Different levels of CD52 antigen expression evaluated by quantitative fluorescence cytometry are detected on B-lymphocytes, CD 34+ cells and tumor cells of patients with chronic B-cell lymphoproliferative diseases
Abstract
Background:
The success of treatment using monoclonal antibodies in oncology is influenced by, among other factors, the level of target antigen expression on tumor cells. The authors analyzed the intensity of the CD52 antigen expression in patients with chronic lymphoproliferative diseases and compared them with B-lymphocytes of a healthy population and CD34+ cells in peripheral blood stem cells (PBSC) grafts.
Methods:
Recently diagnosed and previously untreated patients with B-cell chronic lymphocytic leukemia (B-CLL), mantle-cell lymphoma (MCL), or small lymphocytic lymphoma (SLL) were evaluated and compared with control group and CD34+ cells. The intensity of CD52 was expressed in molecules of equivalent soluble fluorochrome units (MESF) and antibody-binding capacity (ABC).
Results:
In the group of patients with B-CLL, the CD52 level on tumor cells (245 × 103 MESF; 107 × 103 ABC) was significantly lower than on B-lymphocytes of the control group (446 × 103 MESF; 194 × 103 ABC; P < 0.001) and SLL tumor cells (526 × 103 MESF; 229 × 103 ABC; P < 0.001). The CD52 antigen was expressed on a majority of CD34+ cells, but its expression intensity was low (101 × 103 MESF; 44 × 103 ABC).
Conclusions:
Our data demonstrate differences in the intensity of the CD52 antigen expression between B-lymphocytes and tumor lymphocytes of B-CLL patients, and between B-CLL and SLL tumor cells. CD52 antigen is expressed at low level on CD34+ cells. © 2007 Clinical Cytometry Society
Applications of monoclonal antibodies in clinical medicine have become widespread since 1979, when they were first used in treating patients (1), and today they are being applied to treat both malignant and nonmalignant diseases (2-8). Monoclonal antibodies are used in hematooncology in graft versus host disease (GvHD) treatment and in purging malignant cells in vitro (9, 10, 11, 12).
CD52 antigen is a glycosylphosphatidylinositol (GPI) bound glycoprotein of very low molecular weight (21–28 kDa) (13). This antigen exists in two forms, CD52-I and CD52-II, both recognized by Campath-1H antibody (14). CD52 antigen is found on the surface of the majority of lymphocytes, macrophages, monocytes, and eosinophils in relatively high density (15, 16).
Campath-1H (alemtuzumab) is the humanized version of Campath-1 and causes cell death by complement-dependent cytotoxicity (CDC) or by antibody-dependent cell cytotoxicity (ADCC) (17, 18, 19). Alemtuzumab is used today in the treatment of B-cell chronic lymphocytic leukemia (B-CLL) (6, 20, 21), prolymphocytic leukemia (PLL) (2, 21, 22), Waldenstrom's macroglobulinemia (23), T-cell large granular lymphocyte leukemia (7), and T-cell non-Hodgkin's lymphomas (3). Therapeutic potential of alemtuzumab was also suggested in multiple myeloma, primary systemic amyloidosis and monoclonal gammopathies of undetermined significance with high expression of the clonal CD38+CD45+ plasma cells (24).
Some publications suggest that a treatment's success may depend on the antigen expression intensity on the target cells and their binding affinity to monoclonal antibodies (25, 26). In these cases, quantitative flow cytometry is being used. However, different approaches are included in this category. Determination of molecules of equivalent soluble fluorochrome (MESF), used in our work, is based on comparison of the fluorescence intensity of fluorochrome-labeled particles with the fluorescence intensity of fluorochrome-labeled antibodies bound on cells from sample. MESF values, unlike antibody-binding capacity (ABC) units, do not provide information about the precise amount of bound ligand on the cells (27). Nevertheless, the use of this unit is still appropriate in clinical practice, especially as its determination is easy and quick.
The presence of CD52 antigen on CD34+ cells is not yet clear. Although some studies deny its expression on hematopoietic cells (28, 29), others have proven its presence both on CD34+ hematopoietic cells (30) and on CD34+ cells in the blastic phase of chronic myeloid leukemia (31). Because of these controversial results, we decided to investigate CD52 antigen expression on CD34+ cells quantitatively to provide clarification.
Small lymphocytic lymphoma (SLL) and B-CLL are known to express the same antigens, although their intensity of expressions may differ. The CD52 expression intensity in mantle cell lymphoma (MCL) had not to date been analyzed quantitatively. In our study, B-CLL, SLL, and MCL patients were examined for CD52 levels and the impact of results for clinical application is discussed. In addition, the CD52 antigen expression in CD34+ cells from autologous peripheral blood stem cells was analyzed.
MATERIALS AND METHODS
Patients
Peripheral blood samples from a total of 55 patients and 9 healthy subjects were evaluated. B-CLL, with 36 cases, was the prevalent diagnosis, while other evaluated diagnoses included SLL (10 cases) and MCL (9 cases). The diagnoses were determined based on clinical criteria, cell morphology, histology, and immunophenotyping according to WHO classification. In B-CLL diagnosis, criteria of the International Workshop on Chronic Lymphocytic Leukemia (IW-CLL) were used. Only previously untreated, recently diagnosed patients were evaluated. More detailed information is presented in Table 1.
| Case | Diagnosis | Age | Sex | Clinical stage | Lymphocyte count (×109/l) | Percentage of CD5+19+ cells in peripheral blood | Percentage of CD5+19+ cells in bone marrow |
|---|---|---|---|---|---|---|---|
| 1 | B-CLL | 56 | M | 2 | 30.7 | 60.1 | |
| 2 | B-CLL | 43 | M | 1 | 17.3 | 75.5 | 73.0 |
| 3 | B-CLL | 67 | M | 2 | 23.1 | 66.5 | |
| 4 | B-CLL | 39 | M | 2 | 485.0 | 96.9 | |
| 5 | B-CLL | 59 | M | 2 | 157.3 | 97.6 | |
| 6 | B-CLL | 72 | F | 1 | 11.2 | 79.9 | |
| 7 | B-CLL | 72 | M | 1 | 78.5 | 93.8 | |
| 8 | B-CLL | 60 | M | 0 | 43.0 | 92.3 | 68.8 |
| 9 | B-CLL | 58 | M | 1 | 43.3 | 94.7 | 93.3 |
| 10 | B-CLL | 69 | M | 0 | 16.4 | 90.2 | 73.4 |
| 11 | B-CLL | 88 | M | 3 | 22.3 | 90.1 | |
| 12 | B-CLL | 58 | M | 1 | 7.1 | 84.4 | 86.9 |
| 13 | B-CLL | 61 | F | 3 | 40.3 | 83.3 | |
| 14 | B-CLL | 54 | M | 4 | 11.8 | 80.5 | |
| 15 | B-CLL | 65 | F | 4 | 47.9 | 95.1 | 98.7 |
| 16 | B-CLL | 79 | M | 0 | 14.9 | 84.9 | |
| 17 | B-CLL | 61 | M | 1 | 258.0 | 94.7 | |
| 18 | B-CLL | 57 | F | 0 | 17.0 | 80.8 | |
| 19 | B-CLL | 76 | M | 2 | 38.9 | 76.1 | |
| 20 | B-CLL | 55 | F | 0 | 16.5 | 79.1 | 63.7 |
| 21 | B-CLL | 63 | M | 3 | 215.0 | 96.4 | 81.6 |
| 22 | B-CLL | 49 | F | 0 | 89.1 | 95.8 | |
| 23 | B-CLL | 75 | M | 0 | 19.9 | 77.0 | 98.7 |
| 24 | B-CLL | 64 | M | 1 | 97.7 | 96.8 | 93.2 |
| 25 | B-CLL | 60 | M | 2 | 187.0 | 97.6 | |
| 26 | B-CLL | 71 | F | 1 | 16.5 | 92.5 | |
| 27 | B-CLL | 72 | M | 1 | 67.9 | 92.1 | |
| 28 | B-CLL | 40 | F | 0 | 29.5 | 91.8 | |
| 29 | B-CLL | 58 | M | 1 | 78.2 | 88.3 | |
| 30 | B-CLL | 72 | F | 3 | 95.1 | 94.9 | 89.4 |
| 31 | B-CLL | 47 | M | 0 | 85.6 | 95.8 | |
| 32 | B-CLL | 64 | F | 0 | 58.2 | 95.6 | 46.8 |
| 33 | B-CLL | 61 | M | 0 | 18.6 | 88.2 | |
| 34 | B-CLL | 56 | F | 1 | 34.8 | 78.7 | |
| 35 | B-CLL | 54 | M | 0 | 18.5 | 56.8 | 57.6 |
| 36 | B-CLL | 64 | M | 0 | 29.0 | 55.2 | |
| 37 | SLL | 49 | M | IVA | 4.2 | 22.1 | |
| 38 | SLL | 61 | M | IVA | 1.1 | 24.6 | 25.4 |
| 39 | SLL | 70 | M | IVA | 3.0 | 37.3 | 5.1 |
| 40 | SLL | 68 | F | IVA | 5.4 | 43.5 | |
| 41 | SLL | 62 | F | IVA | 8.9 | 80.2 | |
| 42 | SLL | 62 | M | IIA | 4.2 | 0.5 | |
| 43 | SLL | 66 | F | IIIA | 2.5 | 13.4 | |
| 44 | SLL | 74 | F | IVA | 7.4 | 82.2 | |
| 45 | SLL | 57 | M | IVB | 3.5 | 38.0 | 25.3 |
| 46 | SLL | 80 | F | IVA | 8.7 | 64.9 | |
| 47 | MCL | 76 | F | IVA | 2.6 | 14.8 | |
| 48 | MCL | 64 | F | IIIB | 1.0 | 4.1 | |
| 49 | MCL | 63 | M | IVA | 2.5 | 38.5 | 30.7 |
| 50 | MCL | 82 | M | IIA | 6.1 | 79.4 | |
| 51 | MCL | 72 | M | IIIA | 2.0 | 16.4 | 16.4 |
| 52 | MCL | 56 | M | IVA | 5.7 | 84.7 | |
| 53 | MCL | 74 | M | IVB | 4.4 | 60.4 | |
| 54 | MCL | 72 | M | IVA | 1.2 | 2.3 | |
| 55 | MCL | 64 | M | IVA | 3.2 | 23.0 |
- Clinical stage in patients with B-CLL was determined according to Rai classification. (The missing values in some patients were not measured.) B-CLL, B-cell chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma; MCL, mantle-cell lymphoma.
PBSC Collection
Antigen expression of CD52 on CD34+ cells from peripheral blood stem cells (PBSC) grafts was measured in 15 patients indicated for autologous transplantation of hematopoiesis. The collection was made with the separator COBE Spectra (COBE BCT, Inc., Lakewood, Colorado, USA) via v. femoralis. The PBSC samples were analyzed within 6 h after collection.
Sample Preparation
A peripheral blood or PBSC sample was incubated with a combination of corresponding monoclonal antibodies according to the manufacturer's recommendations. Anti-CD52 conjugated with fluorescein-isothiocyanate (FITC) was used (Caltag Laboratories, Inc., Burlingame, CA, USA). Other antibodies were chosen in such a way as to identify the tumor cell population: anti-CD19 conjugated with R-phycoerythrin (R-PE) and anti-CD5 conjugated with PE-Cy5 (Caltag Laboratories, Inc.) (Fig. 1). In the analysis of the CD34 antigen, anti-CD34 conjugated with R-PE was used (Becton-Dickinson, Franklin Lakes, NJ, USA) (Fig. 2). Negative controls consisted of cells stained with isotype-matched FITC-labeled antibodies.
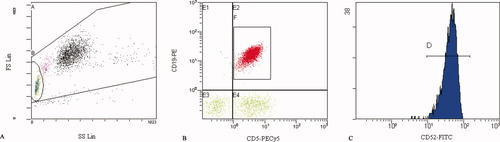
Gating of CD52+ cells in SLL. A: The lymphocyte population was gated on the FS/SS histogram (gate B). B: The SLL 5+19+ tumor cells were evaluated on lymphocyte populations (CD19 PE/CD5 PE-Cy5 histogram; gate F). C: In the histogram, fluorescence intensity of the CD52 antigen (gate D) on CD5+19+ cells is shown.
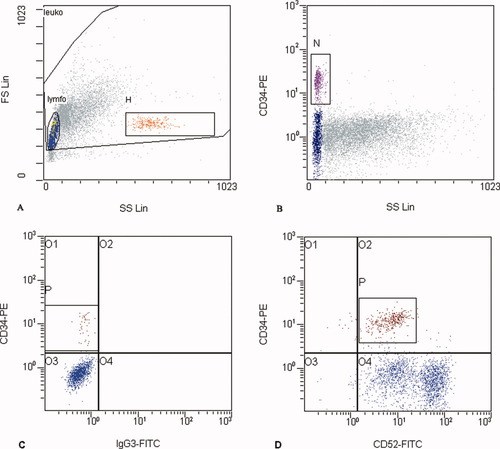
Gating of CD34+ cells in PBSC. A: CD34+ cells were evaluated on lymphocyte populations (gate lymfo on the FS/SS histogram). Gate H includes microbeads added to sample. B: Population of CD34+ cells was gated on SS/R-PE histogram (gate N). C: Gating of CD52+ cells in PBSC. In the histogram, expression of CD34 antigen on all lymphocytes versus FITC-labeled isotype control is shown (gate lymfo; A). D: Histogram shows coexpression of CD34 and CD52 antigen (gate lymfo; A).
Erythrocytes were lysed after incubation with formic acid and osmolarity was afterwards restored using ImmunoPrep Reagent System (Beckman-Coulter, Fullerton, CA, USA). The fluorescent microparticles Quantum™ FITC high level (Bangs Laboratories, Inc., Fishers, IN, USA) were added to each sample (Fig. 3). MESF values of microparticles were the following: 0, 18,599, 39,545, 147,185, and 600,940.
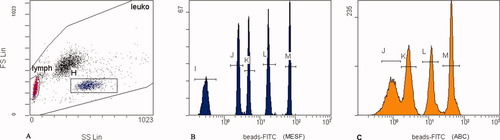
Calibration microparticles on the histograms from flow cytometer. A: Calibration fluorescent microspheres (for MESF units) were gated on the FS/SS histogram (gate H). B: Their mean fluorescence (MF) was measured on the one-parameter histogram. The calibration curve was compiled from the MF and standardized MESF values for the particular batch of microspheres. C: Microbeads for ABC determination were analyzed separately on the same protocol as MESF microbeads. After determination of the fluorescence/protein ratio, ABC units were calculated. Because the blank was measured separately, a peak of blank is not shown on this histogram.
Quantitative Flow Cytometry
Samples were analyzed on the Cytomics™ FC500 flow cytometer (Beckman-Coulter, Fullerton, CA, USA). At least 10,000 cells in the lymphocyte gate were evaluated in each sample (Fig. 3). The median values of fluorescence intensity were converted to MESF using QuickCal® software (Bangs Laboratories, IN, USA). To express the intensity of CD52 antigen in ABC units, MESF values were converted to ABC units using Simply Cellular microbeads (Bangs Laboratories, IN, USA). The intensity of the anti-CD52 fluorescence was measured on the tumor population in patients, on B-lymphocytes in samples from the control group and on CD34+ cells in PBSC samples.
Statistical Analysis
Data were evaluated using the Statistics™ program (StatSoft, Inc., Tulsa, OK, USA) and R-language software. MESF and ABC values of the CD52 antigen among the control group and patients with the aforementioned diagnoses were compared using the nonparametric Kolmogorov–Smirnov test.
RESULTS
The expression density of the CD52 antigen on CD19+ normal peripheral blood cells was evaluated. Median of the expression intensity was 446 × 103 MESF (194 × 103 ABC).
The median of the fluorescence intensity was 245 × 103 MESF (107 × 103 ABC) for patients recently diagnosed with B-CLL. Statistically, the intensity of CD52 antigen expression was significantly lower than in the healthy control group (P < 0.001).
The median fluorescence intensity in patients with a diagnosis of MCL was 513 × 103 MESF (223 × 103 ABC). There was a relatively higher variability among these patients.
The antigen expression was not statistically different in patients with a diagnosis of SLL compared to healthy controls, but it was significantly higher compared to the group of patients with B-CLL (P < 0.001). The median expression intensity was 526 × 103 MESF (229 × 103 ABC).
Surprisingly, the CD52 antigen was present on a majority of the CD34+ cells in peripheral hematopoietic stem cells transplant. Nevertheless, the intensity of the antigen expression was lower than on normal or malignant B-cells: 101 × 103 MESF (44 × 103 ABC). The results are summarized in Table 2 and Figure 4.
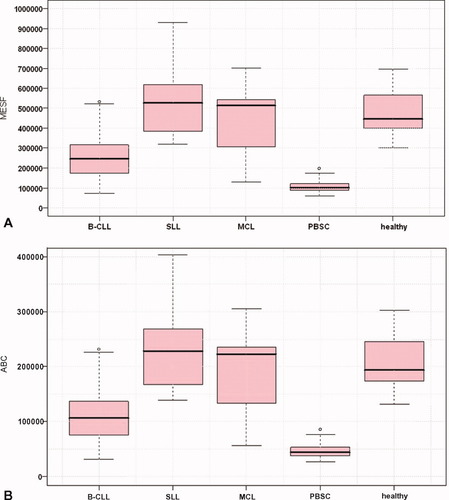
The intensity of CD52 antigen expression, Box–Whiskers plot. A: Antigen levels expressed in MESF units. B: Antigen levels expressed in ABC units. Median, 25%, and 75% quartiles, minimum, maximum, and outlying values are shown in the figures. Intensity of expression was measured on the CD5+19+ cells population in B-CLL, MCL, and SLL diagnoses. Intensity of expression in PBSC was measured on the CD34+ cells from PBSC and on CD19+ B-lymphocytes of peripheral blood in healthy groups. Statistically, CD52 antigen levels in B-CLL and PBSC were significantly lower than on B-lymphocytes of a healthy group.
| Number of samples | CD52 median | p | ||
|---|---|---|---|---|
| MESF (×103) | ABC (×103) | |||
| Normal B-lymphocytes | 9 | 446 | 194 | |
| B-CLL | 36 | 245 | 107 | <0.001 |
| MCL | 9 | 513 | 223 | n.s. |
| SLL | 10 | 526 | 229 | n.s. |
| PBSC | 15 | 101 | 44 | <0.001 |
- The statistical difference is expressed in comparison with normal B-lymphocytes. ABC, antibody-binding capacity; MESF, molecules of equivalent soluble fluorochrome; B-CLL, B-cell chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma; MCL, mantle-cell lymphoma; PBSC, peripheral blood stem cells.
DISCUSSION
The CD52 antigen is expressed on a majority of peripheral blood lymphocytes. The presence of the antigen has been proven on the tumor cell population belonging to both B and T lineages (3, 23, 32-35). Considering the possible correlation between the antigen quantity on the surface of cells and the effects of treatment, several authors have carried out semiquantitative evaluations of CD52 antigen expression during the last few years (23, 25, 32).
In this work, we have evaluated quantitatively the antigen expression. Patients recently diagnosed with chronic B-cell malignancy comprised the target group. Because the antigen expression can be influenced by treatment, patients previously treated were not analyzed together with those patients recently diagnosed. We determined the CD52 expression on B-lymphocytes of peripheral blood from the healthy control group, as well, in order to compare the antigen levels.
The CD52 antigen expression intensity on B-lymphocytes of healthy peripheral blood and on B-CLL cells had been investigated by a Swedish group (32). Those researchers had measured an average density of 409 × 103 MESF on B-cells from a control group. Five untreated patients in their group were found to have a density of 371 × 103 MESF. The difference between B-CLL patients and healthy donors was not found to be statistically significant. In our work, by contrast, the levels of CD52 antigen did differ significantly (P < 0.001) (Fig. 4). It seems possible that the small patient and donor cohort studied in the Swedish group could have led to inaccurate results. Furthermore, the cryopreservation used by this group could have led to change in the antigen expression.
A British group had reported different results (25). In that case, the CD52 antigen levels in healthy donors as well as in patients with B-CLL were lower than that in our work (104 × 103 MESF and 112 × 103 MESF, respectively). As with the Swedish group, they did not note statistical differences between these two groups. In their study, however, over one-third of the analyzed group of patients with B-CLL had more than 10% prolymphocytes, and all patients had been previously treated. The fact that those patients had been treated could account for the difference in comparison with the untreated patients in our study.
Large differences in results between authors can reflect a problem of sample preparation. FITC is very sensitive to pH change. The decline in pH leads to lower fluorescence of FITC, which can be seen mainly between pH 7 and 8. We confirmed this in several assays, when fluorescence intensity of FITC-bound beads was measured in solutions of different pH. However, when fluorescent microparticles are equilibrated and measured together with the sample, recalculation of MESF units gives equivalent results regardless of pH value.
Because we used Immunoprep (based on formic acid and thus having possible impact on surface antigens) for cell lysis in our work, we decided to compare CD52 expression intensity between samples from lysed blood and mononuclear cells prepared on density gradient (Histopaque 1077). The intensity of CD52 expression was higher on Histopaque-prepared cells, but the ratio between CD52 expressions of these two methods remained the same. The level of antigen expression also can be influenced by cryopreservation of cells (36). Thus, when comparing the levels of antigen expression, it is important to state the method of sample preparation.
Until now, very few authors have analyzed the CD52 antigen expression on MCL tumor cells. These few works have demonstrated its presence on a majority of cells (33, 37). In our work, we observed a high variability in CD52 antigen level among the individual patients with MCL.
B-CLL and SLL are very similar diseases. A difference between them is attributed only to definition of diagnostic criteria and in the smaller proportion of malignant cells in peripheral blood of SLL. Although both diseases have the same phenotype, the intensity of expression may be the factor that distinguishes them and explains their different biological behaviors.
We revealed significantly higher CD52 levels in patients with SLL than in patients diagnosed with B-CLL. This difference may be used as an auxiliary parameter to distinguish these two diagnoses. High CD52 intensity of expression on tumor cells from SLL patients suggests suitability to alemtuzumab therapy. However, only a small portion of tumor cells is circulating in peripheral blood and most of these are found in lymph nodes. A multicentric study has shown that after the treatment of B-CLL patients with Campath-1H lymph nodes were less affected by Campath-1H than were blood or bone marrow (20). Therefore, in SLL other aspects must be considered, as accessibility of lymph nodes for monoclonal antibody. Consequently, high CD52 expression in SLL does not mean that response to treatment with alemtuzumab will be as satisfactory in these patients.
Campath-1H is used in stem cell transplantations to prevent GvHD and transplant rejection. The majority of studies have not so far revealed CD52 antigen presence on bone marrow stem cells (28, 29). Other authors investigated the effect of Campath-1H on hematopoietic progenitors in vitro (17). They did not prove a significant decrease in any observed progenitor cells (colony-forming unit—granulocyte macrophages, burst-forming unit—erythroid, colony-forming unit—erythroid, colony-forming unit—mixed lineage). Nevertheless, after the sorting of these cells into Campath-1H positive and negative fractions, it was evident that there were some progenitors in the positive fraction. It is possible that these progenitors bind Campath-1H but do not fix complements to undergo lysis (17). Another study analyzing the phenotypic characteristics of CD34+ cells describes positive expression of CD52 antigen on both fetal and adult bone marrow CD34+ cells (30). Since the results of CD52 presence on CD34+ cells are controversial, we focused also on this problem in our work. In all 15 of our autograft PBSC analyzed, we observed expression of CD52 antigen on almost the entire population of CD34+ cells, but its intensity was relatively low.
The correlation between CD52 intensity of expression and the response to treatment with alemtuzumab has not yet been proven. However, a British group that investigated correlation of CD52 expression (in MESF) with response to therapy describes higher expression in responders than in nonresponders. Nevertheless, the difference was not statistically significant (25). It is supposed that, in addition to the level of antigen expression, the effect of the alemtuzumab treatment is influenced by the antigen distribution on the cell surface and membrane proteins that modify the complement activity (38). Further studies are required to clarify the influence of CD52 expression intensity on treatment with Campath-1H and thus to make decisions easier for the indication of Campath-1H application.
Our work provides new data in the area of level of CD52 antigen expression on tumor cells in chronic malignancies of B-cell lineage.




