Basal levels of CD34 positive cells in peripheral blood differ between individuals and are stable for 18 months†
How to cite this article: Eidenschink L, DiZerega G, Rodgers K, Bartlett M, Wells DA, Loken MR. Basal levels of CD34 positive cells in peripheral blood differ between individuals and are stable for 18 months. Cytometry Part B 2012; 82B: 18–25.
Abstract
Background:
Detection of basal levels of CD34 progenitor cells is a rare event analysis enumerating cells down to 1 cell/μl. A reproducible analytic approach was used in three independent clinical trials in which multiple sequential assays were obtained from the same individual.
Methods:
A 4 color panel combining, HLA-DR, CD34, CD45, and CD11b was used in a dual platform analysis to quantify CD34 progenitor cells in peripheral blood, with quality control focused at the lowest measurements (i.e., basal levels), where assay error is greatest.
Results:
Repeat testing of individuals every 4 h over the course of 6 days provided a unique opportunity to assess the precision of the analytic technique and identified basal differences between individuals. In a second study, the basal levels were stable for 10 weeks while in a third study the individual differences were maintained for 18 months. This approach was then used to monitor the kinetics of mobilization of CD34 cells following G-CSF stimulation every 4 h.
Conclusions:
The differences between individuals in basal levels of CD34 were shown to be a biologic constant, stable for 18 months and not a result of the variability of the assay, shown by low coefficients of variation for each individual. These results can be used to augment a quality control program by monitoring individuals over time to establish intra and inter-laboratory assay precision. In addition, the response of six individuals to G-CSF demonstrated differences in absolute numbers of mobilized CD34 progenitor cells but showed identical kinetics, peaking at 80–110 h. © 2011 International Clinical Cytometry Society
Mobilization of hematopoietic progenitor cells (HPC) expressing CD34 antigen from the bone marrow to the peripheral blood is widely used as a source of hematopoietic stem cells and progenitor cells for autologous and allogeneic bone marrow transplants. In the last 25 years, a monumental effort has been mounted to understand the biology of CD34+ mobilization upon G-CSF treatment. One of the most important aspects of this effort is the precise and accurate counting of CD34+ cells. The ISHAGE protocol (1) is perhaps the most widely used for flow cytometric enumeration of CD34 positive cells and can be used with either dual or single platform methods (2). ISHAGE based single platform bead assays such as ProCOUNT™ and Stem-Kit™ have shown to reduce inter and intra-laboratory variation (2-8). Although the convenience and reproducibility of the single platform bead assays is clear, studies have shown that there is a high correlation between results from dual platform and single platform assays (4, 9) both with and without use of the ISHAGE protocol (9).
Regardless of the protocol used for flow cytometric enumeration of CD34+ cells, it is essential that the platform for CD34 enumeration be established for rare event analysis, as the baseline for CD34 positive cells in normal peripheral blood is one-tenth of 1% of nucleated cells (10, 11). To ensure a precise and accurate CD34 count at the maximum point of mobilization, the assay must be validated at the lowest levels (basal levels), where the error is greatest.
We retrospectively analyzed three independent clinical studies that had in common enumeration of CD34+ cells in the peripheral blood of normal individuals at multiple time points over varying time lengths. These studies are based on a dual platform protocol as the correlation between WBC and CD34 absolute amounts were an essential aspect of the respective studies. While multiple groups (12-15) have shown variability in basal levels of CD34, the question remains if this variability is reflective of biological differences in individuals or is it a part of the variability in measuring very low levels of cells.
The synthesis of the data from these three clinical studies provides answers to very important yet underreported biological questions regarding HPC basal levels and G-CSF mobilization. Namely, what is the coefficient of variation in basal CD34+ levels in the PB of healthy individuals over time? What are the most important aspects of quality control to ensure that these basal levels are precise and accurate? Finally, how does maximum CD34 levels post G-CSF administration vary in comparison with the absolute kinetics of CD34 mobilization?
METHODS
Subjects
All studies were conducted under institutional review board approval with signed informed consent. This retrospective study comprises three separate studies with different time lengths and investigational purposes. The first investigation was a 6 day study comprising 18 normal volunteers, twelve of whom were given Neupogen® (Amgen) subcutaneously at doses of 10 mcg kg−1 daily for 5 days. Volunteer subjects were male and female with age ranges were from 18 to 45. These studies were completed at Charles River Clinical Services (Tacoma, WA).
The second, independent study, comprised peripheral blood specimens from an additional 10 volunteer subjects analyzed by flow cytometry for enumeration of CD34 positive cells pre- and post-ingestion of herbal supplements that had possible effects on progenitor cell mobilization. Specimens were collected prior to supplement ingestion, 1 h and 3 h postingestion over a 10 week period. Blood specimens were collected at weekly intervals for weeks 1–4 and again at weeks 9 and 10. Age range of subjects was 26–62; four of the individuals were female and six of the individuals were male. The supplements included: Marine Lipid Concentrate (EPA, DHA, Other Omega-3 Fatty Acids, total 2,200 mg (EPAX AS, Aalesund Norway); Reishi Mushroom (Ganoderma lucidum) Powdered Extract (20:1) 500 mg (Pharmanex, Provo, UT); Cordyceps Cs-4 mushroom mycelia (Cordyceps sinensis [berk.] Sacc.) 2,000 mg (Pharmanex, Provo, UT); Aphanizomenon flos-aquae powdered extract (5:1) 1,000 mg (Cell Tech., Klamath Falls, OR).
In the third study, peripheral blood specimens from six volunteer individuals were analyzed by flow cytometry for enumeration of CD34 positive cells. Specimens were collected over a period of 6 months at 5-week intervals with a final time point taken 18 months after the initial test. Sextuplicate tests were completed to measure the intrinsic analytical variance. The age range of subjects was 22–65; two of the individuals were female and four of the individuals were male.
Blood specimens (3–5 ml) were collected in sodium heparin. Volunteer requirements for all subjects in each of the three substudies included individuals in good health, a WBC in normal range, and otherwise normal complete blood counts.
Flow Cytometric Analysis
Monoclonal antibodies in this study were obtained from the following sources: fluorescein isothiocyanate (FITC-conjugated) HLA-DR (L243), Phycoerythrin (PE-conjugated) CD34 (8G12), Peridinin Chlorophyll (PerCP-conjugated) CD45 (2D1), Allophycocyanin (APC-conjugated) CD11b (D12) (Becton Dickinson Biosciences, San Jose, CA). To label cells, 100 μl of blood was added to pretitered monoclonal antibodies and incubated in the dark for 20 min. The erythroid cells were then lysed using 3 ml 37°C NH4CL (160 mM) for 5 min. The cells were then washed with 2 ml phosphate buffered saline containing 2% fetal calf serum and then fixed with 1% paraformaldehyde.
The specimens were analyzed on a FACS Calibur flow cytometer (Becton Dickinson BioScience, San Jose, CA) at Hematologics (Seattle, WA). The two flow cytometers used in this study were cross calibrated using fluorescent microspheres (RFP-1 and RCP-1, Spherotech, Libertyville, IL). Daily instrument quality controls including fluorescence standardization, linearity assessment, and spectral compensation were performed to ensure identical operation from day to day (16). The data were collected using CellQuest software (Becton Dickinson) with the list mode data analyzed using WinList (Verity Software House, Topsham, ME). For the CD34 enumeration, 200,000 events were collected to determine the total CD34+ cells per total nucleated cell (CD45 positive). The gating strategy used to determine the total CD34+ cells is shown in Figures 1A and 1B.
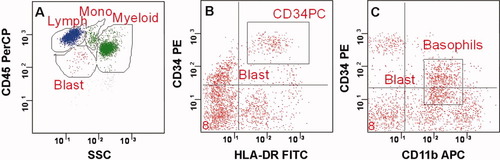
Gating strategy used to identify CD34 positive progenitor cells. Progenitor Cells are identified in the Blast gate by CD45/SSC (A). Cells that are positive for CD34 and HLA-DR (B) excluding the cells that are positive for CD11b (C) are used for the total CD34 count in a specimen. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Enumeration of CD34 Positive Cells
Data were acquired by a 4-color immunofluorescence protocol, forward and log right angle light scatter were collected along with the 4-color antibody combination described above. Therefore, each cellular event consists of six parameters which are coordinates for that event in six-dimensional space (17-19).
Positivity of CD34 cells was determined by a series of electronic gating steps. Cellular debris and nonviable cells were excluded on the basis of FSC and SSC (20). Discreet populations of cells were determined by CD45 staining and SSC as described (Fig. 1A, 21). Cells co-expressing CD34 and HLA-DR that are included in the Blast Region define the CD34+ population (Fig. 1B). These cells were confirmed to be CD11b negative, eliminating basophils (Fig. 1C). Single measurements were taken for all three studies. To measure the intrinsic analytical variability of the assay, sextuplicate testing was completed for subjects in the third study.
As the absolute CD34 counts were calculated based on the total white blood cell count, WBCs were collected for the 6 day study on a Sysmex XE2100 at Charles River Laboratories (Tacoma, WA). WBCs for the 10 week and 6 month study were obtained on a separate Sysmex XE2100 at Fred Hutchinson Cancer Research Center (Seattle, WA).
RESULTS
Two clinical trials were completed to assess the efficacy of drug candidates to mobilize CD34+ cells from the bone marrow. A third study was completed to assess if the amount of CD34+ cells in the peripheral blood remained constant over 18 months. These studies all had multiple tests per individual with variations including time, specimen draw, processing technologist, data collection, and analysis. Multiple tests define the reproducibility and precision for monitoring an individual over time and for comparison of results between individuals. Table 1 demonstrates the mean, median, and range of CD34 basal levels for all 34 subjects studied.
| Subject | Mean | Median | Range | Longitudinal %CV | Analytical mean | Analytical %CV |
|---|---|---|---|---|---|---|
| 1 | 2.2 | 2.0 | 1.4–3.1 | 23 | ||
| 2 | 8.8 | 8.9 | 6.7–11.5 | 14 | ||
| 3 | 1.4 | 1.3 | 0.5–2.7 | 39 | ||
| 4 | 1.9 | 1.6 | 1.0–3.8 | 38 | ||
| 5 | 2.2 | 2.2 | 1.3–3.7 | 26 | ||
| 6 | 3.8 | 3.7 | 2.6–6.1 | 21 | ||
| 7 | 7.3 | 7.1 | 4.9–9.6 | 25 | ||
| 8 | 4.0 | 3.7 | 2.2–4.9 | 28 | ||
| 9 | 3.4 | 2.5 | 1.8–4.1 | 28 | ||
| 10 | 3.5 | 2.6 | 1.7–3.9 | 29 | ||
| 11 | 6.1 | 4.2 | 2.6–6.1 | 29 | ||
| 12 | 9.3 | 7.1 | 5.1–9.6 | 27 | ||
| 13 | 3.8 | 2.6 | 1.5–3.8 | 22 | ||
| 14 | 4.8 | 3.7 | 2.5–4.8 | 19 | ||
| 15 | 3.7 | 2.6 | 1.1–3.7 | 34 | ||
| 16 | 6.5 | 5.3 | 4.0–6.5 | 14 | ||
| 17 | 4.1 | 2.1 | 1.3–4.1 | 36 | ||
| 18 | 6.5 | 4.8 | 3.7–6.5 | 17 | ||
| 19 | 4.2 | 4.1 | 3.1–5.5 | 18 | ||
| 20 | 1.7 | 1.7 | 0.9–2.2 | 18 | ||
| 21 | 4.3 | 4.3 | 3.5–4.9 | 13 | ||
| 22 | 0.9 | 0.9 | 0.7–1.1 | 14 | ||
| 23 | 1.6 | 1.6 | 1.1–2.3 | 22 | ||
| 24 | 5.3 | 5.4 | 4.1–6.5 | 14 | ||
| 25 | 3.5 | 3.5 | 2.7–5.0 | 14 | ||
| 26 | 2.4 | 2.5 | 0.7–3.7 | 32 | ||
| 27 | 3.1 | 3.0 | 2.7–4.1 | 12 | ||
| 28 | 2.7 | 2.8 | 1.5–3.5 | 19 | ||
| 29 | 1.9 | 1.9 | 1.8–2.3 | 12 | 2.3 | 16 |
| 30 | 1.5 | 1.3 | 1.2–1.5 | 31 | 1.2 | 11 |
| 31 | 1.3 | 2.0 | 1.6–2.5 | 22 | 2.7 | 15 |
| 32 | 2.4 | 2.6 | 2.3–2.7 | 17 | 2.6 | 11 |
| 33 | 1.6 | 1.4 | 1.2–1.7 | 43 | 1.6 | 8 |
| 34 | 1.6 | 1.5 | 1.2–2.1 | 22 | 1.8 | 6 |
- Subjects 1–6 are the control group from study 1 with 26 time points taken over six days. Subjects 7–18 are the pre G-CSF response time points from study 1. Subjects 19–28 are from study 2 and taken over 10 weeks. Subjects 29–34 are from study 3 with five time points over 6 months. Measurements of intrinsic analytical variability were completed for study 3 only.
Basal levels of CD34 Positive Cells are Maintained Over 6 days Even with an Increase in WBC
In the first clinical trial, 18 patients were monitored every 4 h over a 6 day period resulting in 26 time points taken over 6 days. Twelve of these subjects received G-CSF while six did not. The control group not exposed to G-CSF, subjects 1–6 (Fig. 2), did not change the proportion of CD34+ cells in circulation. All data points for these 6 subjects represent basal CD34 amounts in the peripheral blood determined by 26 separate assays performed over 6 days. The precision of these data demonstrates the reproducibility of the assay with coefficients of variation ranging 14%–39% at CD34 counts ranging between 1–9/μl (Table 1). From these data, it is evident that basal levels of CD34 differ from individual to individual. Therefore, the differences in CD34 absolute counts observed between normal, unstimulated individuals is not a function of the assay but is a biologic constant that is maintained over 6 days.
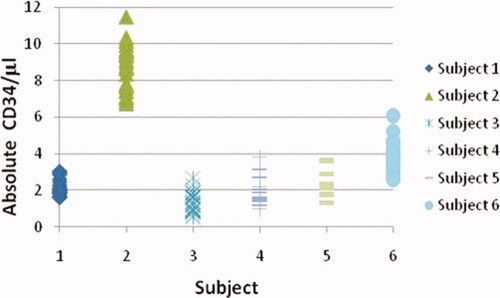
Absolute CD34/μl values for individual subjects assayed 26 times in the 6 day study. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The analytical contribution to the coefficients of variation is dependent on the dual platform technique used. Therefore, the statistical coefficient of variation is the sum of the coefficient of variation of CD34 enumeration and the coefficient of variation of the WBC assessment (Table 2). The coefficient of variation for the CD34/μl measurement is approximately double that of the WBC indicating that the contribution of the flow cytometric analysis does not greatly exceed that of the WBC analysis.
| Subject # | CD34/μl %CV | WBC %CV |
|---|---|---|
| 1 | 23 | 11 |
| 2 | 14 | 11 |
| 3 | 39 | 9 |
| 4 | 38 | 19 |
| 5 | 26 | 14 |
| 6 | 21 | 14 |
| Average | 27 | 13 |
Interestingly, the precision did not change with increasing WBC. An additional 12 subjects were assayed in the early stages of G-CSF administration. Figure 3 shows an increase in WBC for 6 of these subjects treated with G-CSF, showing the CD34 levels remain constant, while the WBC increases. CD34 counts remain at basal levels until 28–40 h (subject dependent) post G-CSF administration. The coefficients of variation for these subjects, 14%–36% (Table 1), are not different from the subjects who had no increase in WBC. An additional six subjects were also treated with G-CSF (data not shown in Fig. 3) but are nearly identical to those depicted with CD34 amounts ranging from 2 to 6/μl with white counts approaching 50,000/μl. The individual fluctuations in basal CD34 amounts were not related to time after G-CSF administration nor WBC. These data reveal there are observable differences in basal levels of CD34 between individuals and the technique is invariant of the WBC up to 50,000/μL. The two platform technique is highly reproducible and consistent even with a 10-fold increase in WBC.
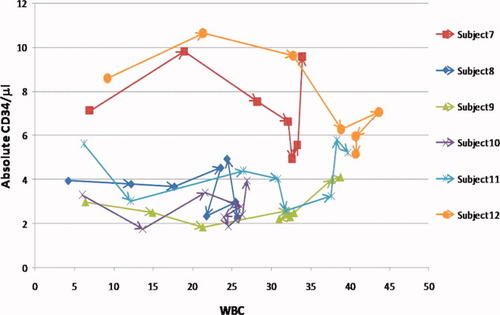
Absolute CD34/μl obtained every 4 h versus WBC for six of the twelve subjects receiving G-CSF before CD34 mobilization. Over the first 20–40 h the WBC dramatically increases; however, the CD34 counts remain constant. The six subjects treated with G-CSF not shown are nearly identical to those shown here with CD34 amounts ranging from 2–6/μl with white counts approaching 50,000/μl.
Basal Levels of CD34 are Maintained for at Least 18 Months
A second study testing whether certain nutrient supplements could mobilize CD34+ cells to the peripheral blood revealed the supplements failed to alter CD34 levels for 10 individuals (Fig. 4). CD34 counts remained constant for a 10-week period independent of the nutrient administered and the time after ingesting the supplement (either pre-ingestion, 1 or 3 h post-ingestion). The coefficients of variation ranged from 9.0% to 37% for the CD34 counts and are not different than those observed for the 6 day study (Table 1). Low coefficients of variation of the data illustrate that individual differences in the basal levels of CD34 could be identified in the peripheral blood and that these individual differences remained constant for 10 weeks. There was no effect from the various supplements tested.
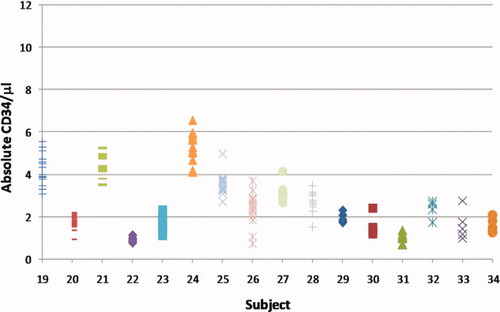
Absolute CD34/μl values for individual subjects assayed 14 separate times during a 10 week study (subjects 19–28) and absolute CD34/μl values for individual subjects assayed six different times over an 18 month period (subjects 29–34).
Having identified individual differences in absolute CD34 counts that were stable over 10 weeks, a further study was initiated to see if this parameter was stable for longer periods of time. The basal level of CD34 was monitored for six individuals approximately every 5 weeks for 6 months (Fig. 4) and 18 months after the initial measurement, with no consistent changes over time observed (Table 1). In addition, the coefficients of variation do not differ from the shorter term studies (12%–43%). Therefore, low coefficients of variation for each individual illustrate that basal levels of CD34 differ between individuals and these levels are stable over at least 18 months.
A Validated Normal Range of Basal CD34 Amounts
The intrinsic analytical variance was measured by sextuplicate testing of the subjects in the third study with coefficients of variation ranging from 6% to 16% (Table 1). One subject did have a higher intrinsic analytical variance than longitudinal variance (16%); however, this individual had a remarkably low longitudinal coefficient of variation (12%). The data indicates that at basal CD34 levels there is essentially no difference between the analytical variance and the longitudinal variance.
From these studies, a unique validated normal range can be developed with the knowledge of variability for the measurement of each individual. Figure 5 demonstrates the distribution of the CD34 absolute counts at basal levels for all 34 subjects in the study. The breadth of this assay reveals the biological variability of basal CD34 levels in the peripheral blood of individuals that appear to remain stable for at least 18 months duration. The difference between individuals is much greater than the variability within an individual, demonstrating that the assay has the precision to distinguish between basal levels of CD34 for different individuals.
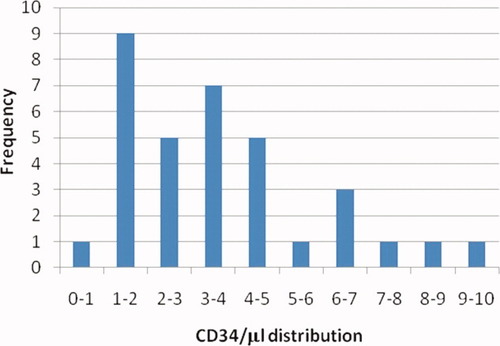
A Normal range distribution of CD34 μl absolute amounts for subjects in all three studies.
The Kinetics of CD34 Mobilization with G-CSF Treatment
Using this approach to enumerate CD34+ cells, six normal individuals were monitored to measure their response to G-CSF (Fig. 6, the same individuals depicted in Fig. 3). The absolute CD34/μl peak maxima vary considerably (Fig. 6A), however the kinetics for reaching the maximum response for individuals are quite precise (Fig. 6B). It should be noted that the increase in WBC was observed almost instantaneously for these individuals, with a 2-fold increase after 4 h (Fig. 3). An observable increase in CD34 levels begins at approximately 48 h with maximums occurring between 80–115 h. The maximum absolute number of CD34+ cells is variable for each subject, and the kinetics do not correlate with maximum WBC values. The data also suggests that subjects with the highest WBCs do not correspond to the subjects that have the highest CD34 counts.
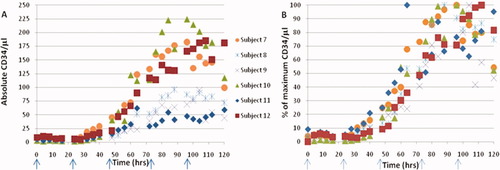
(A) Absolute CD34 counts in response to G-CSF treatment of subjects 7–12. Arrows indicate time points when G-CSF was administered. (B) Kinetics of CD34 mobilization with G-CSF treatment of subjects 7–12 as a percent of the maximum of each subject. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The CD34 basal levels do not predict which subject will have the greatest maximum counts post G-CSF administration (Table 3). Individual subjects basal CD34/μl levels do not predict which subjects will have the highest levels of circulating CD34 positive cells post G-CSF treatment. Although subjects 7 and 12 have relatively high basal and maximum CD34 levels, the subject with the highest maximum has the lowest basal average.
| Subject # | CD34/μl basal average | CD34 maximum |
|---|---|---|
| 7 | 7.3 | 177 |
| 8 | 3.5 | 96 |
| 9 | 2.8 | 95 |
| 10 | 2.7 | 224 |
| 11 | 4.2 | 61 |
| 12 | 7.6 | 181 |
DISCUSSION
Most studies analyzing the kinetics of response to G-CSF usually have only a single time point pre-drug administration and the variability between subjects at this low end of assessment may well be a result of technical artifacts and assay variability in the assessment of target cell populations at levels of 1 cell/μl. The clinical studies described in this article provided an opportunity to define the assay precision, a requirement to detect low levels of progenitor cell mobilization. The results of all three independent studies reveal reproducible differences between individuals in basal levels of circulating CD34+ progenitor cells. This conclusion could only be made by establishing the uncertainty of the assay itself. With an individual's coefficients of variation as low as 9% at levels of 1–9 CD34+ cells/μl, small changes in circulating CD34+ cells could have been detected. Combining data from all three studies provides a unique, validated normal range for basal levels of CD34 progenitor cells with each point a result of multiple independent assessments of the same individual over time. The results illustrate that there are biological, not technical differences between individuals in basal levels of CD34 progenitor cells that remain stable for at least 6 months. The biological basis for these differences have yet to be elucidated.
Rare event assays must be quality controlled at the lowest levels, where the error is greatest. Simply by repeat analysis of peripheral blood from a set of the same individuals over time, the precision of the analysis can be defined. Therefore, by determining the CD34 count monthly from the same individual, it is possible to establish a measure of precision of the assay at the lowest end of the expected range. In this manner, one can be confident measurements at maximum levels are accurate, whether an individual's maximum is only slightly higher or much greater than the lowest levels. Our finding provides an opportunity to better quality control data for CD34 progenitor cell enumeration. Such studies are essential for interpretation of the data from CD34 counts at low levels. An understanding of assay precision is important to investigate the basis of low levels of CD34 progenitor cell mobilization, particularly following chemotherapy. CD34+ cell measurements in the peripheral blood are also used to monitor patients with myelodysplastic syndrome and myelofibrosis at levels of less than 10 cells/μl (22, 23). It is essential to separate analytic variability from biologic variability especially in monitoring patients who have a difficult time mobilizing CD34+ cells, as many of these patients are just above the basal range.
The assay is robust as the absolute CD34 count remains constant during the early phase of G-CSF administration when the WBC undergoes dramatic elevation. Within 4 h of G-CSF administration the WBC increases 2-fold, most likely a result of demargination of cells in circulation. The WBC continues to climb, sometimes reaching a 10-fold increase from the starting value. Interestingly, the CD34 progenitor cell count does not change during the first 28–40 h illustrating that these are clearly different effects of the G-CSF. The CV of the CD34 count did not change nor was there any relationship between assay fluctuation and time (Fig. 3). The proportion of CD34 progenitor cells per nucleated cell dropped during these early time points but was compensated by the increase in WBC.
The requirement to count 200,000 events in our assay is based on the expected total CD34 progenitor cells being detected. Assuming a WBC concentration of 7,000/μl and a CD34 count of 1/μl, the total CD34 positive events in 200,000 event analysis is 29 events. If the WBC increases to 49,000, the total CD34 cells counted in 200,000 events is only 4. If fewer total events were counted, no CD34 positive progenitor cells would be detected during the early phases of G-CSF stimulation or in patients who have poor mobilization. The reason for the absence of CD34 positive cells under such conditions would be unknown and could be a result of technical error or poor instrument setup. To eliminate this source of error, the minimum number of total events that must be collected for this assay is 200,000.
The clinical study monitoring the kinetics of G-CSF every 4 h was a unique opportunity to precisely identify the individual responses to this drug. The assessment at these frequent time points allowed a clear definition of the response in these six normal volunteers. Although there was a dramatic difference in the maximal CD34 levels, normalizing the data to the percent of maxima reveals a constancy of the kinetics of the response, peaking between 80 and 110 h. Although most studies indicate that the maximum is achieved on day 4 or 5 (24-28), a closer look at the data suggests a change in the timing of the dosing may yield better recovery. If the initial dose is administered in the late afternoon or evening of the first day with apheresis scheduled for the mornings of the third and fourth days, the peak of CD34 positive cells would be achieved on both days rather than just 1 day. Such a schedule would then maximize the total collection during the peak of response so that 2 rather than 1 collections were at the maximum.
In conclusion, we have demonstrated herein that individual basal CD34 levels remain constant within individuals for at least 18 months with a low coefficient of variation. Counting at least 200,000 events is essential for proper quality control of this and any rare event flow cytometric assay, as the error is greatest at an assay's lowest levels of measurement. G-CSF stimulation of six individuals demonstrated quite adequately that the absolute kinetics between individuals is very precise, even though the actual maximum values vary greatly. In addition, there is a lack of agreement if basal CD34 levels are predictive of mobilization of CD34+ cells with G-CSF treatment (13, 29); our findings do not support that basal CD34 levels are predictive of HPC mobilization. These data provide further insight regarding the variability of basal CD34 levels and G-CSF mobilization providing a greater understanding of the biology and the tools used to measure HPCs and their mobilization from the bone marrow to the periphery.




