Binding of mannose-binding lectin to fructosamines: a potential link between hyperglycaemia and complement activation in diabetes
Abstract
Background
Complement activation via the MBL pathway has been proposed to play a role in the pathogenesis of diabetic complications. As protein glycation is increased in diabetes, we tested the possibility that the glycation product fructoselysine is a ligand for MBL and that its interaction with this protein may initiate complement activation.
Methods
We investigated the binding of MBL to fructoselysine by chromatography of human serum on fructoselysine-Sepharose, followed by Western blot and mass spectrometry analysis. We also performed enzyme-linked immunosorbent assays using purified MBL and fructoselysine-derivatized (binding assay) or mannan-coated plates (inhibition assay). Complement activation was determined by the fixation of C3d following incubation of fructoselysine-derivatized plates with serum from subjects with different levels of MBL.
Results
MBL and its associated proteases were selectively purified from serum by chromatography on fructoselysine-Sepharose. Competition experiments indicated that MBL had a similar affinity for mannose, fructose and fructoselysine. MBL bound, in a highly cooperative manner, to fructoselysine-derivatized plates. This binding was associated with complement activation and was much lower with serum from subjects with low-MBL genotypes.
Conclusions
MBL binding to fructoselysine and the ensuing complement activation may provide a physiopathological link between enhanced glycation and complement activation in diabetes. The cooperative character of this binding may explain the high sensitivity of diabetic complications to hyperglycaemia. Copyright © 2010 John Wiley & Sons, Ltd.
Introduction
MBL is a serum protein involved in the innate immune response. It binds in a Ca2+-dependent manner to carbohydrates (e.g. on the surface of pathogens), most particularly mannose and N-acetylglucosamine, and initiates the so-called lectin pathway of complement activation, through activation of MASPs 1, 2. The serum level of MBL is highly dependent on sequence variations that are commonly found in the first exon and in the promoter region of the mbl2 gene 3. The importance of MBL in the immune response has been illustrated by clinical studies demonstrating an aggravated outcome of several infectious diseases in individuals possessing ‘low-MBL’ genotypes 4, 5. However, the high prevalence of mutations in the mbl2 gene suggests that low MBL levels may in some situations confer a biological advantage 6.
Recent data suggest that there may be a connection between the complement system and the vascular dysfunction occurring in diabetes. Complement deposits have indeed been observed in diabetic tissues 7-10. Furthermore, several studies point to MBL as a potential initiator of complement activation in diabetic complications: diabetic nephropathy, cardiovascular disease and mortality are associated with high circulating levels of MBL 11-15. It seems therefore that a ‘low-MBL’ genotype may constitute an advantage for diabetic patients. This idea was corroborated by studies on streptozotocin-induced diabetic mice, showing that MBL deficiency protects diabetic mice from nephropathy and cardiomyopathy 16, 17.
Although it is clear that MBL is somehow involved in the ‘pathway’ between hyperglycaemia and the complement activation observed in diabetes, the causal link remains unclear. Glucose is known to react spontaneously with amines, forming Schiff bases, which rearrange to fructosamines. This process, known as glycation, is proportional to glucose concentration and is therefore enhanced in diabetes 18, 19. The purpose of this work was to test if MBL binds to fructosamines, which are structurally analogous to mannose, and thereby initiates complement activation.
Materials and methods
Affinity chromatography
Fructoselysine- and hexitollysine-Sepharose were prepared by incubating 1 mL of CNBr-activated Sepharose 4B (GE Healthcare, Diegem, Belgium) with 20 µmol of fructoselysine (prepared as in Ref 20) or hexitollysine (actually a mixture of sorbitol- and mannitol-lysine prepared by borohydride reduction of fructoselysine, as described in Ref 21), according to the manufacturer's instructions. The same procedure was applied to prepare control Sepharose, except that no ligand was added in the coupling solution. The amount of fructoselysine incorporated to Sepharose was ≈ 3 µmol/mL wet gel (determined through the assay of unreacted fructoselysine, as described in Ref 22). Mannan-Sepharose (termed ‘mannan-agarose’) was purchased from Sigma-Aldrich.
The binding of MBL to fructoselysine-, hexitollysine-, mannan- and control-Sepharose, was tested by incubating, for 1 h at 4 °C under gentle agitation, 2 mL of human serum, diluted twofold in buffer A (50 mM Tris, pH 8, 150 mM NaCl, 20 mM CaCl2, 0.05% Tween-20, 2 µg/mL leupeptin and 2 µg/mL antipain), with 0.1 mL of the indicated gel equilibrated with buffer A. The gel was then washed with 3 × 4 mL of buffer A and elution was performed with 0.4 mL of buffer B (same as buffer A except that 20 mM CaCl2 was replaced by 10 mM EDTA). The elution fractions were analysed by Western blot, using ECL™ products (GE Healthcare) and rabbit MBL H-50 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) as primary antibody (dilution 1 : 250 in phosphate-buffered saline + 0.1% Tween-20 + 5% milk). The buffer of the eluted fractions was replaced with buffer A using NAP-5 columns and the preparations were rechromatographed on the same type of gel. Fractions were analysed by SDS–PAGE and silver-staining with the PageSilver™ (Fermentas, St. Leon-Rot, Germany) or SilverQuest™ (Invitrogen, St. Leon-Rot, Germany) kit.
For the purification of MBL, 10 mL of human serum was diluted twice in buffer A, mixed with 0.5 mL of mannan-Sepharose equilibrated with the same buffer, and allowed to stand for 1 h at 4 °C with gentle agitation. The gel was then washed with buffer A (3 × 20 mL), and proteins were eluted with 4 × 0.5 mL of buffer B. The fractions containing MBL (as determined by Western blot) were buffer-exchanged on a NAP-5 column equilibrated with buffer A.
Enzyme-linked immunosorbent assays
Unless otherwise indicated, enzyme-linked immunosorbent assays (ELISAs) were made with Maxisorp plates™ (Nunc) coated with the appropriate molecule diluted in 0.1 M sodium carbonate, pH 9.6. Blocking and dilution of antibodies were done in Tris-buffered (pH 7.4) saline containing 0.5% bovine serum albumin. The plates were incubated for 1 h at 37 °C at each step and washed with 0.1 M NaCl + 0.1% Tween-20 between steps. Primary antibodies were purchased from Santa Cruz Biotechnology, and peroxidase-conjugated secondary antibodies and streptavidin were purchased from Sigma–Aldrich and GE Healthcare, respectively. Peroxidase was detected with 1-Step™ Ultra TMB-ELISA (Pierce). Antibodies were biotinylated with EZ-Link® Sulfo-NHS-Biotin kit (Pierce).
The concentration of MBL was determined by sandwich ELISA. Mouse monoclonal MBL-C (3E7) antibody was coated (1 µg/mL) and biotinylated MBL-C antibody (1 : 100) was used for the detection of MBL. SER 101 (AntibodyShop) was used as a standard. For the inhibition assay, the plates were coated with mannan (10 µg/mL) and incubated with 0.03 µg/mL MBL and the indicated concentrations of competitor in buffer C (25 mM Tris, pH 8, 150 mM NaCl, 0.5 mg/mL bovine serum albumin and 20 mM CaCl2). MBL was detected with MBL-C antibody (1 : 500).
Fructoselysine-derivatized plates were prepared by allowing ReactiBind™ maleic anhydride-activated plates (Pierce) to react with fructoselysine at the indicated concentrations. Purified MBL was diluted in buffer C and incubated for 1 h at 37 °C; serum samples were diluted in buffer C plus 5 mM MgCl2 and incubated for 2 h at 37 °C; the detection of MBL and C3d was performed with MBL-C or C3d (6F6), both diluted 1 : 500. Washings and incubation of antibodies were performed in the presence of 1 mM CaCl2.
In-plate phosphorylation by fructosamine-3-kinase
To evaluate the amount of fructoselysine bound to plates, 50 µL of a mixture containing 25 mM HEPES, pH 7.1, 1 mM MgCl2, 1 mM EGTA, 0.5 mg/mL ovalbumin, 10 µM ATP·Mg, 100 000 cpm [γ-32P]ATP and 0.2 µg of recombinant fructosamine-3-kinase 23 was added to each well, and the plate was incubated for 1 h at 37 °C. After several washings to remove unreacted ATP, 100 µL of 0.2 N NaOH was added to the wells, and the plate was incubated for 1 h at 37 °C to cause alkaline degradation of fructoselysine 3-phosphate 24 and the release of its radioactivity as 32P inorganic phosphate. The resulting solutions were removed from the wells and counted for radioactivity, which was used to calculate the fructoselysine density.
Results
Affinity chromatography on fructoselysine-Sepharose
To test if MBL binds to fructoselysine, we prepared fructoselysine-derivatized Sepharose beads, and incubated this gel as well as mannan-, hexitollysine- and control-Sepharose with diluted human serum in the presence of Ca2+. After washing with a Ca2+-containing buffer, elution was performed with EDTA. Western blot analysis showed the presence of MBL in the elution fractions of fructoselysine- and mannan-Sepharose, but not in the fractions eluted from control or hexitollysine-Sepharose (Figure 1A). This result indicated that MBL was retained on the fructoselysine affinity column.
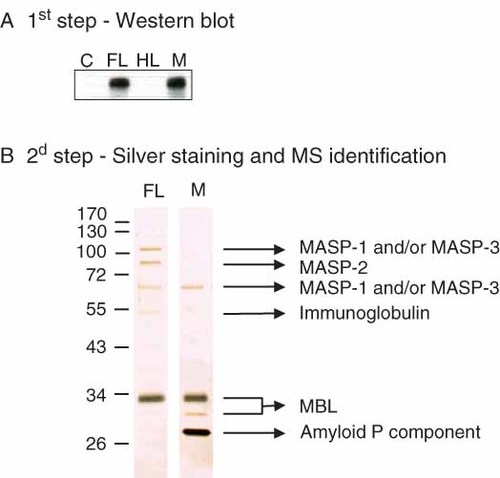
Affinity chromatography and mass spectrometry identification. Serum samples were chromatographed on control (C), fructoselysine (FL)-, hexitollysine (HL)- and mannan-Sepharose (M) columns in the presence of Ca2+. MBL was detected by Western blot (A) in the fractions eluted with EDTA buffer. The proteins eluted with EDTA from fructoselysine- and mannan-Sepharose were loaded on fresh columns containing the same stationary phase. The retained proteins were eluted again with EDTA buffer and analysed by SDS–PAGE and silver-staining, followed by mass spectrometry identification of proteins (B). The top band slightly above 100 kDa most likely corresponds to the intact zymogen MASP-1/3, whereas the band between 55 and 72 kDa corresponds to the A-chain of the activated molecules. It is not known if the binding of immunoglobulin truly corresponds to recognition of fructosamines or if it is non-specific. This figure is available in colour online at www.interscience.wiley.com/journal/dmrr
The proteins eluted from fructoselysine- and mannan-Sepharose were submitted to a second cycle of affinity purification on the same gel. Proteins eluted with EDTA from this second chromatographic step were submitted to SDS–PAGE analysis (Figure 1B). Remarkably, the affinity-purified preparations contained in both cases a limited number of proteins, which were identified through in-gel trypsin digestion and mass spectrometry analysis. The main bands of the preparation eluted from fructoselysine-Sepharose corresponded to MBL and three of its associated serine proteases (MASP-1, 2 and 3). These proteins were also identified in the preparation obtained by chromatography on mannan-Sepharose, although in this case the major band corresponded to serum amyloid P component, a protein known to bind to some polysaccharides (see Discussion).
Inhibition assay
To compare the affinity of MBL for fructoselysine and other monosaccharides, we measured their inhibitory effect on the binding of purified MBL to mannan-coated plates. As expected, mannose was a good competitor for MBL binding. Fructose and free fructoselysine were almost as potent as mannose in competing MBL binding to mannan, whereas glucose and galactose were much poorer competitors and lysine had no effect (Figure 2).
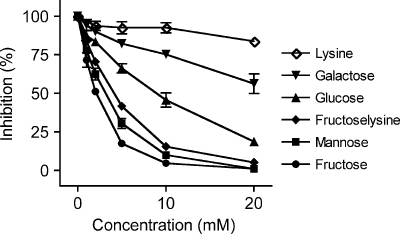
Inhibition assay. The binding of purified MBL to mannan-coated plates was measured (by ELISA) in the presence different concentrations of the indicated compound. Means ± SEM (n = 4)
Binding of purified MBL to fructoselysine-derivatized plates
To measure a direct binding of MBL to glycation products, fructoselysine was covalently attached through its α-amino group to maleic anhydride-activated plates. The amount of bound fructoselysine, determined using fructosamine-3-kinase and radio-labelled ATP, was dependent on the concentration of fructoselysine in the ‘coating buffer’ (Figure 3A), and reached a plateau with 30 mM fructoselysine at a density of ≈ 1.25 molecules/nm2 (not shown). This density corresponds to the nominal binding capacity of the plate (1.3 molecules/nm2).
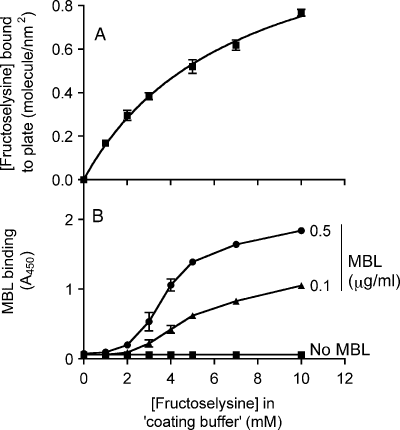
Phosphorylation by fructosamine-3-kinase and binding of MBL to fructoselysine-derivatized plates. Maleic anhydride-activated plates were incubated with different concentrations of fructoselysine. The density of fructoselysine on these plates was measured by phosphorylation with fructosamine-3-kinase (A), and MBL binding was measured by ELISA (B)
Incubation of fructoselysine-derivatized plates with purified human MBL led to a binding of this protein that was dependent on its concentration and on the concentration of fructoselysine in the ‘coating buffer’ (Figure 3B). At physiological MBL concentrations (0.1 and 0.5 µg/mL), significant binding was detected in wells pre-incubated with 3 and 2 mM fructoselysine, which correspond to densities of ≈ 0.4 and 0.3 molecule/nm2, respectively. Binding of MBL to the plates was sigmoidal with respect to fructoselysine density. Hill plots were derived from these data to quantify the degree of cooperativity and thereby estimate the minimal number of fructoselysine binding sites involved in this interaction. These plots indicated that the Hill coefficient was ≥ 4, leading to the conclusion that the interaction involves at least four fructoselysines and therefore, four carbohydrate-recognition domains per MBL molecule.
MBL binding to fructoselysine and complement activation
It was of interest to check if MBL is able to activate the complement following binding to fructoselysine. The use of affinity-purified MBL was not appropriate for this experiment, as this preparation presumably contained activated MBL/MASP complexes. We therefore used sera from various individuals with different mbl2 genotypes and, hence, with widely different serum MBL levels.
As expected, the concentration of MBL in the sera from individuals possessing the A/A genotype was higher than that in the sera from A/B and A/D individuals (Figure 4A). Incubation of fructoselysine-derivatized plates with dilutions of serum induced a binding of MBL that was dependent on the MBL genotype (Figure 4B), proportional to the serum MBL concentration (not shown), and inhibited by mannose.
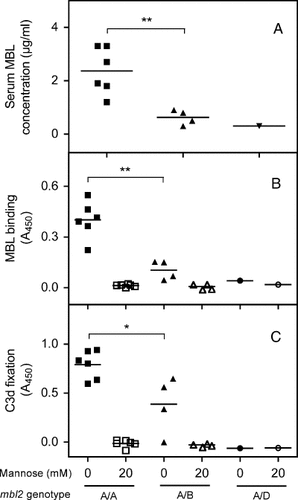
Effect of mbl2 genotype on fructoselysine-induced C3d fixation. Eleven individuals were grouped according to their mbl2 genotype: A/A (wild-type), A/B (Gly54-Asp) or A/D (Arg52-Cys). The concentration of MBL was determined in serum samples (A). ELISA was performed to measure MBL binding (B) and C3d fixation (C) after incubation of a fructoselysine-derivatized plate (and a control plate) with 10% serum and the indicated concentration of mannose. Results are expressed as the difference of A450 between a plate coated with 30 mM fructoselysine and a control plate. Each point is the mean of three A450 values obtained in one representative experiment; horizontal lines represent mean values; ** and * indicate statistically significant differences (Student's t test; p < 0.01 and 0.05, respectively)
Complement activation was determined through assay of C3d fixation. C3d fixation was the highest with sera from individuals with the A/A genotype, much lower with sera from A/B individuals, and nearly undetectable with the serum from the individual with the A/D genotype. Accordingly, C3d fixation was proportional to the MBL concentration in the serum sample (not shown). We also observed that C3d fixation was completely prevented when MBL binding was inhibited by mannose.
Discussion
Several theories have been proposed to explain the development of complications in diabetic patients. These include the formation of advanced glycation end-products, the enhancement of the formation of polyols, the activation of protein kinase C, the increased hexosamine pathway flux and the activation of complement 7, 25. It is likely that these theories are complementary and that multiple mechanisms contribute to the pathogenesis of complications. A fascinating aspect of the development of diabetic complications is their extreme sensitivity to the blood glucose concentration: a doubling of the mean blood glucose concentration leads to a substantial increase in the development of nephropathy, retinopathy and vascular complications. There must therefore be mechanisms that ‘amplify’ the toxic response to glucose.
The spontaneous formation of fructosamines from glucose and their linear dependence on glucose concentration led to suggest that they could participate in the development of diabetic complications 26, but the mechanism of their action has not been elucidated. Based on our in vitro findings, we propose a mechanism (Figure 5) involving the binding of MBL to cell surface fructosamines and the activation of complement resulting from this high-affinity binding, as previously suggested by Hansen et al. 27.
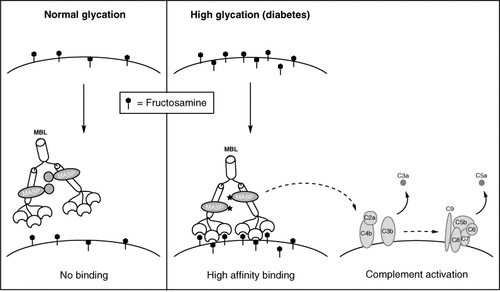
Proposed mechanism for complement activation in diabetes. The increase of fructosamine density on the cell surface, as observed in diabetes, induces MBL binding and hence activation of MASPs and other components of complement
We show indeed that MBL binds to fructoselysine, either attached to Sepharose beads or covalently bound to ELISA plates. We also demonstrate with an inhibition assay that MBL has comparable affinities for free mannose, fructose and fructoselysine. This is consistent with crystallographic data indicating that MBL binds to the trans-hydroxyl groups bound to C3 and C4 of mannose in its hexopyranose configuration 28. Fructose and the sugar portion of fructoselysine mostly exist under their pyranose configuration, which, when flipped by 180° along an O6–C4 axis, resemble mannose to a greater extent, with C3 and C4 in fructose or fructoselysine corresponding to C4 and C3, respectively, in mannose (Figure 6). Thus, MBL presumably binds to the hydroxyl groups bound to C4 and C3 in fructose and fructoselysine.
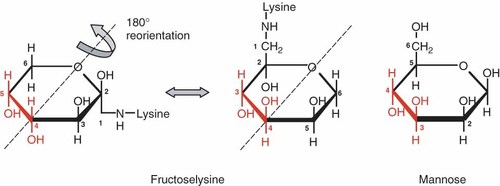
Structure of fructoselysine and mannose. Both molecules are represented under their β-pyranose conformation, and fructoselysine has been flipped by 180° to show the similarity with mannose. Carbons that are important for MBL recognition are in red. This figure is available in colour online at www.interscience.wiley.com/journal/dmrr
Remarkably, the only sugar-binding protein that we could detect after two rounds of purification on fructoselysine-Sepharose is MBL. The other proteins (MASPs) that we detected in the preparation are known to be physiologically associated with it. This suggests that MBL is the only abundant protein in serum that binds fructoselysine in a Ca2+-dependent manner. Fructoselysine was more specific than mannan to purify MBL: affinity chromatography on mannan-Sepharose led to the purification of two proteins with lectin-like properties, MBL and serum amyloid P component, whereas the latter protein was not purified by chromatography on fructoselysine-Sepharose. This indicates that serum amyloid P component has no affinity for fructoselysine, consistent with specificity studies indicating that this protein binds to anionic sugars and related compounds, rather than to neutral sugars 29. On this basis, we propose that serum amyloid P component binds to phosphomannan, a component of mannans 30, rather than to uncharged mannan.
We also demonstrate that complement is activated, as indicated by deposition of C3d, when fructoselysine plates are incubated with serum. The involvement of MBL in this process is indicated by the fact that it is inhibited by mannose and highly dependent on the serum MBL concentration and on the mbl2 genotype. Thus, binding of MBL to a surface covered with fructoselysine leads to complement activation, presumably via MASPs activation.
The question is to know whether the binding of MBL to fructosamines occurs in diabetic patients. Although the cell surface fructosamine ‘concentration’ is difficult to evaluate, it is probably similar to the serum fructosamine concentration, i.e. about 1.6 mM in normal subjects and up to 2.4 mM in diabetic patients 31. Binding of MBL to glycated membrane proteins is likely, as MBL is extremely well retained on a fructoselysine-Sepharose column, in which the fructoselysine concentration (3 mM) is slightly above the serum fructosamine concentration in diabetic patients.
The high affinity of MBL for a polysaccharide like mannan is explained by the multiplicity of its carbohydrate binding sites (≈6–18) per MBL molecule 1. Multivalent ligand binding also occurs between MBL and fructoselysine bound to plates, as indicated by the highly cooperative character of this interaction (Hill coefficient ≥ 4). Consequently, high-affinity binding of MBL to fructosamines requires the interaction of at least four fructoselysines with one MBL molecule.
Complement deposits (C3d and C5b-9) have been found in the choriocapillaris of eyes of patients with diabetic retinopathy, but neither MBL nor C1q could be detected 7. This observation did not allow the authors to conclude by which pathway (classical, lectin or alternative) the complement was activated. It is possible that the amounts of MBL present in these lesions were too low to be detected or that MBL escaped from the complexes after initiation of complement activation. Moreover, the observation that mouse deficient in MBL develops less diabetic nephropathy and cardiomyopathy, argues in favour of the contribution of the lectin pathway in the appearance of diabetic complications.
In conclusion, the binding of MBL to fructosamines and the ensuing complement activation are a mechanism that deserves consideration among the potential mechanisms linking hyperglycaemia with diabetic complications. The cooperative character of this binding makes MBL/fructosamine hypothesis attractive, as it may explain the high sensitivity of these complications to the mean blood glucose concentration.
Acknowledgements
This work was supported by grants from the Belgian Fonds National de la Recherche Scientifique and the Interuniversity Attraction Poles Programme—Belgian Science Policy (P06/05 and P06/28). J. F. is Chargé de Recherches and D. V. is Collaborateur logistique of the Belgian Fonds National de la Recherche Scientifique.
Conflict of interest
The authors have no conflicts of interest.
References
Abbreviations
-
- MBL
-
mannose binding lectin
-
- MASP
-
MBL-associated serine proteases




