A case report: Concurrent chronic myelomonocytic leukemia and T-cell large granular lymphocytic leukemia-type clonal proliferation as detected by multiparametric flow cytometry†
How to cite this article: Song S. A case report: Concurrent chronic myelomonocytic leukemia and T-cell large granular lymphocytic leukemia-type clonal proliferation as detected by multiparametric flow cytometry. Cytometry Part B 2011; 80B: 126–129.
Abstract
Background:
Chronic myelomonocytic leukemia (CMML) is a subtype of myelodysplastic/myeloproliferative neoplasm. Although several studies have reported concurrent myelodysplastic syndrome (MDS) and T-cell large granular lymphocytic leukemia (T-LGL), coexistence of CMML and T-LGL has not been characterized. We describe here a unique case of CMML plus an underlying T-LGL-type clonal proliferation in a patient with a long standing history of severe anemia and recent pancytopenia.
Methods:
Multiparametric immunophenotyping by flow cytometry was conducted using fresh peripheral blood collected in EDTA. In addition, morphologic evaluation of the peripheral blood smear and T-cell gene rearrangement studies by polymerase chain reaction (PCR) were performed.
Results:
Flow cytometric analysis revealed abnormal monocytosis with multiple aberrancies including expression of cross-lineage markers CD2 and CD56, plus reduced expression of multiple antigens. In addition, abnormal CD8+ T-cells were identified, demonstrating dim expression of CD5 and dim to complete loss of CD7. In correlation with clinical history and morphologic review, a diagnosis of CMML plus underlying abnormal CD8+ T-cell lymphoproliferation was made. The clonality of these abnormal T-cells was confirmed by T-cell gene rearrangement studies.
Conclusions:
We have identified a unique case of CMML in association with subclinical T-LGL, neither of which, alone, could fully explain the clinicopathologic features identified. Our findings suggest that the coexistence of these two entities may not be coincidental, and it is likely that they may share a common pathogenic pathway related to immune-dysregulation. © 2010 International Clinical Cytometry Society
Chronic myelomonocytic leukemia (CMML) is heterogeneous disease with characteristics of both myelodysplastic syndrome (MDS) and myeloproliferative neoplasm (MPN). According to the current WHO classification scheme (1), a diagnosis of CMML is made with the following criteria: (1) Persistent monocytosis >1 × 109 L; (2) No Philadelphia chromosome or BCR-ABL1 fusion gene; (3) No rearrangement of PDGFRA or PDGFRB; (4) Less than 20% blasts in blood and bone marrow; (5) Dysplasia in one or more myeloid lineages. Because CMML and MDS share overlapping clinicopathologic features (2, 3), diagnosis of CMML can be difficult and requires multidisciplinary studies including immunophenotypic analysis by multiparametric flow cytometry.
A few studies have reported that MDS is occasionally seen in concurrence with T-cell large granular lymphocytic leukemia (T-LGL) (4-7), a clonal disorder characterized by persistent increase in the number of large granular lymphocytes (LGL) (between 2 to 20 × 109 L) in peripheral blood without a clearly identifiable cause (8). Both MDS and T-LGL are marrow failure disorders that are manifested by variable degree of cytopenias. A common mechanism of pathogenesis has been suggested in cases of concurrent MDS and T-LGL by occasional studies (6, 7). To our best knowledge, no coexistent CMML and T-LGL or T-LGL-type clonal proliferation has been characterized so far.
We report a unique case of CMML with an underlying T-LGL-type clonal proliferation, and describe its clinical presentation plus pathologic studies including laboratory data, morphologic evaluation, as well as detailed diagnostic findings by multiparametric flow cytometry.
CASE REPORT
A 75-year-old gentleman presented to a hematologist at UCLA in 2008 with a long standing history of refractory anemia for 10 years, and was found to have a more recent pancytopenia for about 1 year. His anemia was severe, requiring frequent transfusion support and administration of Procrit. At the time of presentation at UCLA, the CBC data revealed pancytopenia with WBC at 3.5 × 103 μL with 25% neutrophils (neutropenia), RBC 2.3 × 106 μL, Hgb 7.3 g dL−1, Hct 22.7%, and Plt 21 × 103 μL. The differential count was 25% neutrophils, 1% eosinophils, 1% basophils, 39% lymphocytes, and 34% monocytes, with an absolute monocyte count of 1.2 while lymphocyte count of 1.4. Review of prior CBC results indicated absolute monocytosis dated back to 2004. Prior to 2008, the patient had had two bone marrow biopsies performed at outside institutions, both of which were apparently nondiagnostic. A bone marrow biopsy collected at UCLA in 2008 showed hypercellular marrow with markedly reduced erythropoiesis, myeloid, and megakaryocytic dysplasia, plus 9% myeloblasts. Cytogenetic studies were normal. These features were, at the time, thought to be consistent with MDS/refractory anemia with excess blasts-1 (RAEB-1).
Several months later, a peripheral blood sample was submitted for flow cytometry. Results of multiparametric immunophenotyping by flow cytometry demonstrated abnormal monocytosis (35% of the total) with multiple aberrancies (Fig. 1A) including expression of cross-lineage markers CD2 and CD56, significantly reduced expression of CD15, heterogeneously reduced coexpression of CD14 and CD64, as well as reduced CD11b and HLA-DR. In addition, two abnormal subsets of CD8+ T-cells were detected, accounting for ∼50% of the T-cells (10% of the total). One subset revealed expression of dim CD5 and dim CD7, while the other showed dim CD2, bright CD3, dim CD5, dim CD57, and complete loss of CD7. Morphologic review of the peripheral blood smear demonstrated pancytopenia, absolute and abnormal monocytosis, frequent large granular lymphocytes (LGL), plus occasional pseudo Pelger-Huët anomaly (Fig. 1B). There was no increase of blasts. In correlation with clinical history, the CBC data of persistent absolute monocytosis, as well as morphologic features of the blood smear, and flow cytometric results, a diagnosis of CMML with an underlying CD8+ T-cell lymphoproliferative disorder was made. Follow-up PCR studies of the peripheral blood were positive for clonal T-cell gene rearrangement. Subsequent bone marrow biopsy collected in 2009 illustrated similar findings to those seen in 2008, and cytogenetic studies were again normal.
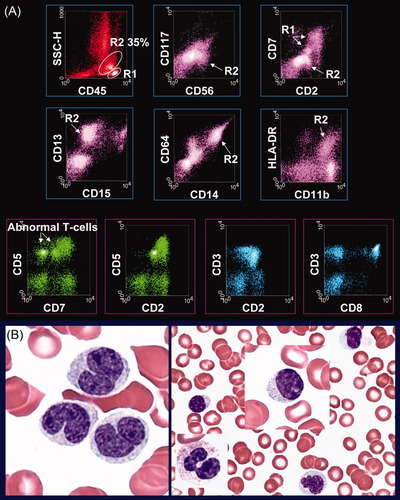
Peripheral blood evaluation by flow cytometry and morphology. A: Results of multiparametric immunophenotyping by flow cytometry. The open gate display (the top two panels) reveals abnormal monocytosis (R2—35% of the total) with cross-lineage aberrancies of CD2 and CD56, significantly reduced expression of CD15, heterogeneously reduced coexpression of CD14 and CD64, plus reduced CD11b and HLA-DR. Abnormal lymphocytes (R1) are also noted. The lymphocyte gate display (the bottom panel) shows two abnormal subsets of CD8+ T-cells (50% of the T-cells; 10% of the total) with dim expression of CD5 and dim to complete loss of CD7. B: Morphologic examination of the peripheral blood demonstrates pancytopenia, absolute and abnormal monocytosis, frequent large granular lymphocytes, plus occasional pseudo Pelger-Huët anomaly. No increased blasts are detected. [Color figure can be viewed in the online issue which is available at wileyonlinelibrary.com.]
DISCUSSION
The diagnosis of CMML can be challenging because of its clinicopathologic presentations, often intersecting with those of MDS. Presence of blood monocytosis, the hallmark of CMML (1), may sometimes be overlooked by clinicians as well as pathologists, especially in the setting of neutropenia or pancytopenia as demonstrated in this case, which can be a compounding factor to the diagnostic impediments. Considering the long history of severe refractory anemia and more recent onset of pancytopenia, as well as the marrow findings of erythroid aplasia, myeloid and megakaryocytic dysplasia, and 9% myeloblasts, a diagnosis of MDS/RAEB-1 seems quite logical. Besides the clinical and morphologic similarities between MDS and CMML, a recent flow cytometric study (3) has shown only one statistically significant difference in the immunophenotype of MDS and CMML, while multiple differences are identified between MPN and CMML.
Although a few studies have reported the concurrence of MDS and T-LGL, an association of CMML with T-LGL or T-LGL-type clonal proliferation has not been described specifically so far. Using an approach of multidisciplinary correlation with clinical history, thorough review of the previous CBC data, and careful evaluation of the morphologic features, the results of multiparametric flow cytometry on the peripheral blood sample are diagnostic of CMML. The immunophenotypic features of CMML by flow cytometry have been described in recent publications (9-12). Interestingly, further flow cytometric analysis and follow-up PCR studies reveal an underlying T-LGL-type clonal proliferation of cytotoxic T-cells, even though the patient does not have clinically apparent T-LGL. A very recent study of concurrent MDS and T-LGL cases has also found several patients who do not have T-LGL by clinical criteria (7). However, the coexistence of CMML and T-LGL-type clonal proliferation in the present case may not be by chance, since neither of the two, by itself, could fully explain the clinicopathologic findings. For example, it is uncommon for CMML patients to present with a long standing history of severe anemia that requires frequent transfusion and administration of Procrit. At the same time, although T-LGL is known to be associated with acquired red cell aplasia (13-17), which is evident in this case with the history of severe anemia and lack of erythropoiesis in the marrow, it could not explain the significant dysplasia observed in megakaryocytes and some myeloids.
The pathogenic mechanism of marrow failure disorders like MDS and T-LGL is not clear. Combined clinical and experimental data suggest that immune dysregulation mediated by expanded cytotoxic T-cell clones, may result in intrinsic stem cell defect and suppression of normal hematopoiesis (18-21). These studies, along with the two largest reported cases of nine patients with concurrent MDS and T-LGL (6, 7), corroborate an etiologic link between the two entities. Our findings imply that the simultaneous CMML and T-LGL-type clonal proliferation may not be coincidental, further supporting the proposed hypothesis that a common pathway of pathogenesis may contribute to the development of both disorders. However, additional studies with larger case numbers are needed to advance our understanding of this distinctive occurrence of simultaneous CMML and T-LGL.




