Immunologic features of HIV-1-infected women on HAART at delivery† ‡
Presented in part at the 21st Annual Clinical Cytometry Meeting and Course, Long Beach, CA, October 6–10, 2006.
How to cite this article: Ono E, dos Santos AMN, Machado DM, de M Succi RC, Amed AM, Salomão R, Kallás EG, de Moraes-Pinto MI. Immunologic features of HIV-1-infected women on HAART at delivery. Cytometry Part B 2008; 74B: 236–243.
Abstract
Background:
The conjoint effect of HIV infection and pregnancy on the immune system of women submitted to the prophylactic antiretroviral therapy presently recommended is still poorly understood.
Methods:
We evaluated 44 HIV-infected women (HIV) and 45 HIV-negative women (CT) at parturition and we compared them to 20 healthy nonpregnant women (NP). Immunophenotyping of lymphocytes was done by four-color flow cytometry.
Results:
All HIV-infected women received HAART during pregnancy and 56.8% had viral load <50 copies/mL at delivery. CD4+T cells/mm3 were lower in HIV (447) than CT (593) and NP (738) (P < 0.05). CD8+T cells/mm3 were higher in HIV (799) than CT (384) and NP (395) (P < 0.05). NK cells/mm3 were lower in HIV (146) than in CT (253) and NP (198) (P < 0.05). CD38 expression on CD4+T and on CD8+T cells was higher in HIV (CD4:12.1; CD8:14.9) than in CT(CD4:9.2; CD8:10.2) and NP(CD4:8.6; CD8:6.0) (P < 0.05). However, CD56 expression on CD8+T cells (a marker of cytolytic effector function) was lower in HIV(7%) than in CT(12%) and NP(9%) (P < 0.05).
Conclusions:
Even with low levels of viremia, HIV-infected women at delivery showed a different immunologic profile from both healthy non-HIV-infected women in the puerperium and nonpregnant women, with lower CD4+T and higher CD8+T cells, high levels of CD38 expression, but low CD56 expression on CD8+T cells and low NK cell numbers. © 2008 Clinical Cytometry Society
Over the past few years, we have seen an increase in the epidemic of HIV/AIDS among women. Today worldwide, females account for nearly half of the 38.6 million people living with HIV/AIDS (1).
In Sub-Saharan Africa, females constitute 59% of HIV-infected individuals, many of whom are of childbearing age (1). In resource-rich countries, although men still outnumber women among HIV-infected individuals (2), higher rates of pregnancy have actually been observed in HIV-infected women over the last years (3). Factors such as the clinical improvements in the treatment of HIV disease and an increase in longevity due to the use of highly active antiretroviral therapy (HAART) as the standard of care may have increased the chances of pregnancy in HIV-infected women. On the other hand, the decrease in vertical HIV infection to rates as low as 0.99% due to prophylactic interventions to prevent mother-to-infant transmission (4), may have influenced the reproductive decisions of that population.
Many immunologic modifications occur during pregnancy, and they are essential for its success (5, 6). The conjoint effects of HIV infection and pregnancy on the maternal immune system are still poorly understood, but a previous study suggested an increased immune activation on HIV-infected women throughout pregnancy (7). However, no data is available on the immunologic profile of HIV-infected women who received HAART during pregnancy.
The objective of this study was to analyze the peripheral blood mononuclear cells in HIV-infected parturient women on HAART and to compare them with HIV-soronegative parturient women and healthy nonpregnant women. All HIV-infected parturient women were on triple therapy and 84.1% were submitted to elective cesarean sections. Using flow cytometry analyses, we evaluated peripheral blood mononuclear cells, including CD34+ progenitors, B, NK, CD4+ T and CD8+ T subsets, their subpopulations and activation markers, and we compared them with peripheral blood cells from HIV-seronegative parturient women and from healthy nonpregnant women.
METHODS
Patients and Study Design
The protocol was approved by the Ethics Committee of the Federal University of São Paulo. All women gave written informed consent prior to enrolment in the study. Eighty-nine parturients were evaluated from March 2004 to October 2005. HIV-1-infected women (HIV, n = 44) and HIV-seronegative women (CT, n = 45) were assessed on admission to hospital just before delivery at Maternity units linked to the Federal University of São Paulo, Brazil.
Twenty healthy HIV-seronegative nonpregnant women (NP) were also evaluated and compared with the other two groups.
Information collected included age, number of gestations, type of delivery (for pregnant women) antiretroviral treatment, and viral load (for HIV group).
Blood Collection
A single blood sample (10 mL) was drawn by peripheral venous puncture from each individual; for pregnant women, blood was collected at admission to the maternity hospital. Samples were put into EDTA-treated vacuum tubes for complete blood count (CBC), phenotypic analysis of peripheral blood mononuclear cell subsets using the flow cytometry assays and plasma IL-7 assessment.
Complete Blood Count
All samples were tested using Advia 120 automatic counter (Bayer, Germany) and confirmed by a slide smear performed for manual count on the same blood sample used for flow cytometry analysis within a few hours of blood collection.
Flow Cytometry Analysis
Blood mononuclear cells were assessed by flow cytometry (FACSCalibur, BD Biosciences, USA) within 18 h of blood collection and analyzed using the CellQuest software (BD Biosciences, USA). Four-color phenotypical characterizations were performed in fresh blood using lyse-wash protocol. Appropriate isotypic controls (IgG1-FITC, IgG1-PE, IgG1-APC, IgG2a-PE) (BD Biosciences, USA) were used to evaluate nonspecific staining. For each sample, multiparametric data were acquired for 10,000 events.
Cell numbers per cubic milliliter of blood were obtained using the lymphocyte counts from CBC.
Cell Subsets
CD4+ and CD8+ T lymphocytes
The markers to assess the subpopulations within the CD4+ (CD3-APC and CD4-PerCP labeled) and CD8+ (CD3-APC and CD8-PerCP labeled) populations were CD45RA-FITC and CCR7-PE (BD, USA). In both the CD3+CD4+ and the CD3+CD8+ populations, the “naïve” cells were CD45RA+CCR7+. The “central memory” cells were CD45RA-CCR7+ and the “effector memory” cells were CD45RA-CCR7-. Finally, the “terminally differentiated memory” cells, more abundantly seen among CD8+ cells than in the CD4+ cohort, were CD45RA+CCR7- (8, 9).
CD4+ and CD8+T lymphocyte activation
Antibodies used to evaluated T cell activation were: CD3-APC, CD4-PerCP or CD8-PerCP, CD38-FITC and CD25-PE or CD56-PE. CD38 expression was evaluated using the mean fluorescence intensity (MIF) of single parameter histograms with no cutoff setting. CD25 expression was evaluated in CD4+ T cells and CD56, in CD8+ T cells. For those markers, the percentage of positive cells was assessed with the use of isotype controls.
B lymphocytes and NK cells
B lymphocytes were identified as CD3-CD19+ cells. NK cells were quantified by the CD45+CD3-CD56+CD16+ phenotype.
CD34+ progenitors
Progenitor cells were identified as CD45+CD34+ cells.
Figure 1 shows an illustrative example of flow cytometry plots used for data analysis.

Illustrative example of flow cytometry plots used for data analysis.
Detection of HIV Antibodies in Nonpregnant Women
Commercially available indirect ELISA kits were used to assess HIV (Detect HIV™ V2; ADALTIS; Canada) according to the instructions of the manufacturer.
Measurement of Plasma IL-7
IL-7 was measured in plasma samples using a high sensitivity enzyme-immunosorbent assay (ELISA) kit (Quantikine HS human IL-7; R&D; USA) according to the instructions of the manufacturer. The minimum detectable dose of IL-7 by this kit is typically less than 0.1 pg/mL.
Statistical Analysis
Group characteristics at study entry were compared using ANOVA, t-test, and Chi-square test. For peripheral blood leucocytes and phenotype analyses, ANOVA was used, with multiple comparisons performed by Tukey test. Logarithmic transformation was done when necessary in order to normalize the distribution. Level of significance was set at P < 0.05.
RESULTS
Characteristics of Study Subjects
HIV-infected mothers were older than HIV-negative women and nonpregnant women (mean age: 29.6 years, 25.2 years, and 24.7 years; Tukey, P = 0.01 for the first comparison and P = 0.01 for the second comparison). All 44 women received highly active antiretroviral prophylaxis during gestation, which consisted of two nucleoside reverse transcriptase inhibitors (NRTI) and one protease inhibitor in 29/44 (65.9%) of the women and of two NRTI and one non-nucleoside reverse transcriptase inhibitor (NNRTI) in 15/44 (34.1%) of the women. Fifteen out of the 44 HIV-infected women (34.1%) received some antiretroviral treatment before gestation, which was then modified in order to reduce viral load in some of them or to avoid toxic drugs, e.g., efavirenz. Twenty-eight out of 44 (63.6%) reached HIV viral load levels below 400 copies/mL and maintained those levels for 10.7 weeks in mean during gestation. Viral load at delivery was below 50 copies/mL in 25 of 44 (56.8%). Mean viral load in the other 19 HIV-infected women was 1569.4 copies/mL. Elective caesarean section was performed in 37 out of 44 of the HIV-positive women (84.1%) and in 3 out of 45 (6.7%) women from the control group. The characteristics of study subjects are summarized in Table 1.
| Characteristic | HIV (N = 44) | CT (N = 45) | NP (N = 20) | P value |
|---|---|---|---|---|
| Mean age in years (range) | 29.6 (17–40) | 25.2 (15–38) | 24.7 (15–34) | 0.01a,b* |
| Women on HAART prophylaxis (%) | 44/44 (100%) | |||
| Mean gestational age in weeks at the introduction of HAART (range) | 19.5 (0–36.3) | |||
| Mean gestational age in weeks at first HIV viral load <400 copies/mL (range) | 28.2 (1–39.1) | |||
| Women who reached viral load <400 copies/mL during pregnancy (%) | 28/44 (63.6%) | |||
| Mean weeks of gestation since viral load <400 copies/mL (range) | 10.7 (1–35.7) | |||
| Women with HIV viral load <50 copies/mL at delivery | 25/44 (56.8%) | |||
| Mean viral load in women with >50 copies/mL at delivery (range) | 1569.4 (55–5660) | |||
| Caesarean section | 44/44 (100%) | 9/45 (20%) | <0.001** | |
| Elective caesarean section | 37/44 (84.1%) | 3/45 (6.7%) | <0.001** |
- * Tukey: a-HIV × CT; b-HIV × NP; c- CT × NP; d-all significant.
- ** Chi-Square test.
Peripheral Blood Leucocytes
HIV-infected mothers had lower mean white blood cells when compared to HIV-seronegative mothers, but higher mean values if compared with nonpregnant women (HIV:8800 cells/mm3; CT:12,300 cells/mm3; NP: 6600 cells/mm3, Tukey; P = 0.01 for both comparisons). All 3 groups differed in hemoglobin levels, with the lowest values being found in the HIV-infected mothers (HIV: 11.0 g/dL; CT: 12.0 g/dL; NP: 13.4 g/dL; Tukey; P = 0.01 for all comparisons). Highest neutrophil values were found among HIV-seronegative mothers when compared to the two other groups (HIV: 6594 cells/mm3; CT: 10,700 cells/mm3; NP: 4200 cells/mm3; Tukey; P = 0.01). Monocytes were elevated in HIV-seronegative mothers when compared to nonpregnant women (HIV: 423 cells/mm3; CT: 500 cells/mm3; NP: 400 cells/mm3; Tukey; P = 0.034). Basophil numbers were lower in HIV-infected mothers when compared to HIV-seronegative mothers (HIV: 37 cells/mm3; CT: 49 cells/mm3; NP: 33 cells/mm3; Tukey; P = 0.02). Lymphocytes and platelets did not differ among the three groups (Table 2).
| Characteristic | HIV (N = 44) | CT (N = 45) | NP (N = 20) | ANOVA/ Tukey (P) |
|---|---|---|---|---|
| White blood cells (cells/mm3) | 8800 (2060–18900) | 12300 (5600–25100) | 6600 (3800–10000) | 0.01a,c |
| Hemoglobin (g/dL) | 11.0 (8.0–15.3) | 12.0 (6.6–15.4) | 13.4 (11.4–15.0) | 0.01d |
| Platelets/mm3 | 224727 (110000–395000) | 231300 (94000–408000) | 251400 (150000–375000) | 0.375 |
| Neutrophils (cells/mm3) | 6594 (1300–17200) | 10700 (3800–23900) | 4200 (2200–6500) | 0.01a,c |
| Monocytes (cells/mm3) | 423 (150–1190) | 500 (200–800) | 400 (200–700) | 0.034c |
| Basophils (cells/mm3) | 37 (20–120) | 49 (10–106) | 33 (0–70) | 0.02a |
| Lymphocytes (cells/mm3) | 1799 (290–8900) | 1700 (500–10600) | 1800 (900–2500) | 0.416 |
- Mean values and ranges are shown.
- a-HIV × CT; b-HIV × NP; c- CT × NP; d-all significant.
T Cells, CD4+ T Cells and CD8+ T Cells, B Cells, NK Cells and CD45+CD34+ Progenitors
Mean CD3+T cell numbers did not reach a statistically significant difference in the 3 groups of women. Mean CD4+T cells were lower in HIV-infected mothers when compared with HIV-seronegative mothers and nonpregnant women (HIV: 447.1 cells/mm3; CT: 593.2 cells/mm3; NP: 738.3 cells/mm3; Tukey; P = 0.02, P = 0.01, respectively). Mean CD8+T cells were higher in HIV-infected mothers when compared to both HIV-seronegative mothers and nonpregnant women (HIV: 799.9 cells/mm3; CT: 384.8 cells/mm3; NP: 361.7 cells/mm3; Tukey; P = 0.01, P = 0.02, respectively). Mean B cells were higher in nonpregnant women when compared with both HIV-infected mothers and HIV-seronegative mothers (HIV: 168.6 cells/mm3; CT: 173.9 cells/mm3; NP: 230.9 cells/mm3; Tukey; P = 0.04, P = 0.03, respectively). NK cells were lower in HIV-infected mothers if compared to both HIV-seronegative mothers and nonpregnant women (HIV: 146.8 cells/mm3; CT: 253.0 cells/mm3; NP: 198.2 cells/mm3; Tukey; P = 0.01, P = 0.02, respectively). Mean CD45+CD34+ progenitors were higher in HIV-seronegative mothers than in HIV-infected mothers and nonpregnant women (HIV: 21.8 cells/mm3; CT: 46.5 cells/mm3; NP: 15.5 cells/ mm3; Tukey; P = 0.01, for both comparisons) (Table 3).
| Subset (cells/mm3) | HIV (N = 44) | CT (N = 45) | NP (N = 20) | ANOVA/Tukey (P) |
|---|---|---|---|---|
| CD3+ T cells | 1297.2 (115.0–6983.8) | 975.3 (218.6–5046.7) | 1189.4 (559.6–1695.1) | 0.053 |
| CD4+ T cells | 447.1 (45.3–2038.1) | 593.2 (117.7–2887.4) | 737.8 (347.0–1049.7) | 0.02a; 0.01b |
| CD8+ T cells | 799.9 (46.8–4698.3) | 384.8 (74.0–2071.2) | 395.2 (139.7–717.8) | 0.01a; 0.02b |
| B cells | 168.6 (27.2–496.6) | 173.9 (27.9–1134.2) | 230.8 (98.3–524.4) | 0.04a; 0.03c |
| NK cells | 146.8 (23.3–659.2) | 253.0 (44.6–1764.9) | 198.2 (76.5–336.6) | 0.01a; 0.02b |
| CD45+CD34+ | 21.8 (2.6–111.8) | 46.5 (7.4–169.4) | 15.5 (4.8–28.0) | 0.01a,c |
- Mean values and ranges are shown.
- a-HIV × CT; b-HIV × NP; c- CT × NP; d-all significant.
CD4+ and CD8+ T Cells Subsets
When CD4+ and CD8+ T cell subsets were compared among groups using CD45RA and CCR7 markers, nonpregnant women had higher terminally differentiated CD4+ T cells (Fig. 2A) than HIV-infected mothers (HIV:9.0%; CT:6.2%; NP:13.6%; Tukey; P = 0.01). HIV-infected mothers had lower naïve CD8+ T cells (Fig. 2B) than the other two groups (HIV: 16.2%; CT: 27.1%; NP: 24.9%; Tukey; P = 0.02, P = 0.01, respectively). Higher effector memory CD8+ T cells (Fig. 2B) were found in HIV-infected mothers when compared with the two other groups of women (HIV: 53.9%; CT: 40.9%; NP: 39.4%; ANOVA; P = 0.01, for both comparisons).
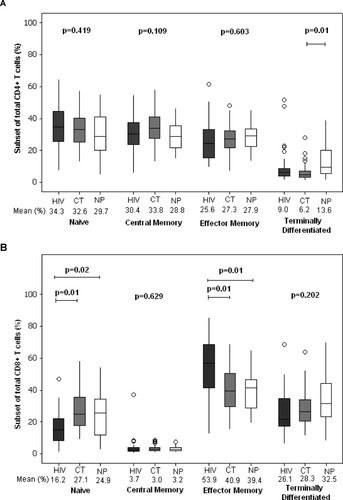
CD4+ (A) and CD8+ T cell subsets (B) using CCR7 and CD45RA markers in HIV-infected mothers (HIV), HIV-seronegative mothers (CT) and control healthy uninfected nonpregnant women (NP).
Expression of Activation Markers on CD4+ and CD8+ T Cells
When assessed for activation markers, a higher mean fluorescence intensity of CD38 was observed in CD4+ T cells and CD8+ T cells of HIV-infected mothers when compared to both HIV-seronegative mothers and nonpregnant women (MFI in CD4+; HIV:12.1; CT:9.1; NP:8.6; Tukey, P = 0.01, for all comparisons) (MFI in CD8+; HIV: 14.9; CT: 10.2; NP: 6.0; Tukey; P = 0.01 for both comparisons) (Fig. 3A).
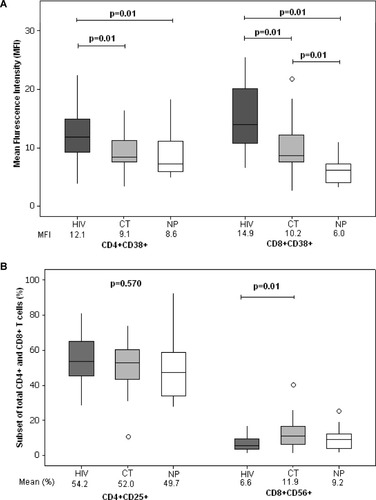
Mean fluorescence intensity (MFI) of CD38+ cells in CD4+ and CD8+ T cells (A) and expression of CD25 in CD4+ T cells and CD56 in CD8+ T cells (B) in HIV-infected mothers (HIV), HIV-seronegative mothers (CT) and control healthy uninfected nonpregnant women (NP).
For both HIV and CT group, no increase in the CD38 MFI was observed for women with more than 3 gestations when compared with women with 3 or less gestations. Actually, for CT group, women with multiple gestations had even lower CD38 MFI for both CD4+ and CD8+ T cells than those with a smaller number of gestations (Table 4).
| HIV(N = 29) ≤ 3 gestations | HIV (N = 15) > 3 gestations | NP (N = 20) | ANOVA/Turkey | |
|---|---|---|---|---|
| CD4+CD38+ MFI | 12.1 | 12.2 | 8.6 | 0.01b; 0.02c |
| CD8+CD38+ MFI | 14.8 | 15.0 | 6.0 | 0.01b,c |
| CT (N = 33) ≤ 3 gestations | CT (N = 12) > 3 gestations | NP (N = 20) | ANOVA/Turkey | |
| CD4+CD38+MFI | 9.9 | 7.0 | 8.6 | 0.01d |
| CD8+CD38+MFI | 11.2 | 7.6 | 6.0 | 0.01d,e |
- Healthy control non-pregnant women (NP) were used as controls.
- a-HIV ≤ 3gestations × HIV > 3gestations; b-HIV ≤ 3gestations × NP; c- HIV > 3gestations × NP; d- CT ≤ 3gestations × CT > 3gestations; e-CT ≤ 3gestations × NP; f- CT > 3gestations × NP; g-all significant.
CD4+CD25+ T cells did not differ among the three groups. Interestingly, higher mean CD8+CD56+ T cells were found in HIV-seronegative mothers and that was statistically significant when compared to HIV-infected mothers (HIV: 6.6%; CT: 11.9%, NP: 9.2%; Tukey; P = 0.01) (Fig. 3B).
Interleukin 7
Plasma IL-7 concentrations did not differ among the three groups of women, although HIV-infected parturients showed a tendency to higher values (HIV: 7.2 pg/mL; CT: 5.4 pg/mL; NP: 3.9 pg/mL; Tukey; P = 0.066).
DISCUSSION
We have shown that, despite the use of HAART during pregnancy and low or undetectable HIV viral load at delivery, HIV-infected women present a different immunologic profile from HIV-seronegative parturients and control nonpregnant women.
HIV-infected parturients had lower hemoglobin levels and neutrophil counts than the other two groups of women. Anemia was found in some women on zidovudine prophylaxis, especially where it was administered in high doses. Neutropenia, on the other hand, was seen less frequently (10). As neutrophils are directly involved in the control of infection caused by extracellular bacteria, it is possible that the low neutrophil counts might be related to higher rates of infection at parturition in HIV infected women (11).
Similar to our results, other authors have observed high leucocyte numbers close to parturition in healthy women, and have considered these changes as physiologic (12).
Monocytes and basophils were higher in seronegative parturients. We did not find any similar data in the literature in relation to pregnancy. It is known that basophils are elevated in conditions associated with Th2 responses (13). As pregnancy has been associated with a Th2 switch of the immune system (5), that would not be unexpected. Interestingly, we found that HIV-infected parturients on HAART had lower basophil counts than HIV-seronegative parturients, suggesting a return of the Th1 profile with a lower viral load (14).
The analysis of the lymphocytes showed that HIV-infected women had lower CD4+T cells and higher CD8+T cells than the other two groups of women. That is in accordance with HIV infection pathogenesis with the destruction of CD4+ T cells (15, 16). Even after 12 months of HAART and viral replication control, CD4+ T cells do not reach values comparable to age-matched controls (17). Similarly, CD8+ T cells might remain moderately elevated despite HAART (18, 19).
NK cell numbers were lower in HIV-infected women than in the other two groups of women. Other studies have already noticed that low NK cell numbers in HIV-infected individuals were associated with viral replication (20). Reduced NK cell cytolytic activity has been found in HIV infection as well (21, 22).
Our results also showed reduced numbers of CD34+ progenitors in peripheral blood of HIV-infected parturients. Low CD34+ cells in the bone marrow of HIV-infected patients have already been found, even after HAART (23), Others have suggested that programmed cell death of CD34+ cells might take place during HIV infection (24, 25). That seems of especial relevance during pregnancy, because it is a condition when cell production is most necessary.
When analysis of T cell subsets was performed, both HIV-infected and HIV-seronegative parturients showed lower percentages of terminally differentiated CD4+ T cells (CD45RA+ CCR7-), suggesting an effect of pregnancy. Functional studies assessing cytokine production by these cells should be performed to investigate further the practical implications of that finding.
As for CD8+ T cells, HIV-infected parturients had lower percentages of the naïve subset and higher percentages of the effector memory subset, which probably indicates an effort to fight HIV infection, possibly with a blockage of differentiation to terminally differentiated CD8+ T cells (CD45RA+ CCR7-) (26).
Consistent with a previous study (7), HIV-infected women had the highest levels of CD38 expression in both CD4+ T and CD8+ T cells, a marker of immune activation. HIV-seronegative parturients also showed higher levels of CD38 than nonpregnant women, although lower levels when compared to HIV-infected women.
We then evaluated CD38 expression in CD4+ and CD8+ T cells in parturients classified according to the number of gestations. Women with more than 3 gestations did not show higher CD38 expression on lymphocytes than those who got pregnant 3 times or less. That spoke against an increase in the state of immune activation triggered by gestation.
Despite the high activation state, the low percentage of CD56+ cells among CD8+ T cells of HIV-infected parturients, consistent with a low cytolytic activity of those cells (27), suggests a poor effective control of the infection.
We have also assessed plasma IL-7 levels in the three groups of women. It is known that IL-7 is a cytokine involved in the maintenance of homeostasis of CD4+ T cells. Conditions associated with lymphopenia such as HIV infection lead to a rise in plasma IL-7 levels (28). When lymphocyte numbers augment in response to antiretroviral treatment, CD4+ T cells increase and IL-7 levels are reduced. In our study, plasma IL-7 values were not statistically different among the three groups of women. This might indicate an increase in total CD4+ T cells for many HIV-infected women after the introduction of antiretroviral treatment (29).
It is important to emphasize, however, that antiretroviral prophylaxis introduced or modified during pregnancy aims to reduce mother-to-child-transmission of HIV infection (4, 30). Since none of the children born to those 44 HIV-infected women were HIV-infected, that was a fully accomplished objective. On the other hand, because antiretroviral drugs are often modified or introduced in the second trimester, most pregnant women do not have enough time on a maintained undetectable HIV viral load to show expressive immune reconstitution. In our study, a sustained viral load under 400 copies/mL was achieved in 63.6% of the women for a mean time of 10.7 weeks.
Our conclusion is that despite good virologic control, many HIV-infected women on HAART during pregnancy still present clear immunologic alterations when compared to HIV-seronegative parturients and nonpregnant women. As recent publications stress the link between immune activation and progression to AIDS (31), it is reassuring to observe that no cumulative effect of CD38 expression on lymphocytes occurs with multiple pregnancies. However, further work should help clarify whether the conjoint effects of pregnancy and HIV infection might have other long-term consequences not noticeable in a transversal evaluation such as the present study.




