Does abnormal insulin action or insulin secretion explain the increase in prevalence of impaired glucose metabolism with age in populations of different ethnicities?
Abstract
Background
Age is associated with both impaired glucose and insulin metabolism. To what extent the age-related changes in insulin resistance (IR) and β-cell function contribute to the increase in prevalence of impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) is less known, and this is investigated in this study.
Methods
This study included 6610 men and 7664 women of different ethnic groups aged 30-69 years. IR and β-cell function were examined by the homeostasis model assessment of insulin resistance (HOMA-IR) and homeostasis model assessment of β-cell function (HOMA-B). Odds ratios (ORs) and 95% confidence intervals (95% CIs) were estimated using logistic regression analysis adjusting for body mass index and study.
Results
In Chinese men, the ORs (95% CIs) for IFG were 2.69 (1.70, 4.26), 2.51 (1.49, 4.21) and 2.89 (1.68, 4.97), respectively, in age groups of 40–49, 50–59 and 60–69 years compared with 30–39 years (p < 0.001 for trend); the corresponding figures for IGT were 1.73 (1.25, 2.38), 2.54 (1.78, 3.63) and 3.57 (2.46, 5.19) (p < 0.001 for trend). Similar trends for IGT were observed also in Chinese women and other ethnic groups, but not for IFG in Mauritius Indian and Creole men. Adjustment for HOMA-IR and HOMA-B reduced the ORs in all age groups of all ethnicities for both IFG and IGT, but the risk gradient between age groups remained particularly for the IGT.
Conclusions
The age-related increase in glucose intolerance may not be fully explained by the defect in HOMA-IR and HOMA-B. As HOMA-IR and HOMA-B are only surrogate measures of insulin sensitivity and insulin secretion, the results need to be further investigated. Copyright © 2010 John Wiley & Sons, Ltd.
Introduction
The prevalence of type 2 diabetes and impaired glucose tolerance (IGT) increases with age 1. Several risk factors for type 2 diabetes, including ageing, increased adiposity and physical inactivity, predispose elderly people to develop glucose intolerance and insulin resistance (IR). The progression from normal glucose tolerance to IGT and type 2 diabetes is characterized by progressive defect in β-cell function or impaired β-cell compensation for IR 2. Different opinions exist, however, on whether IR increases with age as does IGT 3, 4. Similarly, a positive correlation of fasting plasma glucose (FPG) with age has been reported from some studies 5, 6 but not in others 7, 8. The ageing of populations may further increase the burden of type 2 diabetes and pre-diabetes [impaired fasting glucose (IFG)/IGT] on health care systems worldwide 9. Knowledge of the impact of age on IR together with β-cell function and glucose metabolism may have clinical implications in intervention and management of pre-diabetes and diabetes in elderly populations.
In this study, we aim to examine to what extent the age-related IR and β-cell dysfunction contribute to the increase in the prevalence of IFG and IGT based on the cross-sectional data of the Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Asia (DECODA) study.
Materials and methods
Study populations
The DECODA study is based on collaborative analysis of existing databases of different study populations of Asian origin and Creole 10. The Creole ethnic group is comprised of African and Malagasy ancestry with some European admixture 11. Researchers who had carried out population-based cross-sectional or large occupational surveys on diabetes were invited to join the DECODA collaboration. Data on history of diabetes, FPG, 2-h plasma glucose (2hPG), fasting insulin, body mass index (BMI) and other variables were sent to the Diabetes Prevention Unit, Department of Chronic Disease Prevention of National Institute for Health and Welfare in Helsinki, Finland for collaborative data analysis. In this study, data from 11 studies including 6610 men and 7664 women aged 30–69 years were analysed. Informed consent of participants complying with the Declaration of Helsinki or other ethical standards was obtained in all studies.
To study glucose metabolism and insulin function in pre-diabetic status, subjects with diabetes previously diagnosed or detected on screening with FPG ≥ 7.0 mmol/L and/or 2hPG ≥ 11.1 mmol/L were excluded from this data analysis. Individuals with FPG of 6.1–6.9 mmol/L were categorized as IFG and those with 2hPG of 7.8–11.0 mmol/L were categorized as having IGT 12. Fasting insulin assays differed among the 11 studies: 5 used conventional radioimmunoassays (RIA) that measured immunoreactive insulin and cross-react with proinsulin and its split products, whereas others used either an enzyme-linked immunosorbent assay (ELISA) or a chemiluminescence immunoassay for intact insulin (Table S1, supplementary information). Homeostasis model assessment of insulin resistance (HOMA-IR) and β-cell function (HOMA-B) were calculated based on FPG and fasting insulin. HOMA-IR = (fasting insulin × FPG)/22.5, and HOMA-B = 20 × fasting insulin/(FPG − 3.5) 13. Fasting insulin concentration was measured in picomoles per litre and FPG concentration in millimoles per litre.
Statistical analysis
The 10-year age-specific prevalence of IFG and IGT was calculated for each study. Differences in proportions were evaluated by Chi-square test. Considering the difference in laboratory assays of fasting insulin and FPG between studies, Z score (Z = [χ− µ]/σ) transformation for HOMA-IR, HOMA-B and glucose concentrations was made for each study before the studies of the same ethnic group were pooled together. Logistic regression analysis was used to estimate age-specific odds ratios (ORs) and 95% confidence intervals (95% CIs) for IFG and IGT in each ethnic group subsequently adjusting for BMI, study, HOMA-IR and HOMA-B. All analyses were performed using SPSS for Windows (Version 15.0; SPSS Inc., Chicago, IL, USA). A probability (p) less than 0.05 (two tailed) was considered statistically significant.
Results
The characteristics of participants differed slightly among studies of the same ethnicity (Table 1). Among the three Chinese studies, the Qingdao study population was the oldest, and had the highest FPG, 2hPG and BMI (p < 0.001 for all). There was no difference between the two Hong Kong Chinese studies, except that mean of fasting insulin, HOMA-IR and HOMA-B were lower in the Hong Kong Cardiovascular Risk Factor Prevalence Study (HK-cvrfps) than in the Hong Kong Workforce Survey on Cardiovascular Risk Factors (HK-wcvdrf). Asian Indians from the Chennai Urban Rural Epidemiology Study (CURES) in Chennai, India, in 2001 had higher FPG, 2hPG, fasting insulin and HOMA-IR than the Chennai Urban Population Study in 1997 (CUPS1997) (p < 0.001 for all).
| Ethnicity/ country | Studies | Age (years) mean (range) | N (% men) | BMI (kg/m2) | FPG (mmol/L) | 2hPG (mmol/L) | Fasting insulin (pmol/L) | HOMA-IR | HOMA-B | Year of screening |
|---|---|---|---|---|---|---|---|---|---|---|
| Chinese/China | HK-wscvdrf | 41 (30–66) | 1065 (58.5) | 23.8 (0.11) | 4.94 (0.02) | 5.75 (0.05) | 61.5 (1.05) | 13.9 (0.27) | 940.0 (17.66) | 1991 |
| HK-cvrfps | 45 (30–69) | 1896 (48.7) | 24.0 (0.08) | 5.10 (0.01) | 6.16 (0.04) | 40.5 (0.77) | 9.4 (0.20) | 525.5 (12.91) | 1995–1996 | |
| Qingdao2006 | 48 (30–69) | 2162 (41.7) | 25.4 (0.07) | 5.29 (0.01) | 6.32 (0.03) | 45.3 (0.73) | 10.8 (0.19) | 604.8 (12.28) | 2006 | |
| All | 45 (30–69) | 5123 (47.8) | 24.5 (0.06) | 5.15 (0.01) | 6.16 (0.02) | 46.8 (0.62) | 11.0 (0.15) | 639.0 (18.98) | ||
| Creole/Mauritius | Mauritius1987 | 45 (30–69) | 889 (46.7) | 24.1 (0.16) | 5.39 (0.02) | 6.39 (0.06) | 47.1 (1.62) | 11.6 (0.42) | 535.3 (18.72) | 1987 |
| Mauritius1992 | 47 (30–69) | 557 (43.3) | 25.5 (0.20) | 5.49 (0.02) | 6.28 (0.07) | 78.1 (2.05) | 19.3 (0.53) | 811.8 (23.73) | 1992 | |
| Mauritius1998 | 40 (30–68) | 271 (43.2) | 25.0 (0.29) | 5.32 (0.04) | 6.17 (0.10) | 64.5 (2.98) | 15.3 (0.77) | 773.1 (34.41) | 1998 | |
| All | 45 (30–69) | 1717 (45.0) | 24.7 (0.10) | 5.42 (0.01) | 6.33 (0.04) | 59.8 (1.07) | 14.6 (0.27) | 655.1 (32.73) | ||
| Indian/India | CUPS1997 | 44 (30–69) | 665 (40.5) | 22.9 (0.16) | 4.38 (0.02) | 5.60 (0.06) | 48.5 (1.74) | 10.0 (0.39) | 1093.0 (45.29) | 1996–1998 |
| CURES | 42 (30–69) | 1326 (45.1) | 23.3 (0.11) | 4.81 (0.02) | 6.11 (0.04) | 59.6 (1.22) | 12.9 (0.27) | 1050.6 (31.76) | 2001 | |
| All | 43 (30–69) | 1991 (43.5) | 23.2 (0.09) | 4.70 (0.01) | 6.00 (0.04) | 55.5 (1.00) | 11.9 (0.25) | 1041.7 (15.53) | ||
| Indian/Mauritius | Mauritius1987 | 43 (30–69) | 2196 (46.3) | 23.4 (0.09) | 5.17 (0.01) | 6.39 (0.03) | 52.5 (1.07) | 12.3 (0.27) | 652.4 (12.61) | 1987 |
| Mauritius1992 | 46 (30–69) | 1110 (47.8) | 24.9 (0.13) | 5.40 (0.02) | 6.57 (0.05) | 78.3 (1.51) | 19.2 (0.39) | 858.4 (17.82) | 1992 | |
| Mauritius1998 | 41 (30–68) | 618 (43.0) | 24.4 (0.18) | 5.33 (0.01) | 6.40 (0.07) | 71.6 (2.02) | 17.1 (0.52) | 822.2 (23.88) | 1998 | |
| All | 44 (30–69) | 3924 (46.2) | 24.1 (0.06) | 5.29 (0.01) | 6.51 (0.03) | 62.4 (0.71) | 15.0 (0.18) | 718.0 (21.80) | ||
| Japanese/Brazil and America | San Paulo1992 | 55 (37–69) | 365 (48.5) | 24.5 (0.18) | 5.16 (0.03) | 6.01 (0.09) | 38.4 (2.47) | 8.9 (0.63) | 506.1 (39.70) | 1992–1993 |
| San Paulo1999 | 52 (31–69) | 696 (42.5) | 24.0 (0.13) | 6.13 (0.02) | 7.29 (0.06) | 54.8 (1.78) | 15.2 (0.45) | 426.9 (28.56) | 1999–2000 | |
| Seattle | 51 (34–69) | 458 (51.3) | 24.1 (0.16) | 5.27 (0.03) | 7.35 (0.07) | 93.7 (2.20) | 22.3 (0.56) | 1212.5 (35.35) | 2001 | |
| All | 52 (31–69) | 1519 (46.5) | 24.3 (0.10) | 5.63 (0.02) | 7.01 (0.04) | 63.6 (1.16) | 15.8 (0.29) | 731.0 (35.40) |
- Data are age-adjusted mean (SE) unless otherwise stated.
- BMI, body mass index; FPG, fasting plasma glucose; 2hPG, 2-h plasma glucose; HOMA-IR, homeostasis model assessment of insulin resistance; HOMA-B, homeostasis model assessment of β-cell function; HK-cvrfps, Hong Kong Cardiovascular Risk Factor Prevalence Study; HK-wscvdrf, Hong Kong Workforce Survey on Cardiovascular Risk Factors; CUPS1997, Chennai Urban Population Study in 1997; CURES, Chennai Urban Rural Epidemiology Study.
In all ethnic groups, the mean Z scores of FPG and 2hPG increased with age among men and women, whereas HOMA-B declined with age, although in some populations this decline did not reach statistical significance. However, no consistent trend was observed for mean HOMA-IR across age groups (Figure 1). The prevalence of IFG (Table 2) and IGT (Table 3) increased significantly with age in men and women of all ethnic groups (p for trends < 0.05 for all) except for IFG in Mauritian Indians and Creole men. The increase was more prominent for IGT than for IFG in both men and women, and the association of age with IFG and IGT was not altered after adjusting for BMI and studies. Adjustment for hypertension did not change the results (data for hypertension not shown).
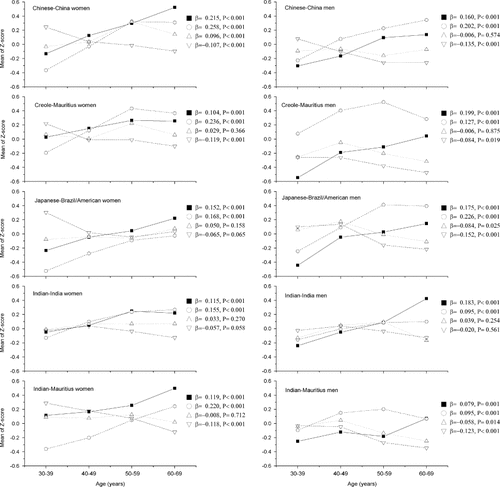
Age-specific mean of Z score of fasting plasma glucose (▪), 2-h plasma glucose (○), HOMA-IR (▵), and HOMA-B(▿) for women and men in five ethnic groups. Standardized β-coefficients of correlations between these variables and age are also shown. HOMA-IR: homeostasis model assessment of insulin resistance, HOMA-B: homeostasis model assessments of β-cell function
| Ethnicity | Age (years) | p for trend | |||
|---|---|---|---|---|---|
| 30–39 ORs (95% CIs) | 40–49 ORs (95% CIs) | 50–59 ORs (95% CIs) | 60–69 ORs (95% CIs) | ||
| Men | |||||
| Chinese-China % (N) | 3.1 (27) | 10.0 (84) | 10.0 (42) | 11.1 (35) | — |
| Model 1 | 1 | 2.69 (1.70, 4.26) | 2.51 (1.49, 4.21) | 2.89 (1.68, 4.97) | <0.001 |
| Model 2: 1 + HOMA-IR | 1 | 2.76 (1.70, 4.46) | 2.72 (1.59, 4.68) | 3.06 (1.73, 5.42) | <0.001 |
| Model 3: 1 + HOMA-B | 1 | 2.39 (1.50, 3.80) | 2.02 (1.19, 3.40) | 2.34 (1.35, 4.06) | 0.012 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 2.06 (0.85, 5.02) | 1.37 (0.53, 3.54) | 1.42 (0.51, 3.94) | 0.977 |
| Creole–Mauritius % (N) | 11.1 (37) | 17.8 (33) | 22.2 (36) | 11.7 (11) | — |
| Model 1 | 1 | 1.64 (0.98, 2.73) | 2.11 (1.27, 3.51) | 1.02 (0.50, 2.10) | 0.140 |
| Model 2: 1 + HOMA-IR | 1 | 1.58 (0.93, 2.70) | 2.28 (1.34, 3.87) | 1.01 (0.47, 2.15) | 0.117 |
| Model 3: 1 + HOMA-B | 1 | 1.59 (0.95, 2.67) | 1.99 (1.19, 3.33) | 0.98 (0.47, 2.01) | 0.212 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 1.01 (0.36, 2.85) | 1.51 (0.54, 4.18) | 0.27 (0.05, 1.43) | 0.497 |
| Indian-India % (N) | 2.1 (8) | 1.6 (4) | 5.4 (8) | 7.5 (6) | — |
| Model 1 | 1 | 0.76 (0.23, 2.55) | 2.70 (0.99, 7.34) | 4.28 (1.40, 13.07) | 0.004 |
| Model 2: 1 + HOMA-IR | 1 | 0.62 (0.18, 2.13) | 2.09 (0.73, 5.99) | 4.17 (1.35, 12.87) | 0.009 |
| Model 3: 1 + HOMA-B | 1 | 0.86 (0.25, 2.91) | 2.92 (1.06, 8.04) | 4.64 (1.49, 14.42) | 0.003 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 0.68 (0.16, 2.88) | 1.09 (0.28, 4.22) | 4.78 (1.24, 18.39) | 0.056 |
| Indian–Mauritius % (N) | 8.3 (67) | 13.1 (64) | 15.7 (52) | 6.7 (12) | — |
| Model 1 | 1 | 1.49 (1.03, 2.15) | 1.89 (1.27, 2.81) | 0.78 (0.41, 1.49) | 0.193 |
| Model 2: 1 + HOMA-IR | 1 | 1.57 (1.07, 2.29) | 2.02 (1.34, 3.05) | 0.86 (0.45, 1.65) | 0.107 |
| Model 3: 1 + HOMA-B | 1 | 1.42 (0.98, 2.06) | 1.73 (1.16, 2.58) | 0.71 (0.37, 1.36) | 0.429 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 1.17 (0.55, 2.46) | 1.25 (0.58, 2.71) | 0.68 (0.24, 1.91) | 0.768 |
| Japanese-Brazil/America % (N) | 23.1 (27) | 28.5 (55) | 41.8 (79) | 34.5 (71) | — |
| Model 1 | 1 | 1.33 (0.71, 2.48) | 3.12 (1.67, 5.85) | 2.25 (1.22, 4.16) | 0.001 |
| Model 2: 1 + HOMA-IR | 1 | 1.35 (0.72, 2.52) | 3.29 (1.75, 6.20) | 2.35 (1.27, 4.34) | 0.001 |
| Model 3: 1 + HOMA-B | 1 | 1.24 (0.65, 2.37) | 2.74 (1.44, 5.20) | 1.98 (1.06, 3.70) | 0.006 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 1.06 (0.51, 2.22) | 2.76 (1.32, 5.75) | 1.98 (0.96, 4.08) | 0.008 |
| Total % (N) | 6.6 (166) | 12.2 (240) | 17.3 (217) | 15.4 (135) | — |
| Model 1 | 1 | 1.66 (1.33, 2.07) | 2.26 (1.79, 2.86) | 1.74 (1.32, 2.28) | <0.001 |
| Model 2: 1 + HOMA-IR | 1 | 1.67 (1.33, 2.10) | 2.41 (1.90, 3.07) | 1.83 (1.38, 2.42) | <0.001 |
| Model 3: 1 + HOMA-B | 1 | 1.58 (1.26, 1.98) | 2.02 (1.59, 2.56) | 1.54 (1.17, 2.03) | <0.001 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 1.26 (0.94, 1.69) | 1.54 (1.14, 2.08) | 1.19 (0.84, 1.70) | 0.086 |
| Women | |||||
| Chinese-China % (N) | 4.1 (34) | 6.3 (65) | 16.2 (90) | 13.4 (35) | — |
| Model 1 | 1 | 1.20 (0.78, 1.86) | 2.58 (1.68, 3.98) | 2.61 (1.55, 4.39) | <0.001 |
| Model 2: 1 + HOMA-IR | 1 | 1.29 (0.82, 2.02) | 2.45 (1.57, 3.84) | 2.69 (1.58, 4.60) | <0.001 |
| Model 3: 1 + HOMA-B | 1 | 1.03 (0.66, 1.60) | 2.06 (1.33, 3.21) | 2.04 (1.20, 3.48) | <0.001 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 1.24 (0.55, 2.81) | 1.77 (0.79, 3.99) | 1.88 (0.72, 4.96) | 0.107 |
| Creole–Mauritius % (N) | 5.5 (21) | 11.5 (24) | 19.0 (40) | 19.3 (28) | — |
| Model 1 | 1 | 2.04 (1.10, 3.79) | 3.32 (1.87, 5.90) | 3.60 (1.95, 6.62) | <0.001 |
| Model 2: 1 + HOMA-IR | 1 | 2.36 (1.25, 4.46) | 3.63 (2.01, 6.55) | 4.17 (2.22, 7.85) | <0.001 |
| Model 3: 1 + HOMA-B | 1 | 1.76 (0.94, 3.28) | 2.83 (1.57, 5.07) | 2.94 (1.58, 5.49) | <0.001 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 1.71 (0.36, 8.18) | 2.48 (0.57, 10.75) | 4.01 (0.81, 19.94) | 0.076 |
| Indian-India % (N) | 0.6 (3) | 2.5 (9) | 1.7 (3) | 5.2 (5) | — |
| Model 1 | 1 | 4.26 (1.14, 15.94) | 3.11 (0.61, 15.81) | 11.04 (2.49, 45.98) | 0.003 |
| Model 2: 1 + HOMA-IR | 1 | 4.59 (1.18, 17.87) | 3.33 (0.64, 17.49) | 12.45 (2.66, 58.32) | 0.003 |
| Model 3: 1 + HOMA-B | 1 | 3.88 (1.03, 14.57) | 2.96 (0.58, 15.09) | 9.96 (2.25, 44.08) | 0.005 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 3.21 (0.69, 14.99) | 3.14 (0.51, 19.23) | 11.77 (2.18, 63.52) | 0.006 |
| Indian–Mauritius % (N) | 3.5 (33) | 6.7 (37) | 10.7 (41) | 14.1 (31) | — |
| Model 1 | 1 | 1.65 (1.01, 2.69) | 2.77 (1.71, 4.50) | 4.21 (2.49, 7.10) | <0.001 |
| Model 2: 1 + HOMA-IR | 1 | 1.90 (1.15, 3.16) | 3.11 (1.88, 5.14) | 4.97 (2.89, 8.56) | <0.001 |
| Model 3: 1 + HOMA-B | 1 | 1.45 (0.88, 2.38) | 2.29 (1.40, 3.76) | 3.37 (1.98, 5.74) | <0.001 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 0.86 (0.20, 3.71) | 2.34 (0.66, 8.26) | 1.29 (0.32, 5.21) | 0.396 |
| Japanese-Brazil/America % (N) | 14.7 (17) | 24.3 (52) | 32.5 (80) | 24.7 (57) | — |
| Model 1 | 1 | 1.90 (0.97, 3.73) | 2.43 (1.27, 4.68) | 2.42 (1.23, 4.77) | 0.013 |
| Model 2: 1 + HOMA-IR | 1 | 1.87 (0.95, 3.69) | 2.56 (1.32, 4.93) | 2.37 (1.20, 4.67) | 0.014 |
| Model 3: 1 + HOMA-B | 1 | 1.92 (0.97, 3.81) | 2.32 (1.20, 4.49) | 2.46 (1.24, 4.89) | 0.015 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 1.72 (0.71, 4.15) | 2.47 (1.05, 5.80) | 2.42 (1.01, 5.83) | 0.040 |
| Total % (N) | 3.9 (108) | 7.9 (187) | 16.2 (255) | 16.4 (157) | — |
| Model 1 | 1 | 1.66 (1.28, 2.14) | 2.86 (2.22, 3.68) | 3.30 (2.49, 4.36) | <0.001 |
| Model 2: 1 + HOMA-IR | 1 | 1.82 (1.40, 2.37) | 3.03 (2.34, 3.92) | 3.57 (2.68, 4.76) | <0.001 |
| Model 3: 1 + HOMA-B | 1 | 1.49 (1.15, 1.93) | 2.42 (1.87, 3.13) | 2.80 (2.11, 3.72) | <0.001 |
| Model 4: 1 + HOMA-IR+ HOMA-B | 1 | 1.35 (0.96, 1.90) | 1.73 (1.24, 2.41) | 2.04 (1.41, 2.93) | <0.001 |
- Model 1 adjusted for body mass index and studies.
| Ethnicity | Age (years) | p for trend | |||
|---|---|---|---|---|---|
| 30–39 ORs (95% CIs) | 40–49 ORs (95% CIs) | 50–59 ORs (95% CIs) | 60–69 ORs (95% CIs) | ||
| Men | |||||
| Chinese-China % (N) | 8.2 (71) | 13.7 (115) | 18.5 (78) | 24.4 (77) | — |
| Model 1 | 1 | 1.73 (1.25, 2.38) | 2.54 (1.78, 3.63) | 3.57 (2.46, 5.19) | <0.001 |
| Model 2: 1 + HOMA-IR | 1 | 1.72 (1.25, 2.38) | 2.61 (1.82, 3.74) | 3.58 (2.45, 5.22) | <0.001 |
| Model 3 : 1 + HOMA-B | 1 | 1.67 (1.21, 2.30) | 2.40 (1.67, 3.44) | 3.39 (2.32, 4.93) | <0.001 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 1.53 (1.10, 2.12) | 2.15 (1.49, 3.10) | 2.97 (2.02, 4.35) | <0.001 |
| Creole–Mauritius % (N) | 12.3 (41) | 16.2 (30) | 21.0 (34) | 25.5 (24) | — |
| Model 1 | 1 | 1.31 (0.78, 2.18) | 1.83 (1.10, 3.04) | 2.59 (1.45, 4.62) | 0.001 |
| Model 2: 1 + HOMA-IR | 1 | 1.25 (0.74, 2.11) | 1.88 (1.12, 3.16) | 2.64 (1.46, 4.74) | <0.001 |
| Model 3: 1 + HOMA-B | 1 | 1.30 (0.78, 2.18) | 1.82 (1.09, 3.03) | 2.58 (1.44, 4.61) | 0.001 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 1.04 (0.61, 1.79) | 1.58 (0.93, 2.69) | 2.29 (1.26, 4.18) | 0.004 |
| Indian-India % (N) | 7.0 (27) | 13.7 (35) | 20.1 (30) | 21.3 (17) | — |
| Model 1 | 1 | 2.22 (1.30, 3.81) | 3.56 (2.01, 6.29) | 4.78 (2.40, 9.54) | <0.001 |
| Model 2: 1 + HOMA-IR | 1 | 2.06 (1.20, 3.56) | 3.27 (1.83, 5.84) | 4.58 (2.28, 9.20) | <0.001 |
| Model 3: 1 + HOMA-B | 1 | 2.27 (1.33, 3.90) | 3.61 (2.04, 6.39) | 4.84 (2.42, 9.66) | <0.001 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 2.07 (1.19, 3.61) | 3.15 (1.74, 5.70) | 4.50 (2.22, 9.12) | <0.001 |
| Indian–Mauritius % (N) | 11.9 (97) | 18.4 (90) | 18.7 (62) | 20.0 (36) | — |
| Model 1 | 1 | 1.54 (1.12, 2.12) | 1.75 (1.22, 2.49) | 2.07 (1.35, 3.20) | <0.001 |
| Model 2: 1 + HOMA-IR | 1 | 1.57 (1.14, 2.17) | 1.80 (1.25, 2.59) | 2.20 (1.42, 3.40) | <0.001 |
| Model 3: 1 + HOMA-B | 1 | 1.56 (1.14, 2.15) | 1.79 (1.25, 2.56) | 2.13 (1.38, 3.29) | <0.001 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 1.50 (1.08, 2.08) | 1.63 (1.12, 2.35) | 2.00 (1.29, 3.11) | <0.001 |
| Japanese-Brazil/America % (N) | 17.9 (21) | 26.9 (52) | 36.0 (68) | 38.9 (81) | — |
| Model 1 | 1 | 2.13 (1.17, 3.90) | 3.62 (2.00, 6.56) | 4.05 (2.29, 7.17) | <0.001 |
| Model 2: 1 + HOMA-IR | 1 | 2.19 (1.19, 4.04) | 3.93 (2.15, 7.18) | 4.37 (2.45, 7.82) | <0.001 |
| Model 3: 1 + HOMA-B | 1 | 2.14 (1.17, 3.90) | 3.65 (2.00, 6.63) | 4.07 (2.29, 7.24) | <0.001 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 2.17 (1.15, 4.07) | 3.46 (1.86, 6.43) | 3.95 (2.17, 7.19) | <0.001 |
| Total % (N) | 10.2 (257) | 16.4 (322) | 21.7 (272) | 26.8 (235) | — |
| Model 1 | 1 | 1.65 (1.38, 1.98) | 2.33 (1.92, 2.83) | 3.04 (2.46, 3.76) | <0.001 |
| Model 2: 1 + HOMA-IR | 1 | 1.64 (1.36, 1.97) | 2.39 (1.97, 2.91) | 3.11 (2.51, 3.85) | <0.001 |
| Model 3: 1 + HOMA-B | 1 | 1.64 (1.37, 1.97) | 2.30 (1.90, 2.80) | 3.01 (2.43, 3.72) | <0.001 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 1.52 (1.26, 1.83) | 2.07 (1.70, 2.53) | 2.73 (2.20, 3.39) | <0.001 |
| Women | |||||
| Chinese-China % (N) | 9.2 (76) | 17.2 (177) | 22.2 (123) | 35.1 (92) | — |
| Model 1 | 1 | 1.72 (1.29, 2.31) | 1.92 (1.39, 2.66) | 3.84 (2.68, 5.47) | <0.001 |
| Model 2: 1 + HOMA-IR | 1 | 1.80 (1.34, 2.42) | 1.84 (1.32, 2.56) | 3.93 (2.74, 5.64) | <0.001 |
| Model 3: 1 + HOMA-B | 1 | 1.71 (1.27, 2.29) | 1.89 (1.36, 2.63) | 3.77 (2.63, 5.41) | <0.001 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 1.61 (1.19, 2.17) | 1.51 (1.07, 2.11) | 3.22 (2.23, 4.65) | <0.001 |
| Creole–Mauritius % (N) | 17.8 (68) | 22.1 (46) | 27.6 (58) | 26.9 (39) | — |
| Model 1 | 1 | 1.19 (0.78, 1.82) | 1.45 (0.96, 2.20) | 1.51 (0.95, 2.39) | 0.039 |
| Model 2: 1 + HOMA-IR | 1 | 1.29 (0.84, 2.00) | 1.49 (0.98, 2.28) | 1.62 (1.01, 2.60) | 0.023 |
| Model 3: 1 + HOMA-B | 1 | 1.23 (0.80, 1.89) | 1.51 (0.99, 2.30) | 1.57 (0.99, 2.51) | 0.027 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 1.19 (0.77, 1.84) | 1.24 (0.80, 1.92) | 1.37 (0.85, 2.22) | 0.178 |
| Indian-India % (N) | 8.8 (43) | 11.6 (42) | 17.6 (31) | 16.7 (16) | — |
| Model 1 | 1 | 1.57 (0.99, 2.49) | 3.03 (1.79, 5.11) | 3.31 (1.72, 6.38) | <0.001 |
| Model 2: 1 + HOMA-IR | 1 | 1.50 (0.94, 2.40) | 2.89 (1.70, 4.90) | 3.16 (1.63, 6.12) | <0.001 |
| Model 3: 1 + HOMA-B | 1 | 1.54 (0.97, 2.45) | 3.00 (1.77, 5.06) | 3.24 (1.68, 6.26) | <0.001 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 1.38 (0.86, 2.22) | 2.74 (1.61, 4.66) | 2.87 (1.48, 5.58) | <0.001 |
| Indian–Mauritius % (N) | 19.6 (187) | 22.6 (125) | 27.4 (105) | 35.5 (78) | — |
| Model 1 | 1 | 1.03 (0.79, 1.34) | 1.37 (1.03, 1.82) | 2.19 (1.57, 3.04) | <0.001 |
| Model 2: 1 + HOMA-IR | 1 | 1.12 (0.85, 1.47) | 1.46 (1.08, 1.95) | 2.40 (1.71, 3.37) | <0.001 |
| Model 3: 1 + HOMA-B | 1 | 1.05 (0.80, 1.37) | 1.41 (1.05, 1.88) | 2.28 (1.63, 3.19) | <0.001 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 1.04 (0.79, 1.37) | 1.27 (0.94, 1.71) | 1.98 (1.40, 2.80) | <0.001 |
| Japanese-Brazil/America % (N) | 24.1 (28) | 28.2 (61) | 33.5 (83) | 41.4 (96) | — |
| Model 1 | 1 | 1.49 (0.87, 2.55) | 2.01 (1.18, 3.42) | 2.82 (1.67, 4.78) | <0.001 |
| Model 2: 1 + HOMA-IR | 1 | 1.50 (0.87, 2.58) | 2.05 (1.20, 3.50) | 2.79 (1.64, 4.75) | <0.001 |
| Model 3: 1 + HOMA-B | 1 | 1.49 (0.87, 2.56) | 2.01 (1.18, 3.43) | 2.83 (1.67, 4.80) | <0.001 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 1.42 (0.82, 2.47) | 1.92 (1.12, 3.31) | 2.60 (1.52, 4.46) | <0.001 |
| Total % (N) | 14.5 (402) | 19.0 (451) | 25.4 (400) | 33.6 (321) | — |
| Model 1 | 1 | 1.31 (1.13, 1.53) | 1.66 (1.41, 1.96) | 2.48 (2.07, 2.98) | <0.001 |
| Model 2: 1 + HOMA-IR | 1 | 1.38 (1.18, 1.61) | 1.67 (1.41, 1.97) | 2.56 (2.13, 3.08) | <0.001 |
| Model 3: 1 + HOMA-B | 1 | 1.32 (1.13, 1.55) | 1.68 (1.42, 1.98) | 2.51 (2.09, 3.02) | <0.001 |
| Model 4: 1 + HOMA-IR + HOMA-B | 1 | 1.27 (1.08, 1.48) | 1.45 (1.22, 1.71) | 2.20 (1.83, 2.66) | <0.001 |
- Model 1 adjusted for body mass index and studies.
Further adjustment for either HOMA-IR or HOMA-B or both simultaneously reduced the OR for IFG and IGT in all age groups of all ethnic groups (Tables 2 and 3). The reduction was slightly larger in the middle and old age groups than in the young age group for IFG in some ethnic groups but not in others. The results based on pooled data analysis of all ethnic groups showed that the OR for IGT was reduced when both HOMA-IR and HOMA-B appeared simultaneously in the model, but the risk gradient for IGT across age groups still remained (Table 3), indicating an independent effect of age. The age-related increase in IFG also remained after the multivariate adjustment for HOMA-IR and HOMA-B although decreased substantially.
Discussion
We found that the prevalence of IFG and IGT, particularly the prevalence of IGT, increased with age even after the adjustment for HOMA-IR and HOMA-B. The mean of HOMA-IR did not increase with age in most studies, except for Asian Indian and Chinese, Creole and Japanese-Brazil/American women, whereas β-cell function declined with age in almost all studies. The age-related decline in β-cell function and the IR may contribute to the presence of glucose intolerance across age groups, not only in the elderly population. As HOMA-IR and HOMA-B were only surrogate measures of insulin sensitivity and insulin secretion, further investigations are warranted.
IR and decreased insulin secretion are the major factors contributing to the deterioration of glucose metabolism 14, 15. Elderly individuals are apparently able to maintain normal glucose tolerance by secreting more insulin to overcome the IR 16. This compensatory response can be maintained, despite the presence of subtle defect in the ability of elderly individuals to increase their insulin secretion rate in response to a given increment in plasma glucose concentration revealed by the graded glucose infusion study 17. Studies 18-21 have also shown that insulin sensitivity does not decrease with age and the decline in insulin sensitivity is most likely secondary to changes in body composition and physical fitness 22, 23. Obesity and physical inactivity are two major risk factors contributing to the deterioration of glucose intolerance. It is plausible that the effect of obesity and physical inactivity is mediated through IR, but IR could not fully explain the age-related increase in IGT and IFG in this study, and the association of age with IGT and IFG was also independent of body fat composition (measured as BMI). The same was also observed in our previous report on European population 3. To further check whether such an association will be altered by central obesity measured by waist circumference, the data analysis was also performed in a subgroup of individuals who had the waist circumference measured at baseline (men = 6583, women = 7651). This did not change the results substantially, and the results are shown in Tables S2 and S3. A sensitivity analysis by including previously undiagnosed diabetes did not change the observations based on the non-diabetic population reported in this article. However, it should be kept in mind that the upper age limit of the present study is 69 years. Whether the impact of age on IR and glucose metabolism remains the same in people above 69 years needs to be further investigated.
A negative impact of ageing on β-cell function has been shown in many studies 18, 24, 25. It appears to be highly attributable to an impairment of proinsulin conversion to insulin 26. Our study also revealed a slightly declining trend in HOMA-B with age, but this only partly explained the increase in IGT/IFG in older age groups. HOMA as a surrogate marker of β-cell function may underestimate the magnitude of the β-cell defect across declining glucose tolerance status, compared with a direct measure of insulin secretion 27. However, both HOMA-IR and HOMA-B correlated reasonably well with clamp tests when used to assess the risk of type 2 diabetes in both cross-sectional and prospective studies 28-31. On the other hand, hyperglycaemia may accelerate the loss of β-cell mass because β-cell from older individuals appears to be more sensitive to adverse effects of glucose-induced apoptosis 32. Studies also showed that β-cell function declined in elderly population even though their glucose remained in the normal range 1, 20. To what extent ageing contributes to the deterioration of insulin action and insulin secretion observed in the elderly population remains uncertain. It will be of considerable interest to determine the effect of ageing on insulin secretion and IR and their relationship with the deterioration of glucose intolerance in the elderly population. It should also be noted that HOMA-IR is calculated based on fasting values and may reflect hepatic IR better than peripheral IR. The latter would require the evaluation of glucose and insulin values after a glucose load. It is therefore possible that this unmeasured component of IR contributes to the increase in IGT observed with age and was missed.
Evidence has shown that individuals with a family history of diabetes have lower insulin sensitivity and decreased β-cell compensation than those without; diabetes in first-degree relatives may increase IR that was independent of the degree of obesity 33. Familial factors play an effect on the relationship between insulin sensitivity and glucose effectiveness, which may modulate the risk for the development of pre-diabetes and diabetes. It is well known that insulin is capable of preventing protein breakdown by increasing amino acid availability needed for protein synthesis in muscle tissue. Ageing is associated with impaired substrate utilization and IR, probably due to a sedentary lifestyle and elevated body fat causing impaired mitochondrial function. An age-related decline in physical activity may contribute to the decreased ability of muscle to metabolize and oxidize fat, which would lead to defects in muscle insulin sensitivity. Unfortunately, data on family history of diabetes and physical activity were not available in the current study, and their effect cannot be evaluated.
The main strengths of our study are: (1) the collaborative analysis was based on individual data rather than aggregate data; (2) all studies included are population-based except for HK-wcvdrf which is an occupational study; (3) a standard 2-h 75 g oral glucose tolerance test was used to classify individuals with diabetes, IFG and IGT; (4) data analysis has been carried out using standard methods for all ethnic groups. A limitation of our study was that all studies are cross-sectional, the direction of these associations cannot be conclusively determined and a causal relationship cannot be inferred. In addition, there are discrepancies in assays of fasting insulin, FPG and 2hPG between studies. To reduce the discrepancies study-specific Z scores were calculated and used in the data analyses. Both HOMA-IR and HOMA-B are only surrogate indicators of insulin sensitivity and β-cell function which are mainly based on glucose and insulin levels at the fasting status. They do not directly reflect the capacity of the β-cell to cope with the glucose challenge, and thus may be less associated with the IGT. The extent to which this has biased the present study needs to be further explored.
In conclusion, among the non-diabetic adult population included in this study, the deterioration in glucose metabolism with age may only be partly attributed to the defect in HOMA-IR and HOMA-B. Because HOMA-IR and HOMA-B are only surrogate measurement of insulin secretion and insulin sensitivity and have certain limitation, the study question needs to be further investigated.
Acknowledgements
This study was supported by grants from Academy of Finland (118492, 129197) and Finnish Centre for International Mobility Fellowship (CIMO TM-08-5694); Unrestricted grants from AstraZeneca R&D, Mölndal, Sweden for data analysis are also acknowledged.
Conflict of interest
No potential conflicts of interest relevant to this article were declared.
Appendix
Studies and investigators in this collaborative study are:
-
China
Hong Kong Cardiovascular Risk Factor Prevalence Study: T. H. Lam, S. Y. Ho, E. D. Janus, Department of Community Medicine, School of Public Health, University of Hong Kong, Hong Kong SAR, China.
Hong Kong Workforce Survey on Cardiovascular Diseases Risk Factors: G. T. C. Ko, J. C. N. Chan, C. S. Cockram, The Chinese University of Hong Kong, The Prince of Wales Hospital, Hong Kong SAR, China.
Qingdao Diabetes Survey 2006: Q. Qiao1, 2, Z. C. Pang3, SH. J. Wang3, for the Qingdao Diabetes Study Group 2006 (http://www.qddiabetes.org/Organize-6.asp), 1Department of Public Health, University of Helsinki, Helsinki, Finland; 2Diabetes Prevention Unit, Department of Chronic Disease Prevention, National Institute for Health and Welfare, Helsinki, Finland; 3Qingdao Centers for Disease Control and Prevention, Qingdao, China.
-
India
The Chennai Urban Population Study 1997 (CUPS1997): V. Mohan, Madras Diabetes Research Foundation and Dr Mohan's Diabetes Specialties Centre, Chennai, India.
Chennai Urban Rural Epidemiological Study (CURES): V. Mohan, M. Deepa, Madras Diabetes Research Foundation and Dr Mohan's Diabetes Specialities Centre, Chennai, India.
-
Japanese Migrants Cohorts
Japanese American Community Diabetes Study: W. Y. Fujimoto, E. J. Boyko, M. McNeely, J. Shofer, D. Leonetti, University of Washington, Seattle, USA.
Japanese Brazilian Diabetes Study Group 1992, São Paulo, Brazil: S. R. G. Ferriera, L. Franco, A. Hirai, S. Gimeno, Preventive Medicine Department, Federal University of São Paulo, São Paulo, Brazil.
Japanese Brazilian Diabetes Study Group 1999, São Paulo, Brazil: S. R. G. Ferriera, L. Franco, A. Hirai, S. Gimeno, Preventive Medicine Department, Federal University of São Paulo, São Paulo, Brazil.
-
Mauritian Indian and Creole Cohorts
Mauritius Non-Communicable Disease Study: P. Zimmet1, J Tuomilehto2, 3, J. Shaw1, K. G. M. M. Alberti4, S. Söderberg1, 5, Sudhir Knowlessur6, 1Baker IDI Heart and Diabetes Institute, Melbourne, Australia; 2Department of Public Health, University of Helsinki, Helsinki, Finland; 3Diabetes Prevention Unit, Department of Chronic Disease Prevention, National Institute for Health and Welfare, Helsinki, Finland; 4Imperial College, Mint Wing, St Marys Hospital, London, UK; 5Department of Public Health and Clinical Medicine, Cardiology, University of Umeå, Umeå, Sweden; 6Ministry of Health, Port Louis, Mauritius.




