Tropical infectious diseases and the skin: Diagnostic and treatment updates since the WHO's integrated campaign against neglected tropical skin diseases
Correspondence Stephen K. Tyring, Department of Dermatology, McGovern Medical School at UTHealth, Houston, TX 6431, USA.
Email: [email protected]
Abstract
Introduction
The World Health Organization (WHO) initiated a unified effort to address skin-related neglected tropical diseases (NTDs) in 2017. This effort increased attention and resources allocated toward decreasing the burden of tropical skin diseases. It emphasized an “integrated approach” to detect and treat multiple co-existing NTDs at once. This article will outline new diagnostic tests, treatment options, and vaccine development for neglected tropical skin diseases since the initiative began.
Data Sources
A PubMed search of clinical trials, randomized controlled trials, systematic reviews, and meta-analyses published since 2017 was performed.
Results
The WHO's initiative has already seen success, increasing the surveillance of leprosy and the distribution of treatment for yaws. It has encouraged the development of new point-of-care tests and better-tolerated treatment options. It has also brought new challenges, with the rise of resistant organisms. Development of point-of-care DNA-RNA-based testing may improve drug resistance monitoring. New vaccines are needed for long-term control of skin NTDs in areas with high transmission rates.
The World Health Organization (WHO) initiated a unified effort to address skin-related neglected tropical diseases (NTDs) in 2017.1 This effort increased attention and resources allocated towards decreasing the burden of tropical skin diseases. It emphasized an “integrated approach” to detect and treat multiple co-existing NTDs at once. The program has already seen success, increasing the surveillance of leprosy and the distribution of treatment for yaws. It has also brought new challenges, with the rise of resistant organisms. This article will outline new diagnostic tests, treatment options, and vaccine development for neglected tropical skin diseases (Table 1) since the initiative began.
| Skin neglected tropical diseases |
|---|
| Buruli ulcer |
| Cutaneous leishmaniasis |
| Filarial lymphoedema |
| Onchocerciasis |
| Leprosy |
| Mycetoma |
| Yaws |
1 NEW DIAGNOSTIC TESTS
A clinical suspicion based on dermatologic findings remains the first and most important diagnostic step. All skin NTDs then require a confirmatory diagnostic test before treatment except for Yaws, which can be treated at the time of clinical suspicion.1 Although a culture of the organism would be the theoretical gold standard in diagnosis, this is often impractical. For example, Mycobacterium leprae spp. are slow-growing and obligate intracellular organisms, requiring cultures in animal models. Mycobacterium ulcerans, which causes the Buruli ulcer, also grows slowly and has a sensitivity of 60% at most.2 Most diseases can be confirmed by PCR of the skin biopsy (Leishmaniasis spp.), swabs (M. ulcerans, Treponema pallidum pertenue), or smears (Leishmaniasis spp.).1 However, the difficulty with PCR is that many endemic areas are not in proximity to labs with this capability. Sample transfer can take days and these delays may result in patient loss to follow up.
Consequently, point-of-care testing (POCT) has been proposed, with an emphasis on low-cost methods. The only routine POCT used currently is microscopic detection of the organism from a skin biopsy, skin smear, or blood smear. However, the sensitivities and specificities are poor. One developing method for confirming Buruli ulcer is fluorescent thin-layer chromatography (fTLC). This method detects the secreted toxin of M. ulcerans, mycolactone A/B. The assay uses a boronic acid-enhanced fluorescent stain. However, more than a decade after the initial conception of the idea, the interpretation of fluorescent bands has not been automated. The chemical composition of the boronic acid stain is still being refined to minimize interference with human tissue in the sample.3
Other POCT options include DNA-detection methods such as loop-mediated isothermal amplification (LAMP) and recombinase polymerase amplification (RPA). Unlike PCR, both methods do not require a thermocycler and can be performed within an hour. Their sensitivities for detecting disease are promising; the estimated LAMP sensitivity for M. ulcerans is between 73.75% and 91.49%.4 These methods have also been applied to Leishmaniasis and Schistosoma detection.5, 6
Monitoring treatment efficacy is another area that requires better POCT. Early or low-grade parasitic infections are frequently undetected by stool or urine microscopy. Serology is not useful for monitoring treatment response, as antibody titers do not correlate with parasite or mycobacterial burden and remain elevated after treatment. Serologic testing for M. leprae phenolic glycolipid-1 (PGL-1) was explored in the late 1990s but was not sensitive and had a high false-positive rate.7
Alternatively, antigen detection can be used to diagnose early disease and monitor disease progress, as levels correlate with clinical severity and quickly become negative after the infection resolves. For Schistosoma spp. infections, assays detecting circulating anodic antigen (CAA) in serum and urine have been proposed. The up-converting phosphor-lateral flow (UCP-LF) assay performed on serum samples had the highest sensitivity, 97% in a preliminary study, showing promise for use in low-endemic areas.8
However, cost, sample preparation, and scarce laboratory capabilities remain issues with antigen testing. Currently, only the urine test for the Schistosoma CCA is available in the field. It comes in the form of a urine dipstick test that has lower sensitivity than the serum test but is far superior to the standard diagnostic test, the Kato-Katz (KK) stool smear. The urine CCA test was used to demonstrate that the prevalence of Schistosoma mansoni infection in Rwandan children was vastly underestimated based on the use of stool smears alone.9 Furthermore, the urine CCA and serum CCA tests were used to demonstrate that the efficacy of repeated praziquantel treatment was overestimated using egg-based diagnostics (KK and PCR), which detected fewer unresolved cases compared to worm-based testing (CCA assays).10
Lymphatic filariasis also requires testing for diagnostic confirmation. It is caused by three species (Wuchereria bancrofti, Brugia malayi, and Brugia timori). Circulating filarial antigen testing provides the highest sensitivity but is only available for W. bancrofti. These tests can also be falsely positive in patients with a high load of Loa loa.11
Point-of-care ultrasound has also traditionally aided diagnosis by detecting worm movement in lymphatics. One novel modality is infrared thermal imaging, which detects acute lymphadenitis caused by filariasis based on temperature at the patient's heel or calf. It was shown to differentiate between stages of disease and even detect disease at subclinical stages.12 In sum, better diagnostic testing methods for tropical skin diseases are needed to obtain more accurate assessments of disease prevalence and measurements of treatment efficacy.
2 NEW TREATMENT OPTIONS
Great advances in therapies for tropical skin diseases have been made since the WHO's global campaign began. Medical therapy advanced through research on revised regimens that minimize systemic toxicity and improve patient compliance, repurposing old drugs, and new combination therapies. Finally, mass drug administration (MDA) strategies significantly decreased prevalence of disease by treating subclinical or latent cases and targeting co-endemic diseases at once. However, drug resistance is now a concern and has had little surveillance to date.
Treatment of Buruli ulcers is trending toward less invasive approaches. The WHO recommends treating all ulcers with antibacterial therapy initially. This practice minimizes unnecessary tissue destruction that would occur with an early surgery. A study demonstrated that oral antibiotic monotherapy was sufficient for resolving <10 cm ulcers 95% of the time. This approach was also non-inferior to combining oral antibiotics with intramuscular streptomycin.13
A study in Benin further confirmed the benefits of a noninvasive approach. It found no significant difference in functional harm when comparing delayed excision surgery (14 weeks after starting antibiotics) to standard practice (excision surgery 8 weeks after starting antibiotics). Delayed excision resulted in decreased length of hospital stay and fewer wound care days. One important point is that the number and size of the ulcers can worsen during antibiotic therapy (usually around 8 weeks post-initiation) due to a treatment-induced inflammatory response. This should not be confused with a failure to respond to antimicrobial treatment.14
Studies have looked to improve treatment tolerability for other tropical skin diseases as well. The standard therapy for Chagas disease in children and adults is either benznidazole or nifurtimox. However, side effects cause about one-fifth of patients to discontinue treatment. A Bolivian study of adults with chronic Chagas disease suggested that significantly shorter treatment durations of Benznidazole (2 weeks as opposed to 8 weeks) have similar efficacy with no adverse effects leading to discontinuation.15
2.1 MDA: Improving ease of administration through combination therapies
The most successful eradication program set forth by the WHO has been that against dracunculiasis, also known as guinea-worm disease. In 1981, the WHO partnered with the US Centers for Disease Control and Prevention to establish a campaign to monitor new cases, ensure access to clean water, and obtain vector control using larvicide. The program resulted in a drop from nearly 900,000 annual cases of dracunculiasis in 1989 to just 13 cases in 2022 (Figure 1).16, 17
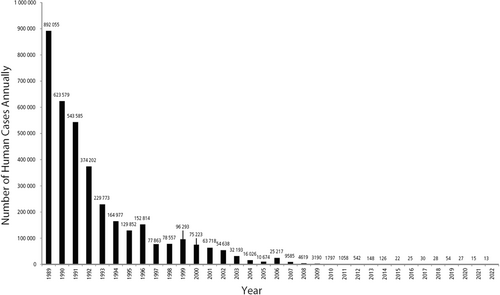
The dramatic success against dracunculiasis inspired larger-scale campaigns. In 2012, a collaborative effort between WHO leadership, government officials from seven countries, pharmaceutical companies, the World Bank, and the Gates Foundation, set to control or eradicate 10 NTDs by 2020.18 The effort achieved partial success: pertaining to skin-related NTDs, the program to eliminate filariasis was among the most successful. It involved repeated rounds of mass drug administration meant to decrease serum microfilariae levels to a level that would prevent transmission by mosquito vectors. During the initiative, 16 countries eliminated filariasis and four eliminated onchocerciasis.19
Combination therapies were explored to support the MDA campaigns. The elimination of filariasis initially began with a two-drug combination, diethylcarbamazine plus albendazole, or ivermectin plus albendazole, depending on whether onchocerciasis was co-endemic. Variations in treatment guidelines were created because patients with high loads of co-existing onchocerciasis are at risk of adverse events in the eyes and skin if they take repeated doses of diethylcarbamazine.20
More recently, a single dose triple combination of ivermectin, diethylcarbamazine, and albendazole (IDA) was found to be as effective as the two-drug therapy taken yearly over 3 years in treating filariasis. The single dose regimen resulted in a sustained clearance of microfilariae: 55 of 57 patients remained clear in 3 years.21
IDA is also a practical regimen that can be safely integrated with mass treatment of other conditions. In areas with co-endemic filariasis and yaws, IDA can be safely combined with azithromycin, the mass treatment for Yaws.22
Work is being completed to determine whether the single-dose triple therapy is safe for patients with co-existing filariasis and onchocerciasis. A recent pilot study demonstrated promising results, showing that IDA could be well tolerated after pretreatment with ivermectin to clear the microfilariae in the skin and eyes.
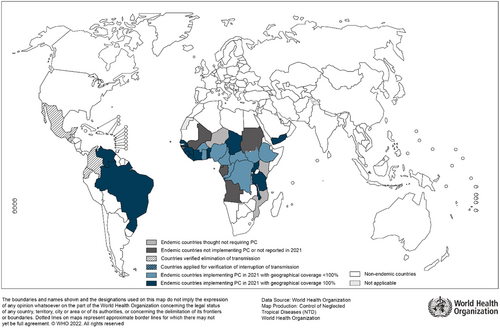
Furthermore, IDA may be more effective than the current standard of care for onchocerciasis (ivermectin alone). Ivermectin only temporarily sterilizes adult female worms. These worms can live up to 15 years in subcutaneous nodules. Thus, MDA with ivermectin alone has not been as successful in areas with high transmission rates of onchocerciasis, like sub-Saharan Africa (Figure 2). IDA shows promise for greater efficacy by reducing the number of living female worms. However, larger studies are needed to confirm this.23
Studies increasingly demonstrate the usefulness of ivermectin for other diseases as well, such as endemic scabies. Ivermectin has been shown to improve control of scabies during MDA compared to topical permethrin alone.24-26 Furthermore, increasing evidence demonstrates its safety in children. Ivermectin has been contraindicated in children less than 15 kg. However, a meta-analysis of 1,088 children under that weight showed that only 1.4% (15/1088) developed adverse effects on ivermectin, all of which were mild.27
While the WHO's efforts against skin NTDs are currently focused on establishing MDA programs, the surveillance of antimicrobial resistance against these treatments is lacking. A systematic review of 11 NTDs designated as “most important” by WHO found that only six NTDs had information on resistance. The majority of studies supplying information about resistance were rated as low quality.28 The mass administration of azithromycin for Yaws was publicized because of its remarkable success in Papua New Guinea. A 2013 study showed that a single dose of azithromycin given to 82.7% of residents dropped the number of active cases by nearly 90%.29 However, infection rates began to increase 2 years later, and five patients were found with azithromycin-resistant strains.29 The MDA campaign for Yaws demonstrates the importance of detecting drug resistance for long-term success of programs fighting skin NTDs.
3 VACCINE DEVELOPMENT
Vaccines against skin NTDs could advance the WHO goals significantly. Although MDA efforts reduce prevalence, they are much less effective in areas with high transmission rates. This problem could be alleviated with vaccination.30 However, vaccines are costly to develop, and financial incentives are lacking as these diseases are endemic in poor countries.
The development of vaccines for skin NTDs has been limited but promising. Four potential target antigens against schistosomes are currently under evaluation in clinical trials. One additional catalyst for the creation of a vaccine could be a new method that was developed to produce male-only schistosomes. This schistosome model can be used to test the efficacy of vaccines by creating a controlled human infection. The male-only line ensures that there are no egg-producing females that would cause pathology in a human host.31
A small vaccine trial against human hookworms demonstrated progress. The study only included five patients in the placebo group and 10 in the vaccine group, but it demonstrated significantly decreased larval burden in vaccinated patients.30
In summary, the global effort to address skin NTDs that began in 2017 instigated improvement of diagnostic tests, new treatment options that are safe and practical, and greater surveillance of these diseases. Since the start of the program, new challenges have arisen. Resistance to antimicrobial therapy is not a current focus but should be monitored more closely during MDA campaigns. Monitoring can be improved through point-of-care DNA-RNA-based testing. Areas where disease transmission rates are high are difficult to treat with repeated MDA and necessitate vaccine development, which may provide greater control of these diseases in the long-term.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




