Characterization of CD4+ T cells specific for glutamic acid decarboxylase (GAD65) and proinsulin in a patient with stiff-person syndrome but without type 1 diabetes
Abstract
Background
Glutamic acid decarboxylase (GAD) is a rate-limiting enzyme in the synthesis of gamma-amino butyric acid (GABA) and an important autoantigen both in patients with type 1 diabetes (T1D) and stiff-person syndrome (SPS). Autoantibodies (GADA) to the 65-kDa isoform of GAD are a characteristic feature in both diseases. Approximately 30% of patients with SPS develop diabetes, yet, it is unclear to which extent co-existing autoimmunity to GAD65 and other islet autoantigens determines the risk of developing T1D.
Methods
In this study, we monitored CD4+ T-cell responses to GAD65 and proinsulin in a patient with SPS who remained normoglycaemic during the 46-month follow-up.
Results
Fluctuating but persistent T-cell reactivity to GAD65 was identified, as well as T-cell reactivity to proinsulin at one time point. The majority of the T-cell clones isolated from the patient with SPS produced high levels of Th2 cytokines (IL-13, IL-5 and IL-4). We also examined levels of GADA, insulin and IA-2 autoantibodies, and epitope specificity of GADA. In both serum and cerebrospinal fluid (CSF), GADA levels were high, and GADA persisted throughout the follow-up. Despite T-cell reactivity to both GAD65 and proinsulin, autoantibodies to other islet autoantigens did not develop.
Conclusions
Further follow-up will determine whether the beta-cell autoimmunity observed in this patient will eventually lead to T1D. Copyright © 2010 John Wiley & Sons, Ltd.
Introduction
Stiff-person syndrome (SPS) is a rare neurological disorder characterized by muscle stiffness, hyperreflexia and elevated serum levels of creatinine kinase. A key feature in the pathophysiology of SPS is the impairment of GABAergic neurotransmission 1. Type 1 diabetes (T1D) results from the destruction of insulin-producing beta cells in pancreatic islets and leads to metabolic derangement which can only be compensated by daily injections of exogenous insulin 2 These two seemingly very different disorders share one pathophysiologic characteristic. A common feature in both is the presence of autoantibodies to glutamic acid decarboxylase (GAD), the rate-limiting enzyme in the synthesis of the inhibitory neurotransmitter gamma-amino butyric acid (GABA). While in T1D, GAD-specific autoantibodies (GADA) are mostly considered as indicators of islet autoimmunity without any proven role in islet destruction 2-4, in SPS, a direct pathogenic role of GADA has been postulated based on the finding that they inhibit the enzymatic activity of GAD in vitro 5 and because reduced GABA levels have been detected in CSF and brain of patients with SPS 5-7. Both SPS and T1D are considered to be autoimmune diseases 3, 8, 9.
GAD is expressed in two isoforms with molecular weights of 65 (GAD65) and 67 (GAD67) kDa 10-12. While GAD65 is a common target of humoral autoimmunity in both T1D and SPS 13-16, the larger isoform is rarely recognized by autoantibodies, possibly due to the differences in the enzymes' conformations 17. Approximately ∼80% of patients with T1D test positive for GADA 18, 19, although the serum levels are 10–1000-fold lower than in SPS 20. GADA in patients with T1D recognize different GAD65 epitopes when compared to GADA in patients with SPS 21, 22, and in strong contrast with SPS-related GADA, do not inhibit GAD65 enzyme activity 14, 22. In SPS, GADA predominantly target the linear parts of GAD65 whereas in T1D, the epitopes are mainly conformation-dependent 14, 19, 20, 22. Patients with SPS also recognize a C-terminal epitope of GAD65, but it is not clear whether this is the same epitope as recognized by patients with T1D. In fact, it is unlikely that the same epitope is recognized as the T1D-associated antibodies do not inhibit GAD65 enzyme activity.
Relatively few studies have investigated GAD65-specific cellular responses in SPS. GAD65 reactive T cells have been identified in the peripheral blood and CSF of some patients with SPS 23-27 although the responses have generally been weak and sometimes detectable only in T-cell lines generated by repeated stimulation with a GAD65 peptide 26, 27. Studies comparing T-cell responses in patients with both T1D and SPS have suggested differences in the T-cell epitope recognition pattern between the two diseases 25, 28-30. However, there are no reports on monitoring of potential changes in the T-cell responses to GAD65 or other islet autoantigens in relation to clinical parameters in SPS. In this longitudinal study, we evaluated the frequency and phenotype of GAD65 and proinsulin-specific T cells in a non-diabetic patient with SPS using MHC class II tetramers, and correlated these findings to autoantibody status and clinical parameters.
Materials and methods
The patient
The patient is a 33-year-old (as of 2010) Caucasian female, previously healthy other than having partial complex epilepsy. In March 2006, she was hospitalized because of lower back pain, walking difficulty and severe abdominal and lower lumbar muscle spasms. Serum creatinine kinase levels increased 10-fold during the first 5 days in the hospital. The clinical picture raised suspicion of SPS, and very high levels of GADA were serially measured from sera (Table 1). GADA was also measured from a CSF sample drawn concomitantly with the second serum sample, and the GADA titer was more than four times higher than in serum. Clonazepam was initiated and the patient was able to walk unassisted upon release from the hospital. Brain and cervical, thoracic and lumbar spinal cord MRIs were normal. CSF leukocyte count and CSF protein concentration and IgG index were normal, but total IgG concentration in CFS was elevated to 59 mg/L (normal value < 45 mg/L). Protein electrophoresis of CFS showed three oligoclonal gamma bands, indicating immunoglobulin production in the CNS, or a compromised blood–brain barrier. No viral, Mycoplasma or Borrelia burgdorferi-antibodies were present in the CSF. IgA deficiency was diagnosed. ENMG examination was normal, and serum autoantibody titers against tissue transglutaminase and gliadin, as well as parietal cell-, nuclear, acetyl choline, insulin and IA-2 autoantibodies were all negative. Anti-thyroid peroxidase antibody titre was intermediately positive 120 IU/mL (normal < 10 IU/mL). Islet cell antibodies were of a very high titer (3033 JDFU, normal value < 2.5 JDFU) which was likely due to the high GADA titer. Blood glucose levels, monitored every 3–6 months for 42 months and oral glucose tolerance test (performed twice) were normal. The clinical symptoms of SPS worsened in September 2006. Intravenous immunoglobulin treatment was contraindicated because of IgA deficiency, and she received oral prednisolone and tizanidine therapy for 3 months in addition to clonazepam. Thereafter, her SPS symptoms have been relatively stable until today (January 2010). The patient was HLA typed for class II alleles 31 and she carried the following genotype: HLA-(DR3)-DQA1*05-DQB1*02/HLA-(DR7)-DQA1*0201-DQB1*0303.
| Sample drawn | ICA (JDFU) | IAA, RU | GADA, RU | IA-2A, RU |
|---|---|---|---|---|
| 3/22/2006 | 3033 | 0.62 | 377 310 | 0.13 |
| 4/26/2006 | 3033 | 0.34 | 493 470 | 0.07 |
| 9/14/2006 | 3033 | 0.00 | 1 100 300 | 0.11 |
| 1/17/2007 | 3033 | 0.72 | 398 200 | 0.09 |
| 6/12/2007 | 3033 | 0.00 | 317 550 | 0.07 |
| 9/3/2007 | 1517 | 0.00 | 488 210 | 0.04 |
- Positive titers are shown in bold.
Peripheral blood samples for this study were obtained from the patient after informed consent, and when possible they were drawn simultaneously to blood samples collected for diagnostic purposes. The use of the blood samples for immunological studies was approved by the Ethical Committee at the Hospital District of Southwestern Finland (20.5.2003, 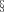 134).
134).
GAD65 enzyme activity assay
GAD65 enzyme activity was measured by the 14CO2-trapping method described previously 32. Recombinant human GAD65 (a kind donation by Amgen, Seattle, WA, USA) (100 ng) was incubated with the reaction buffer (50 mM K2HPO4, 0.03 mM PLP, 0.1 mM DTT, pH 6.8) for 1 h at room temperature, with or without the indicated amounts of isolated IgG. The enzymatic reaction was initiated by the addition of 0.56 mM L-glutamate and 0.018 µCi 14C-glutamate (Perkin–Elmer, Boston, MA, USA) and allowed to continue for 2 h at 37 °C with gentle agitation. During incubation, released 14CO2 was captured on filter paper (Kontes, Vineland, NJ, USA) soaked in 50 µL 1 M NaOH. After the incubation, the absorbed radioactivity was determined in a Beckman scintillation counter. The results are presented as: [1-(residual activity = cpm in the presence of IgG/cpm in the absence of IgG)] × 100.
GADA assay
GADA were quantified with a radioligand assay in two different laboratories as previously described 33, 34. These two laboratories were the University of Washington laboratory (C.H.; University of Washington, WA, USA) and the University of Helsinki laboratory (M.K.; University of Helsinki, Finland). GADA titers (index) presented in Figure 1 were derived using the Washington laboratory assay, while GADA titers presented in Table 1 summarize results obtained in the Helsinki laboratory.
GAD65Ab titer and inhibition of GAD65 enzymatic activity remains stable over time. Longitudinal serum samples taken at the indicated time points were tested for their capacity to inhibit GAD65 enzymatic activity (white squares, right Y-axis) and their GAD65Ab titer (black squares, left Y-axis). GAD65 enzyme activity was measured in the absence and presence of serum samples. Inhibition of GAD65 enzyme activity is shown as percent inhibition. GAD65Ab titer was determined in a RBA and is reported as GAD65Ab index
In the Washington assay 33, the cut-off limit for autoantibody positivity was 0.05 (GAD65Ab index). The sensitivity and specificity for T1D in this assay were 86% and 93%, respectively, in the 2007 Diabetes Autoantibody Standardization Program (DASP) Workshop. In the Helsinki assay 34, the cut-off limit for autoantibody positivity was 5.36 relative units (RU), representing the 99th percentile in 373 non-diabetic subjects. The sensitivity and specificity for T1D were 78% and 95%, respectively, in the 2009 DASP Workshop.
IA-2-Ab and IAA assay
IA-2 antibodies were analysed with a radioligand assay as described 35. The cut-off limit for autoantibody positivity was 0.77 RU, representing the 99th percentile in 354 non-diabetic subjects. The sensitivity and specificity for T1D were 64% and 99%, respectively, in the 2009 DASP workshop.
IAA were measured with a radiobinding microassay as described 36. The cut-off limit for IAA positivity was 2.80 RU, representing the 99th percentile in 354 non-diabetic subjects. The sensitivity and specificity for T1D were 42% and 99%, respectively, in the 2009 DASP workshop.
Recombinant Fab fragments
Monoclonal antibodies b96.11 and b78 were derived from a patient with Autoimmune Polyendocrine Syndrome—type 2 37, and recognize conformational epitopes formed by the 3D structure of amino acid residues 308–365 and 451–585, respectively 38. N-GAD65mAb was raised in mice and recognized linear epitopes representing amino acid residues 4–22 32. DPD was isolated from a patient with T1D 39 and recognized epitopes that mapped between amino acid residues 483–585 and 96–173, respectively. MICA-3 was isolated from a patient with T1D 40 and recognized epitopes of amino acid region 451–585 41. All mAbs recognized GAD65 in its native conformation and do not bind GAD67.
Competition studies using rFab
The capacity of the rFab to inhibit GAD65 binding by human serum GAD65Ab was tested in a competitive radioligand-binding assay using Protein A Sepharose (Invitrogen) as described 39. The rFab were added at the optimal concentration (0.7–1 µg/mL) as determined in competition assays using the intact mAb as a competitor. The background competition for each rFab was established in competition experiments with normal control sera. The background was subtracted prior to calculation of percent inhibition. The cut-off for specific competition was determined as > 15% by using as a negative control rFab D1.3 (a kind gift from Dr J. Foote, Arrowsmith Technologies, Seattle, WA, USA), specific to an irrelevant target, hen-egg lysozyme, at 5 µg/mL. Binding of GAD65Ab to GAD65 in the presence of rFab was expressed as follows: cpm in the presence of rFab/cpm in the absence of rFab × 100.
Peptides
Peptides used in the assay were GAD65 339–352 (TVYGAFDPLLAVAD), GAD65 247–266 (NMYAMMIARFKMFPEVKE) and ProIns B24–C36 (FFYTPMSRREVED).
Generation of tetramers
Generation of the MHC class II tetramers has been previously described 42. Briefly, a site-specific biotinylation sequence was added to the 3′ end of the DRB1*0301 leucine zipper cassette, and the chimeric cDNA was subcloned into a Cu-inducible Drosophila expression vector. DR-A and DR-B expression vectors were co-transfected into Schneider S-2 cells, the class II monomers then purified, concentrated and biotinylated. The desired peptide was loaded for 48–72 h, and tetramers were formed by incubating class II molecules with PE-labelled streptavidin.
Primary culture and stimulation
Peripheral mononuclear cells (PBMCs) were isolated from heparinized blood samples by Ficoll-Hypaque gradient separation (Lymphoprep, Nycomed, Oslo, Norway) and frozen. The frozen PBMC were shipped to Benaroya Research Institute in Seattle, USA on dry ice. PBMCs were thawed and cultured (2.0 × 106 cells/well) in RPMI media with HEPES (25 mM), 15% pooled human serum, 1% L-glutamine, 1% Penicillin/streptomycin and 1% sodium pyruvate. 10 µg/mL of peptide was added to the culture and incubated for 14 days at 37 °C. Human recombinant IL-2 (10 U/mL; Chiron, Emeryville, CA, USA) was added on days 6 and 10.
Tetramer staining of PBMC
PBMCs were stained with a peptide loaded, phycoethrin-labelled DRB1*0301 tetramer for 1 h at 37 °C and subsequently with fluorochrome-labelled CD4 (Ebioscience, San Diego, CA, USA) antibody for 20 min on ice. The cells were then washed with PBS containing 1% BSA and 0.01% sodium azide and analysed using a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA, USA). The data were analysed using FlowJo Software (Tree Star Inc., Ashland, OR, USA).
Isolation of peptide-specific T-cell clones
The peptide stimulated cells were single cells sorted into 96-well plates using a FACSVantage cell sorter (Becton Dickinson). Sorted clones were expanded for 10 days by stimulation with irradiated unmatched PBMC (1.5 × 105/well), 5 µg/mL PHA (Sigma, St Louis, MO, USA) and 10 U/mL IL-2 (Chiron) for two cycles, followed by stimulation with irradiated HLA-DR301 matched PBMC pulsed with 10 µg/mL of the peptide and 10 U/mL IL-2. On days 10–12, clones were selected based on the growth for further expansion. 5 × 104 resting T cells were tested for the specificity by stimulation with irradiated HLA-DR301 matched PBMC (1 × 105/well), with and without the specific peptide in the culture. Proliferation as measured by 3H-thymidine incorporation was tested at 72 h of culture. Supernatants were collected from culture supernatants at 48 h and cytokines were measured by using MSD's electrochemiluminescence MesoScale assay (Mesoscale Discovery, Gaithersburg, MD, USA) according to manufacturer's instructions. The assay was performed using a human Th1/Th2 cytokine kit to measure interferon (IFN)-γ, IL-10, IL12p70, TNF-α, IL-5, IL-4, IL-13, IL-2 and IL-6. T-cell clones were tested for tetramer binding by staining with 10 µg/mL specific or negative control tetramer for 1 h at 37 °C followed by fluorochrome conjugated CD4 Ab on ice for 20 min and analyed by flow cytometry.
TCR sequencing of single cell clones
TCR alpha and beta chain CDR3 sequences of the CD4+ T-cell clones were determined by RT-PCR and sequencing analysis. Briefly, total RNA was isolated from CD4+ T-cell clones using an RNeasy Mini Kit (Qiagen) and cDNA was synthesized with a Taqman Reverse Transcription Kit (Applied Biosystems). A set of five multiplex PCR reactions covering a majority of the human V-beta repertoire were performed as per Akatsuka et al. 43 and two series of amplifications, first, with pooled V-alpha primers and then, with specific V-alpha primers covering the CDR3 regions, were performed as per Seitz et al. 44. PCR products were visualized on an ethidium bromide stained 2% agarose gel, sequenced using a Big Dye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems) with either a TCR constant region 3′ primer or a specific variable region 5′ primer, and then run on an ABI3100 Genetic Analyzer. TCR alpha and beta CDR3 sequence data were analysed using the IMGT/V-QUEST (http://imgt.cines.fr) web-based program from the Université Montpellier, France 34.
Results
Inhibition of GAD65 enzymatic activity by SPS sera
The manifestation of SPS symptoms was associated with high levels of GADA in both serum and CSF (up to 1.1 × 106 RU in serum samples, Table 1). We tested the ability of the patient's sera to inhibit GAD65 enzyme activity by incubating recombinant GAD65 with longitudinally collected serum samples and subsequently measuring GAD65 enzyme activity (Figure 1). The enzymatic activity of GAD65 was inhibited significantly at all time points by at least 57%. A slight, but insignificant decrease of inhibition (65–57%) was observed between months 6 and 10.
Epitope mapping of GADA
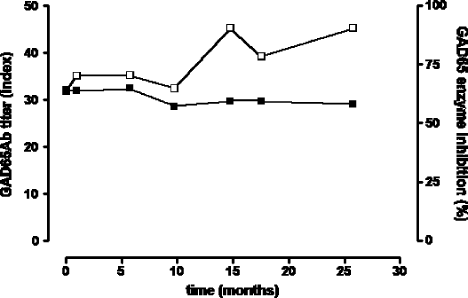
We analysed the GADA epitope specificities present, using rFab derived from GAD65-specific monoclonal antibodies (Figure 2). In our epitope-specific RBA, we determined the binding of the serum sample to radiolabelled GAD65 in the presence of GAD65-specific monoclonal rFab. Competition of serum GAD65Ab with the respective rFab for a GAD65 epitope is reflected in reduced binding of the serum to radiolabelled GAD65. Significant competition (binding of serum sample to radiolabelled GAD65 is reduced by at least 15%) was observed only for rFab b96.11 and rFab b78. The longitudinal analysis showed that the competition with rFab b96.11 was stable throughout the analysis, while competition with rFab b78 increased from 3% at baseline to 20% in the final sample.
Epitope analysis using GAD65-specific rFab. Longitudinal samples taken at the indicated time points were analysed for their epitope binding pattern in our epitope-specific RBA using GAD65-specific rFab b78 (black squares), b96.11 (white squares), MICA-3 (white circles), DPD (diamonds), and N-GAD65 mAb (black circles). Binding to radiolabelled GAD65 by serum samples was tested in the absence (100%) and presence of each of these rFab at half-maximal concentration
T-cell reactivity with GAD and proinsulin tetramers
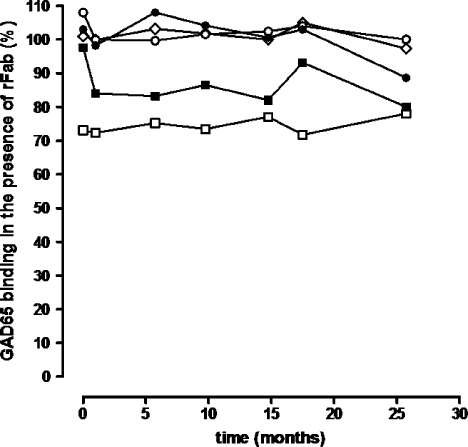
PBMC from four serial blood draws spanning over 40 months were stimulated with two GAD65 peptides (247–266 and 339–352) and a proinsulin peptide B24–C36, and stained with DR301 tetramer containing the same peptide as used in the stimulation. At the first time point, the CD4+ T cells bound to all specific tetramers at frequencies from 2.09 to 6.56% (Figure 3) which was 10–32.8-fold higher than the background staining with the negative control tetramer (∼0.2%). At the subsequent time points, DR301-GAD339–352 tetramer-positive T cells were also identified but at much lower frequencies ranging from 1.06 to 2.00% whereas the T-cell response to proinsulin peptide B24–C36 was observed to be modestly positive (0.88%) and then disappeared. The response to the GAD339–352 peptide seemed to be the most persistent one as tetramer-binding cells were detected in all samples. All the samples were also stimulated with the DR301 binding NS1 peptide derived from flu virus as positive control and showed a strong tetramer binding (data not shown).
DR301-tetramer staining of the PBMC from the patient with SPS. PBMC at 14-day post-stimulation with GAD65 339–352, GAD65 247–266 or proinsulin B24–C36 peptide (10 µg/mL) were stained with both specific DR301 tetramers containing the cognate peptide (upper panels in each section), and DR301-NS1 negative control tetramer (lower panel in each section). The cells were gated on live lymphocytic population and CD3+ cells. The frequency of CD4 and tetramer-positive cells is shown in the quadrant. The tetramer-positive cells were single cell sorted on 96-well plates. This figure is available in colour online at www.interscience.wiley.com/journal/dmrr
Avidity and cytokine profiles of the T-cell clones
Tetramer-positive cells from the cultured CD4+ lymphocytes were single cell sorted and T-cell clones were generated. T-cell clones specific for the GAD65 339–352 peptide were isolated from the first three samples whereas the attempt to raise clones from the fourth time point was not successful. T-cell clones specific for GAD247–266 and proinsulin B24–C36 peptides were isolated only from the first blood sample. Tetramer binding when used at non-saturating concentrations has been shown to correlate with the structural avidity of TCR 45, 46. In Figure 4, tetramer staining of the GAD65 339–352-specific T-cell clones shows that high-avidity T-cell clones with a strong tetramer-binding profile (77–100%) were detected at the first and third time point. However, some T-cell clones, even derived from the same sample, bound the tetramer at much lower level (34%, clone 339–1, sample #3) or, displayed lack of staining (clone 339–201, sample #2). All clones were capable of proliferating when stimulated with the specific peptide (data not shown) and produced cytokines (Figure 4) demonstrating specificity to the given T-cell epitope peptide. When the high-avidity T-cell clones derived from the first blood sample were stimulated with the GAD65 339–352 peptide robust IFN-γ and IL-13 production was observed and two of the clones (#309 and 332) also produced high levels of IL-5 and low amounts of IL-4. Three other clones isolated from the same sample displayed no tetramer binding but produced low levels of IFN-γ, IL-13 and IL-4 (50–80 pg/mL) demonstrating specificity but a low-avidity phenotype (data not shown). GAD339–352-specific T-cell clones derived from samples drawn at the later time points seemed to be biased more towards predominant IL-13 production. T cells specific for the other peptide epitopes, GAD65 247–266 and proinsulin B24–C36, also produced IL-13, and displayed low/intermediate avidity. T-cell clones specific for NS1 flu peptide were isolated from all time points (data not shown). These T cells displayed a strong tetramer binding (∼100%) and significant IFN-γ production upon stimulation with the specific peptide.
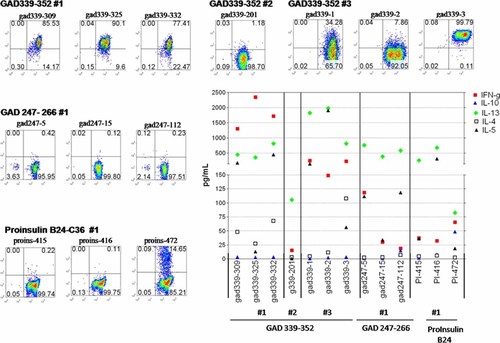
Tetramer staining and cytokine profiles of the T-cell clones. The DR301-GAD339–352 tetramer was used to stain T-cell clones single cell sorted from the PBMC samples obtained at three time points (#1–3, upper panel). Similarly, DR301-GAD247–266 and proinsulin B24–C36 tetramers were used to stain clones derived from the first time point (middle, lower left panels). In all cases, tetramer staining was evaluated on the viable lymphocytic population as gated based on the forward/side scatter profile. The frequency of CD4/tetramer-positive cells is noted in the upper right quadrant. Staining with the control tetramer containing DR301 binding irrelevant peptide (flu, NS-1) was set at 0.2%. Cytokines (pg/mL, Y-axis) were determined from the supernatants collected from triplicate cultures of the T-cell clones at 48 h post stimulation with DR301+ irradiated PBMC pulsed with the cognate peptides (middle-lower right panel). This figure is available in colour online at www.interscience.wiley.com/journal/dmrr
Overall, GAD65 339–352 is likely to be a predominant peptide epitope presented by DRB1*0301 in our patient as T-cell reactivity to this peptide was demonstrated in all four samples studied and, furthermore, T-cell clones with the given specificity, some with a very strong tetramer binding, were derived from three out of four samples. The T-cell clones generated at the first sampling produced high levels of IFN-γ but also IL-13 and IL-5, suggesting a Th1/Th0 profile which shifted more towards Th2 during the follow-up. T-cell responses to GAD247–266 and proinsulin B24–C36 peptides seemed to be more subdominant in nature. In addition, T-cell clones with these specificities displayed low avidity and Th2-biased cytokine profiles and were generated only from the first time point. Sequence analysis of the complementarity determining regions demonstrated that the T-cell clones used distinct TcR-Vβ chains and that the ones derived from different time points were not identical (data not shown).
Discussion
In this study, we analysed CD4+ T-cell responses and autoantibodies in a series of samples from a female patient who developed SPS at the age of 29. Although T-cell reactivity to GAD65 persisted throughout the follow-up, and T-cell reactivity to proinsulin emerged a year from the diagnosis of SPS, this patient did not progress to T1D during the 46-month follow-up. A meta-analysis of published case studies on patients with SPS indicate that T1D may develop from 1 year 24 to more than a decade 26 (and B.O. Roep, personal communication to H.R.) after SPS diagnosis. In individuals genetically predisposed to T1D, the risk to develop T1D depends on both the magnitude and the type of T-cell reactivity to several autoantigens 3. Furthermore, non-progressors are characterized by a regulatory rather than an inflammatory type of T-cell reactivity to islet autoantigens 47.
In our patient, high GADA titers were associated with the presence of GAD and proinsulin-reactive T cells. GAD-reactive T cells displayed some IFN-γ but significant IL-13 production. These findings suggest a non-inflammatory cytokine environment 47, 48 and effective B-cell antibody production with predominantly autoantibody-mediated disease pathogenesis 5, 7, 8. High titers of GADA may lead to functional GABA deficiency and typical manifestations of SPS even in a non-inflammatory cytokine environment which, in turn, may rescue islets from tissue destruction.
Despite that ∼50% of patients with SPS develop T1D 28, 49, there are no longitudinal studies on immune parameters in patients with SPS prior to progression to clinical diabetes. T-cell responses and levels of GADA have been studied only in patients with SPS who were already affected by T1D 24, 28, 50. An early study by Hummel et al. demonstrated a weak, but detectable T-cell response to GAD65 and IA-2 with IFN-γ production that decreased in response to immunosuppressive therapy 24. Schloot et al. reported lack of primary T-cell response to GAD65 in a non-diabetic patient with SPS. However, in vitro stimulation of the GAD65-induced cell line generated T-cell clones responsive to GAD65 peptide 341–360 with production of the anti-inflammatory cytokine IL-10, IFN-γ and low level of IL-4 26. Similarly, in our study T-cell clones specific for an overlapping peptide GAD65 339–352 were predominant, and increasingly Th2-biased cytokine profiles were detected during the course of the follow-up. Consistent with anti-inflammatory environment, a low efficacy of cloning from peripheral blood was observed in a recent study by Skorstad et al. 27. In their study, only one GAD555–565 specific T-cell clone was isolated from the PBMC of a DRB1*0408-positive individual. Interestingly, CD4+ T cells recognizing this epitope have been shown to be prevalent in DR4-positive patients with T1D 46, 51, suggesting that the reported distinct T-cell epitope recognition patterns 28 in patients with T1D and SPS may be associated with the differences in the distribution of HLA alleles rather than the disease mechanisms.
The introduction of the tetramer technology 52 has allowed identification and immune monitoring of autoreactive T cells and their isolation at single cell level with increased efficacy and sensitivity. MHC class II tetramers have turned out to be excellent tools in studies of avidity and phenotype of GAD65-specific T cells in T1D, and these studies have revealed the existence of both low- and high-avidity T cells with various cytokine patterns 46, 53, 54. In this study, GAD-specific T cells with a range of avidities and intermediate/low-avidity (pro)insulin-specific cellular immunity in combination with high levels of GADA were demonstrated. The GADA showed SPS-characteristic inhibition of GAD65 enzymatic activity and showed reactivity towards a SPS-associated antibody epitope (b78) 17. However, reactivity to T1D-associated GADA epitope (b96.11) 39 was also observed. This antibody recognizes a conformational epitope formed by the three-dimensional structure of amino acid residues 308–365, including the peptide sequence that dominated the T-cell response.
In summary, this longitudinal study of T-cell and antibody reactivity against GAD and two islet-antigens, insulin and IA-2, in a patient with SPS may facilitate future efforts to predict the probability of developing T1D after SPS. Although further follow-up is necessary, the transient nature of the (pro)insulin T-cell response, the lack of high-avidity T-cell clones recognizing (pro)insulin and the overall Th2 biased T-cell responsiveness to GAD65 suggest slow or no progression towards the induction of insulin- and other islet-specific (non-GAD65) autoantibodies and clinical manifestation of T1D. Continued follow-up will be needed to determine whether or not this patient eventually progresses to T1D, and to monitor for any apparent changes in T-cell reactivity that may accompany T1D development.
Acknowledgements
This work was supported by the Academy of Finland (A.H.), Päivikki and Sakari Sohlberg Foundation (A.H.), Medical Research Fund of Turku University Hospital (EVO (M. S-H.), Helsinki University Hospital (M.K.), Juvenile Diabetes Foundation (H.R.) and American Diabetes Association (H.R.), and by the National Institutes of Health (DK53004 and DK17047) (C.S.H.). C.S.H. is also the recipient of a Basic Science Award from the American Diabetes Association.
Conflict of interest
None declared.




