Differential modulation of cord blood and peripheral blood monocytes by intravenous immunoglobulin†
How to cite this article: Gille C, Dreschers S, Spring B, Tárnok A, Bocsi J, Poets CF, Orlikowsky TW. Differential modulation of cord blood and peripheral blood monocytes by intravenous immunoglobulin. Cytometry Part B 2012; 82B: 26–34.
Abstract
Background:
Immunoglobulins (IVIG) have been shown to be useful in adults suffering from sepsis. In contrast, prophylactic and curative IVIG trials failed to show beneficial effects in neonates. We tested the hypothesis that IVIG, have different effects on monocytes from cord blood (CBMO) and peripheral blood monocytes from adults (PBMO) with respect to survival, phenotype, and function.
Methods:
Mononuclear cells, or purified monocytes, were cultured in 5% human serum, incubated with polyvalent IVIG (1 mg/ml), stimulated with green fluorescent protein (GFP)-labeled Escherichia coli (E. Coli-GFP), Interferon-γ (IFN-γ, 50 U/ml), or the T cell mitogen anti-CD3 monoclonal antibody, αCD3-mAb, (5 μg/ml). Phagocytosis, phenotype, T cell proliferation, and apoptosis were assessed by flow cytometry.
Results:
IVIG enhanced phagocytosis in PBMO or CBMO when infected directly after isolation, while IVIG had no effect on monocytes cultured 48 h prior to infection. In contrast to PBMO, IVIG inhibited the IFN-γ mediated up-regulation of CD80, CD86, and HLA-DR on CBMO. In the presence of IVIG, stimulation with αCD3 in cord blood enhanced deletion, inhibited blast formation and CD28 up-regulation of T cells (P < 0.05 vs. T cells from adults). IVIG induced monocyte apoptosis, associated with up-regulation of Annexin V and loss of nuclear DNA, which was more pronounced in CBMO. Although phagocytosis induced cell death (PICD) was lower in CBMO (P < 0.05 vs. PBMO), the addition of IVIG enhanced PICD levels of CBMO to the extent of PBMO.
Conclusions:
IVIG inhibits co-stimulatory receptors and functions of CBMO and induces apoptosis. These findings may be of clinical relevance for the failure of IVIG benefit in neonatal sepsis. © 2011 International Clinical Cytometry Society
In adults, intravenous immunoglobulin (IVIG) is known to be beneficial in the treatment of sepsis (1,2), autoimmune and systemic inflammatory diseases (3,4). As an adjunct treatment strategy, IVIG was used frequently in neonatal sepsis (5,6). However, recent trials showed no effect of IVIG on mortality and sepsis-induced organ failure in this age group. Currently, IVIG is not recommended to prevent or treat neonatal sepsis (7), although the discussion has been reopened lately (8). An array of mechanisms, including blockage of cellular receptors for immunoglobulins (Fc receptors), inhibition of the complement cascade, influence on cytokine production, modulation of inhibitory Fc receptor expressions, and cell apoptosis have been attributed to the anti-inflammatory actions of IVIG (9,10).
During sepsis, monocytes are crucial for the initial immune defence by phagocyting and degrading pathogens. This reaction cascade is either terminated through monocyte apoptosis, that is, phagocytosis induced cell death, PICD, (11,12), or may lead to further activation of the immune system (13). The role of apoptotic cell death in the pathophysiology of sepsis in adults is crucial (14-16) and reduced apoptosis of peripheral blood monocytes from adults (PBMO) during the initial phase was shown to be related to poor outcome (17).
Neonatal sepsis often is devastating, and effector cells, for example, monocytes, taken from cord blood (CBMO), differ from PBMO with respect to a variety of aspects: they were found to be less sensitive towards the two most common bacterial species [Escherichia coli (E. coli) and streptococcus group B]-mediated PICD than PBMO (12) and were shown to have deficiencies in both, costimulatory and cytotoxic functions: Due to diminished IFN-γ effects on B7 molecules (CD80 and CD86), CBMO inhibit monocyte-dependent T cell proliferation (18). CBMO have a diminished capacity to induce antibody-dependent cellular cytotoxicity, ADCC, (19) and to remove apoptotic particles (20).
Little is known on the interaction of IVIG and CBMO. Taken into account that IVIG did not show beneficial effects in neonatal sepsis and CBMO are exquisitely sensitive towards drugs such as dexamethasone (21), we expected a more profound effect of IVIG on CBMO as compared to PBMO. Therefore, we tested the hypothesis that IVIG alter monocyte survival and phenotype and monocyte dependent T cell reactions in neonates more profoundly than in adults.
Materials and Methods
Patients
The study protocol was approved by the Ethics Committee of the University of Tuebingen. All mothers gave written consent before they went into labor. Randomly selected, unrelated adults donated blood and served as controls. All term neonates were delivered spontaneously and did not exhibit signs of infection, as defined by the clinical status, white blood cell count and C-reactive protein. Mothers with amnion infections and prolonged labor were excluded. Umbilical cord blood was placed in heparin-coated tubes (4 IU/ml blood), immediately following cord ligation.
Cell Cultures
Peripheral blood and cord blood mononuclear cells (PBMC and CBMC) were isolated by density gradient centrifugation (400g, 25 minutes) using research grade Biocoll solution (density: 1.077 g/ml; Biochrom AG, Berlin, Germany) and following the manufacturer's instructions. Washed cells were resuspended in VLE RPMI-1640 (Biochrom), containing 5% heat-inactivated human AB serum (Invitrogen, Carlsbad, Ca). Cells were counted in an ultraplane Neubauer hemocytometer, placed at 2 × 106 cells/ml in flat bottom 24 well cell culture plates (Costar, Bodenheim, Germany) and incubated at 37°C.
Purification and Depletion of Monocytes
Cell subsets were prepared using a magnetic immunoseparation technique (MACS) according to the instructions of the manufacturer (Dynal, Lake Success, NY). T cells were negatively purified, using an antibody-cocktail of antibodies against CD14, CD19, and CD56; monocytes were negatively enriched by the combination of CD3, CD8, CD19, and CD56. The purity of cell subsets was above 95%, as verified by a CD4/CD8 (T cells) and a CD14 staining (monocytes) and flow cytometric analysis.
Reagents
Interferon-gamma (IFN-γ; 50 U/ml) was purchased from R&D (Minneapolis, MN); anti-CD3 monoclonal antibody (αCD3-mAb; OKT3; 5 μg/ml) from Ortho (Raritan, NJ); bovine serum albumin (BSA) from Roth (Karlsruhe, Germany). Polyvalent IgG (Polyglobin 10%; 100 mg/ml Bayer, Germany) was diluted in RPMI and added freshly. The Ig-levels of pregnant women are between 700 and 1,200 mg/100 ml (22) in serum; in preterm infants due to insufficient maternofetal antibody transport are as low as 200–700 mg/100 ml in serum, depending on gestational age. Since we used IVIG as a replacement therapy to reach maternal serum levels (9), we chose therapeutically active doses with final concentrations of 1–10 mg/ml by dilution of IVIG in RPMI.
Phagocytosis Assay
E. coli DH5α, carrying the green fluorescent protein (GFP)-mut2 encoding plasmid pCD353 (E. coli-GFP), which express a prokaryotic variant of GFP under control of a lactac promoter (23), was a gift of Prof. C. Dehio (Biocentrum Basel, Switzerland). Phagocytosis assay was performed as described before (24). In brief, bacteria were freshly grown on agar plates supplemented with kanamycin (50 μg/ml; Sigma) and isopropyl-b-D-1-thiogalactopyranoside (1 mM; Sigma) for GFP induction. After 24 h, a single colony was grown in Lennox L broth-medium (Invitrogen, Karlsruhe, Germany) until early logarithmic growth phase, then bacteria were added in constant ratios (bacteria : cells = 50:1) for 60 minutes to cell cultures. Free bacteria were removed with the supernatant after centrifugation through an FCS cushion (200g, 5 minutes), cells were fixed (Paraformaldehyde 2%; Merck, Darmstadt, Germany). Phagocytosis index (% CD14+ GFP+ monocytes among all CD14+ monocytes) and phagocytic capacity (mean GFP fluorescence intensity (MFI) on GFP+/CD14+ monocytes) were analyzed.
Immune Phenotyping
Harvested cells were counted in an ultraplane improved Neubauer Hemacytometer and analyzed on a FACScan flow cytometer (BD Biosciences) what was daily calibrated by standard beads (Sphero Rainbow Calibration Particles, Spherotec, Libertyville, IL); mAb to CD4 (SK3), CD8 (SK1), CD14 (MϕP9), CD16 (NKP15), CD28 (L293), CD80 (L307.4), CD86 (IT2.2), HLA-DR (L243), and Ig-matched controls (IgG1 and IgG2b) were from BD Biosciences (BD, Heidelberg, Germany). To prevent nonspecific binding, cells were incubated with 10% human serum on ice for 10 minutes before staining with fluorescein-isothiocyanate (FITC)-, phycoerythrin (PE)-labeled monoclonal antibodies or unspecific isotype immunoglobulins 20 minutes in the dark. Dead cells were discriminated by propidium iodide (PI, Molecular Probes, Eugene, OR, 5 μg/ml, 5 minutes). Monocytes were gated on the basis of forward (FSC) and side scatter (SSC), and CD14 expression, lymphocytes were gated on forward (FSC), and side scatter (SSC) dot plots. PI negative and CD4/CD3, CD8/CD3 double positive lymphocytes were analyzed for expression of CD4 and CD8. Annexin V staining of monocytes was assessed by Annexin V-PE (BD Biosciences) according to the manufacturer's instructions. Cells were washed twice and resuspended in 50 μl in Annexin-buffer (0.02% sodiumazide in HEPES-buffer, pH 7.4), with 1 μl Annexin V-PE for 15 minutes, combined with CD14 FITC staining, washed and analyzed immediately. Determination of lymphocyte apoptosis was performed by Annexin V-FITC-PI staining (Miltenyi Biotec, Berglisch Gladbach, Germany) according to the manufacturer's protocol. Hypodiploid nuclei were assessed according to the method of Nicoletti (25). Washed cells were slowly resuspended in 2 ml of −20°C ethanol 70% with continuous vortexing and stored for 4 h at −20°C. Cells were washed twice, resuspended in 50 μl PBS containing 13 k units RNase (DNase free; Sigma) and incubated for 15 minutes at 37°C. One hundred and eighty microliter of PI (70 μg/ml) were added, incubated for 20 minutes and analysis performed immediately.
T Cell Blast Formation and Deletion
The absolute number of T cells was obtained by multiplying the percentage of CD4+ and CD8hi+ cells with the cell count. T cell blasts were detected as CD14−/CD4+ or CD14−/CD8hi+ cells with enlarged size in the forward scatter, as compared to the corresponding unstimulated controls. The numbers of CD4+ and CD8+ T cells were calculated and the percentage of deleted cells was determined applying the formula: percent deleted cells = (cells cultured in control-cells cultured with αCD3-mAb)/cells cultured in control × 100) as previously described (26). The number of T cell blasts was detected by their enlarged size in the forward scatter, as compared to the corresponding unstimulated controls. T cells were double stained with CD28 and their expression of co-receptors as previously described (27).
Statistics
Mean fluorescence intensities (MFI) were determined by subtracting nonspecific background staining. Experiments were performed in triplicates. Results are expressed as mean +/- SD. Statistical analysis was performed using the decadic logarithm of the values of CD14, CD16, CD80, HLA-DR, and GFP for a student's t-test (Sigmaplot 2000 software for Windows, SPSS, Chicago, IL). Values of P < 0.05 (adjusted according to Bonferroni-Holm for multiple group comparisons) were considered statistically significant.
Results
Effect of IVIG on Bacterial Phagocytosis
PBMC and CBMC were prepared and cultured. One group was infected with E. coli immediately, the second group after 2 days of culture. In each group, IVIG was added prior to infection. As previously shown (12, 19, 20, 24, 28, 29), phagocytic activity of CBMO and PBMO were not different, neither with respect to cells actively participating in phagocytosis (phagocytic index, Fig. 1A, first and third columns) or in the amount of bacteria ingested per cell (phagocytic capacity, Fig. 1B, first and third columns). In freshly cultured cells, IVIG increased the phagocytic index in PBMO from 46 ± 25 to 85 ± 10% and phagocytic capacity from 120 ± 48 MFI to 320 ± 100 MFI and in CBMO from 50 ± 20% to 83 ± 12% and 97 ± 20 MFI to 290 ± 130 MFI (all P < 0.05 vs. without IVIG). If monocytes were cultured for 48 h prior to addition of E. coli, their phagocytic capacity increased slightly, but IVIG showed no additional enhancement on bacterial phagocytic index or capacity (P > 0.05 vs. bacteria only Figs. 1A and 1B, fourth columns).
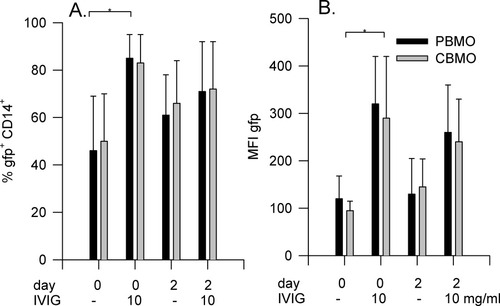
Effect of IVIG on bacterial phagocytosis. PBMC or CBMC (2 × 105) were harvested freshly (day 0) or cultured for 48 h (day 2). Cells were incubated with E. coli-GFP for 60 minutes in the presence or absence of IVIG (10 mg/ml). Cells were harvested, phenotyped, and assayed for phagocytosis index (A) and -capacity (B) of PBMO (black bars; n = 5) and CBMO (gray bars; n = 5). Results are expressed in mean ± SD. * P < 0.05 versus bacteria only.
Effect of IVIG on Monocyte Phenotype
After incubation of PBMO and CBMO with IVIG, a slight down-modulation of CD14 receptors by 29% and fraction of CD14/CD16+ cells by 90% (Table 1) occurred after 24 h. To analyze the B7-family receptor (CD80, CD86), and HLA-DR expression, PBMC and CBMC were incubated for 24 h and monocytes phenotyped (Fig. 2). Besides basal receptor expressions we also analyzed their up-regulatory potential upon stimulation with IFN-γ in the presence or absence of IVIG. As previously described (18), in unstimulated CBMO (Figs. 2A–2C, first columns), we found a lower percentage of CD80+ cells (P < 0.05 vs. PBMO), and slightly less CD86- and HLA-DR- receptor densities (P > 0.05; Fig. 2C) and IVIG down-regulated CD80, CD86, and HLA-DR on CBMO, whereas the phenotype of PBMO did not change significantly (Figs. 2A–2C, second columns). IFN-γ up-regulated the receptors, which with respect to CD80 and CD86 was more pronounced in PBMO compared to CBMO (P < 0.05, Figs. 2A–2C third columns). Addition of IVIG did not influence the IFN-γ mediated up-regulatory effect in PBMO. In contrast, in CBMO, IVIG nearly abolished the IFN-γ mediated up-regulation (Figs. 2A–2C fourth columns). As an internal control, we added BSA in equimolar amounts (1 mg/ml) to one group. BSA had no influence on CD80 and CD86 on PBMO and CBMO, while HLA-DR was upregulated (Figs. 2A–2C third columns). In combination with INF-γ BSA enhanced, the IFN-γ effects (Figs. 2A–2C last columns).
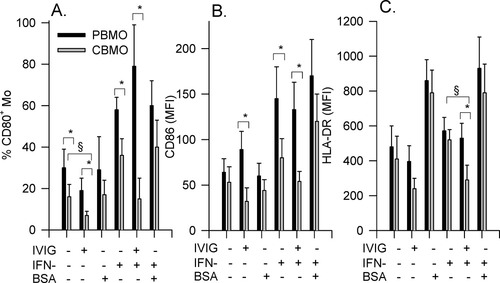
Effect of IVIG on CD80, CD86, and HLA-DR receptor expression. CBMC or PBMC (2 × 105) were incubated for 24 h in the presence or absence of IVIG (10 mg/ml), IFN-γ (50 U/ml), BSA (1 mg/ml), or combinations, and analyzed for % CD80-positive monocytes (A), for CD86 (MFI; B), and HLA-DR expression (MFI; C); n = 6, * P < 0.05 PBMO versus CBMO; § P < 0.05 effect of IVIG on CBMO versus untreated CBMO.
| IVIG (mg/ml) | CD14 PBMO MFI (SD) | CD14 CBMO MFI (SD) | CD16 PBMO (% pos.) | CD16 CBMO (% pos.) |
|---|---|---|---|---|
| 0 | 187 (45) | 166 (48) | 29 (11) | 18 (23) |
| 0.1 | 171 (30) | 148 (35) | 9 (7) | 19 (13) |
| 1 | 145 (34) | 130* (29) | 5* (4) | 0* (5) |
| 10 | 133* (24) | 139* (26) | 0* (3) | 0* (2) |
- CBMC and PBMC were prepared and 2 × 105 cells were incubated for 24 h in the presence or absence of 10 mg/ml IVIG and analyzed for CD14 MFI and percentage of CD16+ cells, n = 5,
- * P < 0.05 versus corresponding nonstimulated group.
Effect of IVIG on Monocyte Dependent CD3-Mediated T Cell Reactions
T cells respond to anti-CD3 monoclonal antibody biphasically: The response is characterized by an initial decline in the number of T cells, followed by a subsequent T cell clonal expansion (27), and a consecutive up-regulation of T cell CD28 receptors (27). CD3-mediated clonal T cell expansion requires the presence of antigen presenting cells, for example, monocytes. The number of monocytes, as well as co-stimulatory signals, for example, via the B7-CD28 axis, influences the proportion of the initial T cell deletion and polyclonal activation (30). To test the influence of IVIG on this monocyte-dependent reaction, PBMC and CBMC were pre-incubated with αCD3-mAb prior to addition of IVIG. T cell αCD3-binding kinetics were unaffected by IVIG as determined by competitive inhibition of fluorescent αCD3-mAb (not shown).
In the absence of αCD3-mAb, neither deletion nor proliferation was detectable (Figs. 3A and 3B, first and second columns). The addition of BSA as well did not induce a T cell reaction (not shown). As already shown (21), in the presence of αCD3-mAb, T cells from CBMC were less deleted and proliferated less compared to T cells from PBMC (Figs. 3A and 3B, third columns). With regard to αCD3-mediated T cell deletion, IVIG induced opposite effects on PBMC and CBMC: It inhibited T cell deletion in PBMC, whereas it enhanced T cell deletion in CBMC (P < 0.05 vs. αCD3-mAb only, Fig. 3A fourth columns). With regard to clonal T cell expansion, IVIG inhibited T cell blast formation in both, PBMC and CBMC with pronounced effect on CBMC, showing almost a paralysis of proliferation (P < 0.05 vs. αCD3 only, Fig. 3B last columns). In parallel to this observation, IVIG in cord blood almost completely inhibited αCD3-mediated CD28 up-regulation on CD4 T cells (P < 0.05 vs. PBMC, Table 2).
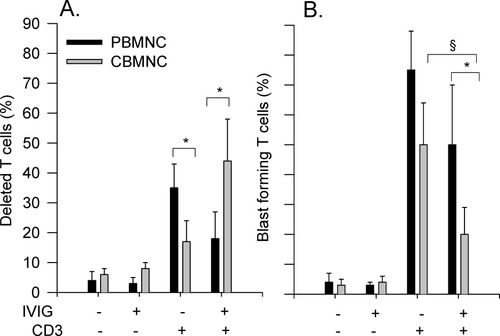
Effect of IVIG on monocyte-mediated T cell reaction. CBMC or PBMC (2 × 105) were pre-incubated with αCD3-mAb (5 μg/ml) for 4 h prior to IVIG (10 mg/ml). T cell deletion (A) and blast formation (B) were assessed after 48 h; n = 4; * P < 0.05 PBMO versus CBMO; § P < 0.05 versus αCD3 effect of corresponding group.
| CD28 MFI (SD) | Ctrl. | IVIG (10 mg/ml) | αCD3 | αCD3 + IVIG |
|---|---|---|---|---|
| CD4 T cells PBMC | 75 (16) | 68 (32) | 209 (74)* | 162 (44)* |
| CD4 T cells CBMC | 89 (24) | 74 (24) | 168 (34)* | 95 (36)§ |
- CBMC or PBMC (2 × 106) were pre-incubated with αCD3-mAb (5 μg/ml) for 4 h prior to IVIG (10 mg/ml). CD28 up-regulation on CD4 T cells was analyzed after 48 h; n = 5,
- * P < 0.05 PBMO versus CBMO; § P < 0.05 versus αCD3-mediated effect.
Effect of IVIG on Monocyte Cell Death
Purified monocytes were treated with different IVIG concentrations and analyzed for Annexin V-binding after 4 h (Figs. 4A and 4B), and for percentage of hypodiploid nuclei after 24 h (Figs. 4C and 4D). Monocytes increased in granularity (SSC) and shrunk in size (FSC) (not shown). Approximately, 8% of untreated monocytes were positive for Annexin V (Fig. 4A, left side and Fig. 4B, left columns). Four hours after addition of IVIG (1 mg/ml), the fraction of Annexin V-positive monocytes rose to 19 ± 5% in PBMO and 30 ± 8% in CBMO (Fig. 4A right side; Fig. 4B; P < 0.05 PBMO vs. CBMO). Phosphatidylserine temporarily is expressed in higher concentrations on the outer membrane layers during phagocytosis (31), so we did not interpret its consecutive up-regulation as an irreversible apoptotic sign. Therefore, in purified monocyte cultures, apoptosis was confirmed by loss of nuclear DNA, resulting in hypodiploid nuclei (Figs. 4C and 4D).
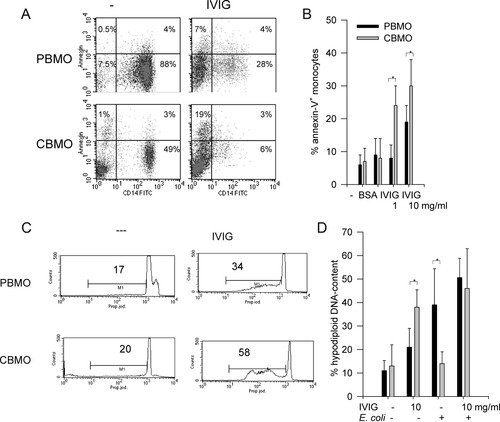
A: Purified 2 × 105 PBMO or CBMO were treated in the absence or presence of IVIG (10 mg/ml) for 4 h. Cells were stained with CD14-FITC/Annexin V–PE, % positive cells are given. B: Annexin V staining (%) on monocytes, n = 7; * P < 0.05 PBMO versus CBMO. C: Hypodiploid DNA content was measured as previously described (25) after 24 h. The marker covers the sub-G1 (apoptotic) fraction. D: Hypodiploid DNA content of purified monocytes, one group pre-incubated with IVIG and infected with E. coli for 60 minutes. Mitomycin-incubated monocytes (mito, 50 μg/ml) served as positive controls for apoptosis. n = 6; * P < 0.05 PBMO versus CBMO.
Addition of IVIG induced a substantial hypodiploid population in CBMO (Figs. 4C and 4D, P < 0.05 vs. corresponding untreated group) after 24 h in the absence of bacteria. After infection with E. coli, more PBMO became apoptotic compared to CBMO (39 ± 15% vs. 14 ± 5%, * P < 0.05, Fig. 4D, third columns), confirming previous results (12,29). Although addition of IVIG to PBMO in the presence of E. coli led to no further enhancement of apoptosis, apoptosis was markedly enhanced in infected CBMO upon addition of IVIG (P < 0.05 vs. IVIG only; Fig. 4D, fourth columns).
Discussion
IVIG enhanced phagocytosis of E. coli in freshly cultured PBMO and CBMO (Fig. 1), affected their phenotype (Fig. 2; Table 1) as well as their ability to orchestrate a T cell response (Fig. 3; Table 2), and induced apoptosis with up-regulation of Annexin V and loss of diploid DNA (Fig. 4).
Besides T cells, immunoglobulins are the key mediators of the adaptive immune response. Deficiencies in either arm of the system increase the susceptibility towards perpetuation and severity of infections (9). This situation occurs in preterm neonates with deficiencies in T cell response and a transient lack of immunoglobulins due to insufficient transplacental transport of IgG antibodies, which mainly occurs during the third trimenon [reviewed in (22)], resulting in a hightened susceptibility towards sepsis. Up to 25% of septic neonates are confronted with long-term sequelae in multiple organ systems, especially when therapy is delayed, resulting from a hyperinflammatory state (32). In neonatal sepsis, IVIG has so fare been administered as replacement therapy to overcome immunoglobulin deficiency. Although rational, this therapeutic approach had no beneficial effects, so that administration of IVIG is not recommended anymore to prevent or treat neonatal sepsis (7).
With respect to phagocytosis, which is a key task of monocytes during sepsis, the addition of IVIG to freshly harvested cells increased their phagocytic capacity, through the number of phagocyting monocytes (Fig. 1A) and the amount of bacteria per cell (Fig. 1B). In neonatal sepsis, where the density of bacteria in the bloodstream often is higher compared to sepsis in adults, this effect may be beneficial. Phagocytosis involves a variety of cell membrane recognition structures, including immunoglobulin receptors (9). One rationale for IVIG in sepsis improved bacterial opsonization. Monocytes ingest E. coli opsonized by IVIG in the absence of complement more effectively: In a similar FACS-based phagocytosis assay, IVIG increased E. coli phagocytosis, and addition of fresh autologous plasma as a complement source resulted in a further boost (33). The basal phagocytic ratio was lower than found here (Fig. 1), however, in their system, fetal calf serum had been used and granulocytes had been present. Parallel to these findings, we also found an increase of bacterial load per monocyte with the use of IVIG (Fig. 1B) in both, freshly harvested CBMO and PBMO. Monocytic differentiation influences periphagocytic functions in response to gram-positive and gram-negative commensal bacteria (34). Thus, it seems plausible that we found no additional effect of bacterial uptake in our system when using 48 h pre-cultured cells (Fig. 1). In clinical practice, the expectation of an enhanced phagocytosis by IVIG does not always hold true: Phagocytic activity of PBMO from adult patients with nosocomial infection was not improved by IVIG infusion (35), however, many of them already were in an anti-inflammatory state of the disease, and timing of IVIG replacement seems to be of great importance (36). Other serum factors, for example, complement (which preterm infants are also depleted of) influence phagocytosis markedly and cannot be substituted via addition of IVIG: Phagocytosis rates of group B streptococci (GBS) were found to be significantly lower when serum of preterm infants was used. Supplementation with IVIG did not enhance phagocytosis (37).
If IVIG is not used as a replacement, but as an anti-inflammatory agent, a high-dose therapy of 1–3 g/kg body weight is used, which is up to 10 times higher than the dose frequently recommended in replacement therapy (36). At higher concentrations, IVIG inhibits inflammatory activation via a diverse array of mechanisms (9, 10, 38).
IVIG treatment suppresses the activation of monocytes by altering transcription of various inflammatory genes and lowers levels of circulating cytokines (10). In our experimental system, we used low concentrations of IVIG, equivalent to a serum level of 10 mg/ml, but found, in CBMO more than in PBMO, an inhibition of the up-regulation of CD80, CD86, and HLA-DR (Fig. 2), reduced T cell proliferation (Fig. 3) and consecutive up-regulation of the corresponding CD28 receptor on T cells (Table 2). It has already been shown that in monocyte derived immature dendritic cells, IVIG partially inhibits expression of CD86, CD40, and CD80, which are markers associated with T cell activation. In addition, it inhibits the production of interleukin (IL)-12, but not of IL-10 (39). We describe phenotypic similar phenomena on monocytes and a strong inhibition of IFN-γ induced up-regulation of these receptors (Fig. 2). These results indicate inhibitory effects of IVIG, to which CBMO are more susceptible. This may be of exquisite importance, since CBMO are per se equipped with less co-stimulatory potential (12, 17, 18, 21); reviewed in (28).
We found a profound down-modulation of the Fcgamma III-receptor, CD16, after addition of IVIG (Table 1). Functional blockage of activating Fcgamma-receptors and up-regulation of inhibitory Fcgamma-receptors are known to trigger many effector functions of cells, including phagocytosis, cytokine production, and antibody-dependent cytotoxicity (9).
IVIG is known to inhibit proliferation of activated B and T lymphocytes. IVIG induced apoptosis in leukemic cells of monocyte lineage and in CD40-activated tonsillar B cells, involving, the CD95/CD95L-pathway via an activation of caspases (40). IVIG-induced apoptosis was higher in Fas-sensitive cells than in Fas-resistant mutant cells, and soluble Fas inhibited IVIG-induced apoptosis (40). We also found an increased T cell deletion and inhibition of T cell activation (Table 2), and proliferation in the presence of IVIG (Fig. 3), indicating functional consequences of IVIG treatment on monocyte-dependent T cell activation. Keeping in mind the critical role of HLA molecules and the co-stimulatory signals delivered by CD80 and CD86 in optimal antigen presentation and T cell activation, inhibition by IVIG offers one plausible explanation for the efficacy of IVIG in immune-mediated inflammatory conditions.
IVIG induced substantial apoptosis in CBMO, even without addition of bacteria (Fig. 4D), indicating a depletory effect for cell survival. In previous experiments, using lower concentrations of IVIG in cells cultured in fetal calf serum, we had seen the same results (data not shown). Since IVIG contains agonistic and antagonistic anti CD95-autoantibodies (40), one may suggest that mediators of the CD95-cell death family induce apoptosis even without a second stimulus. CBMO seem to be more vulnerable to this mechanism, as described with other agents too (26).
This effect also holds true for monocytes, infected with E. coli, the most common species of neonatal sepsis: PICD is a frequent consequence after bacterial phagocytosis, and has been demonstrated in monocytes (12,29) as well as here: Annexin V-binding and hypodiploid DNA indicate cell death by apoptosis (Fig. 4). With equal phagocytic activity (Fig. 1) and production of reactive oxygen species (12,29), CBMO are less sensitive towards E. coli-mediated PICD. We have shown that the CD95/CD95L-axis is involved in this process, and that CBMO up-regulate caspases-8, -9 as well as CD95L mRNA and CD95L protein to a lesser extent than PBMO (12,29). As part of this result, CBMO undergo less apoptosis than PBMO (12,29), as also shown here (Fig. 4). CBMO are not generally resistant towards apoptotic signals, since staurosporine- and mitomycin-induced cell death was equivalent to PBMO (12). Although we again found reduced PICD in CBMO (Fig. 4), their apoptotic response towards IVIG was similar to those in adults, and addition of IVIG to E. coli enhanced PICD of CBMO to adult levels (Fig. 4).
With respect to IVIG mediated effects shown here, there are several limitations: Cytotoxic effects of stabilizing sugars within IVIG preparations against monocyte/macrophage cell lines have been described (41), which we cannot rule out completely. We used an in vitro system with a relatively small sample size. Besides, due to the high sample volume required, we have not yet investigated kinetics of cord and peripheral blood of newborns longitudinally. Our model may therefore not properly reflect the neonatal situation several days postnatally. Although no infant developed early-onset infection, we cannot rule out maternal effects like multipara pregnancy or maternal medication on their immunological profile. To examine these parameters, as well as their inter-individual variation, further experiments are warranted.
Taken together, our findings strengthen previous observations that IVIG influences monocyte functions and suggest that CBMO are more susceptible to these effects than PBMO, underlining the dissimilitude of neonatal and adult monocytes. Taken into account that CBMO were found to be less sensitive towards E. coli- and GBS-induced apoptosis (12,29), addition of IVIG renders these cells more sensitive to apoptosis and bypasses the differences to monocytes from adults. IVIG-induced termination of hyper-inflammatory responses by elimination of effector cells may be the target for controlled future trials.
Acknowledgements
The authors thank Dr. Harald Abele, Bärbel Hahn, and the team of the delivery unit for help with collecting cord blood.




