratification of subjects based upon the shape of the plasma glucose concentration curve identifies physiologically distinct groups with greater severity of insulin resistance and beta cell failure—core defects responsible for the pathogenesis of glucose intolerance in subjects with IFG and IGT 17—and increased risk for future T2DM 21, 22.
We previously have shown 6, 7 that subjects with IFG and/or IGT manifest the two core defects characteristic of type 2 diabetes: insulin resistance in liver, muscle and adipocytes and beta cell dysfunction. The results of the present study extend these observations and demonstrate that insulin resistance and beta cell dysfunction start in the NGT stage, and the relationship between plasma glucose concentration during the OGTT and fasting plasma glucose concentration provides a useful and simple tool to identify NGT subjects with insulin resistance and beta cell dysfunction who are at increased risk for future T2DM.
The results of this study are consistent with a previous study 23 in which we demonstrated that the post-load plasma glucose concentration is superior to the FPG in predicting the future risk for T2DM. Furthermore, addition of the 1-h post-load plasma glucose concentration to prediction models that rely exclusively on fasting measurements significantly increase their prediction power 23. For example, if one were to rely only on the FPG for the assessment of future risk for T2DM, we would have classified NGT subjects in groups III and IV (mean FPG = 92 mg/dL but return of post-load plasma glucose to the fasting level after 60 min) as having a lower risk for future T2DM compared to IFG subjects in groups I and II (mean FPG = 108 mg/dL and return of post-load plasma glucose to the fasting level at 60 min), while the actual risk in the former group is double than that of the latter group (2.7% versus 1.3%, P < 0.0001) (Figure 2). It is well established that progressive beta cell failure is the principal factor responsible for the development of hyperglycaemia and overt T2DM 22. IFG subjects, who returned their post-load plasma glucose concentration to the FPG level within 60 min, have better beta cell function (ΔI0–30/ΔI0–30 and ΔI0–120/ΔG0–120) compared to NGT subjects who returned their post-load plasma glucose concentration to the FPG level after 60 min (20.5 ± 1 vs 54.5 ± 7 and 12.7 ± 0.3 vs 27.7 ± 1.2, respectively, P < 0.0001) (Figure 2). Of note, insulin sensitivity, measured with the Matsuda index, was comparable between the two groups (6.0 ± 0.1 vs 5.9 ± 0.2, P = NS). The reduction in beta cell function in this sub-group of NGT subjects would be expected to contribute to their increased T2DM risk when compared to IFG subjects. This observation underscores the importance of measuring the post-load plasma glucose concentration to more accurately assess the risk for future T2DM. Moreover, without an oral glucose challenge one cannot assess beta cell function, since this parameter cannot be assessed using the fasting plasma glucose concentration.
In summary, we have demonstrated that subjects with NGT and IFG, whose plasma glucose concentration does not return to their baseline FPG level within 60 min following an oral glucose load, have a significantly higher risk of progression to type 2 diabetes compared to subjects whose plasma glucose returns to the fasting level within 60 min. These individuals also manifest greater insulin resistance and reduced insulin secretion compared to subjects whose plasma glucose concentration returns to below their fasting plasma glucose level at 60 min.
Acknowledgements
The Botnia study was funded by grants from the Sigrid Juselius Foundation, the Folkhälsan Research Foundation, the Finnish Diabetes Research Foundation and the Swedish Research Council.
Conflict of interest
None declared.




