Application of immunomagnetic cell enrichment in combination with RT-PCR for the detection of rare circulating head and neck tumor cells in human peripheral blood
Abstract
Detection of rare, circulating tumor cells (CTC's) in human peripheral blood is a potential indicator of prognosis and diagnosis in oncology. Typical methods to detect these CTC's are either by immunocytochemistry (ICCS) or RT-PCR. However without accurate, rapid, and reproducible enrichment processes, these detection techniques are labor intensive and/or unreliable. In this article, a repeatable enrichment process that included a flow-through immunomagnetic cell separation system, the quadrupole magnetic sorter (QMS) was optimized with the aid of a statistical analysis software package. The QMS was operated in a negative mode of operation by immunomagnetically targeting normal human peripheral blood lymphocytes (PBL) through the CD45 surface marker. Three head and neck squamous carcinoma cell lines (HNSCC), Detroit-562, SCC-4, and CAL-27, were used to determine the sensitivity of RT-PCR for the epidermal growth factor receptor (EGFR) in spiked PBL. The detection purity needed for detection was found to be one cell in 104, one cell in 103, and one cell in 105 for the Detroit-562, SCC-4, and CAL-27, respectively. The actual number of cancer cells needed for RT-PCR detection ranged from 30 to 1 cell. To mimic the potential concentration of rare CTC present in peripheral blood of cancer patients, the spiking concentration was chosen to be one cancer cell per 105 total leukocytes from healthy donors. Using a single step immunomagnetic labeling, the final, optimized enrichment process produced a 57.6 ± 30.3-fold (n = 6) enrichment of the rare cancer cells with a final cancer cell recovery of (77.8 ± 6.6)%. © 2007 Clinical Cytometry Society
Head and Neck cancer comprises ∼6% of all cancers which results, globally, in ∼550,000 cases a year. According to the statistics of American Cancer Society, 39,250 new cases of cancer of the oral cavity, oropharynx, pharynx, and larynx are estimated to occur in the US in 2005, and 10,090 deaths were estimated to occur. Greater than 90% of these cases of head and neck cancer are classified as squamous cell carcinomas. For all stages combined, the 5-year survival rate is approximately 58.7%, and this rate has increased slowly in the last 20 years. About 40–50% of the patients will later develop locoregional recurrences, and approximately 80% of these recurrences occur within the first 2 years.
Both recent and historical studies have shown that tumor cells are likely to exist in the bone marrow or in the peripheral blood of various types of cancer patients (1-6). It has been presumed that those tumor cells shed from the original tumors and are considered to contribute to the metastasis and/or recurrence of the original tumor. Collectively, these studies indicate that the presence of cancer cells in human body fluids can be of value as an independent potential prognostic factor for metastatic relapse (7-12).
Immunocytochemistry staining (ICCS), with subsequent human microscopic observation, is the standard method to assess the presence of circulating cancer cells (10, 13, 14). However, without an enrichment procedure for the extremely rare cell, several (up to ten) slides need to be analyzed to accurately detect the cancer cells. As an alternative, several molecular biology methods based on reverse transcriptase polymerase chain reaction, RT-PCR, have been developed for the detection of circulating tumor cells (CTCs) (15-17).
The search for tumor-associated antigens in the detection of circulating head and neck tumor cells in the peripheral blood of patients using conventional RT-PCR has yielded many potential candidates such as cytokeratin family genes (18), matrix metalloproteinases (MMP) (19), squamous cell carcinoma antigen, (SCCA) (12), and epidermal growth factor receptor (EGFR). O'Hara et al. (5) investigated the expression of 37 genes in the peripheral blood of healthy donors, and reported that detection of the EGFR may be used to detect rare CTCs in the peripheral blood of cancer patients.
Generally speaking, the expression of most tumor-associated antigens may be detected using conventionalRT-PCR in peripheral blood and have been shown to function as clinical diagnostic markers associated with metastatic spread (20). However, it has also been reported that illegitimate expression of the so-called tumor cell-specific genes hinders this approach, and it is impossible for the molecular approaches to further characterize the cancer cells when the ratio of cancer cells to total peripheral blood lymphocytes (PBL) is very low (21-25).
Numerous studies have shown that conventional RT-PCR can be used to detect the CTCs in body fluids of patients with various carcinomas, and that the sensitivity of conventional RT-PCR is one cancer cell per 105 or 106 mononuclear cells. Table 1 summarizes some of these examples. However, the detection sensitivity strongly depends on the type of tumor, the choice of the amplified gene, the operative skill of the researcher, and the efficiency of the experiment. For example, variation in the degree of RNA degradation, variation in the efficiency of extraction of RNA, deviation of the RNA amount used in RT-PCR, and the PCR efficiency may lead to false negatives, although a sufficient amount of mRNA template for detection may be present (21). Additionally, the expression level of the target gene is often upregulated or downregulated in cultured cells (21). Zieglschmid et al. (32) also concluded that EGFR appears to be less sensitive in comparison to other gene markers such as CK19.
| Tumor cell type | Pre-enrichment method | Enrichment | Molecular detection approach | Molecular marker | Sensitivity | Reference |
|---|---|---|---|---|---|---|
| Oral SCC line (GRP-SAS-T5) | – | – | Conventional RT-PCR | EGFR | 10 per ml blood | Zen et al., 2003 (12) |
| Oral SCC line (GRP-SAS-T5) | – | – | Real-time quantitative RT-PCR | SCCA or EGFR | 10 per ml blood | Zen et al., 2003 (12) |
| Breast cancer | Magnetic activated cell separation | – | Real-time quantitative RT-PCR | EGFR | 1 per 5 × 106 mononuclear cells | Fellowes et al., 2004 (26) |
| Bladder cancer cell line | – | – | Conventional RT-PCR | EGFR | 5 per ml blood | Gazzaniga et al., 2001 (27) |
| Cervical cancer (Hela) | – | – | Conventional RT-PCR | EGFR | 1 per 105 mononuclear cells | Mitsuhashi et al., 2003 (28) |
| Prostate cancer | Immunomagnetic beads | – | Conventional RT-PCR | Prostate specific antigen (PSA) | 10 per 8 ml blood | Makarovskiy et al., 1997 (29) |
| Colorectal cancer (SW480) | Magnetic activated cell separation | 9 | Nested RT-PCR | K-ras and p53 | 1 per 106 mononuclear cells | Iinuma et al., 2000 (30) |
| Bladder cancer cell line (T24) | – | – | Nested RT-PCR | MUC-7 | 2 per ml blood | Kinjo et al., 2004 (31) |
| Colon carcinoma cell line (SW480) | Immunomagnetic beads | – | GRP-SAS-T5 | K-ras | 1 per 105 mononuclear cells | Hardingham et al., 1993 (3) |
| Lung cancer cell line (Calu-1) | – | – | Nested RT-PCR | Cytokeratin-19 | 1 per 105 mononuclear cells | Ko et al., 2000 (23) |
| Oral SCC line (GRP-SAS-T5) | – | – | Conventional RT-PCR | SCCA | 102 per ml blood | Zen et al., 2003 (12) |
To overcome the aforementioned problems of the present techniques, it is highly desirable to apply an enrichment step prior to the actual detection procedure. In the recent literature, immunomagnetic cell separation has been used as one of the most promising techniques because of its specificity, rapidity, and high efficiency (30, 33-35).
Batch, commercial systems for immunomagnetic cell separation to enrich suspensions containing rare cells are readily available (i.e. MACS system); however, concerns about their limited load capacity and potential irreversible entrapment of cells inside the column have led us to develop and optimize “flow-through” cell separation devices, one of which is referred to as the quadrupole magnetic cell sorter (QMS) for rare cell enrichment (34, 36-38).
In a positive mode of operation (i.e. the cancer cells are immunomagnetically labeled), Nakamura et al. (38) reported the ability to positively select spiked breast cancer cells in human blood with a throughput of 3.29 × 105 cells/s and a recovery of 89% of the cancer cells. Alternatively, in a negative mode of operation (normal blood cells are immunomagnetically labeled), Lara et al. (34) reported a 5.17 log enrichment (log10 of the ratio of final purity to initial purity) and 46.0% recovery of spiked cancer cells in the QMS system. It should be noted that this 5.17 log enrichment included the removal of red blood cells (RBC) with a lysis buffer step.
While the positive selection has proved effective for enrichment and isolation of rare, or not so rare, cells in a cell suspension of various types, there are significant limitations. One of these major limitations is that one frequently lacks information about the phenotype of the rare targeted cell. Moreover, the expression level and frequency of target antigen on the rare cell is potentially diverse. For example, in the case of a rare, circulating cancer cell, one is assuming that the antibody conjugate can label the cancer cell specifically and sufficiently to allow an acceptable separation. Recently, a number of studies have been published which experimentally demonstrate this diversity for a number of commercial antibodies targeting antigens associated with cancer cells. These studies also discuss the implications of this diversity on separation performance (14, 35, 39).
Another potential limitation of a positive selection mode of operation is the fact that the targeted cells are labeled. Such a labeling can, in some cases, illicit physiological responses within the cell, or just limit further labeling for analysis if it is so desired (i.e. specific sites are already bound with antibodies).
Based on these potential limitations of positive enrichment, and our previous success at negative enrichment, we are continuing to optimize the conditions using statistical software to improve the enrichment performance. For this specific report, the performance of detecting rare cancer cells using RT-PCR was investigated and optimized. Specifically, the expression of EGFR in three head and neck squamous carcinoma cell lines (HNSCC), Detroit-562, SCC-4 and CAL-27, was analyzed by RT-PCR. Previously published studies of head and neck cancers have reported that most patients had 1–10 tumor cells/106 mononuclear cells in circulation (40). Therefore, to mimic the potential concentration of CTCs in the peripheral blood of head and neck cancer patients, Detroit-562 cells were cultivated and spiked into the peripheral blood leukocytes (PBL) of healthy donors, where the tumor cell concentration was one cell in 105 PBL. The enrichment results of CTC were assessed by conventional ICCS and regular RT-PCR.
MATERIALS AND METHODS
Cell Sources
HNSCC, Detroit-562, SCC-4, and CAL-27, were purchased from ATCC (Manassas, VA). Detroit-562 cells were grown in Earle's minimum essential medium (ATCC, Manassas, VA) supplemented with 10% fetal bovine serum (FBS; JRH Biosciences, Lenexa, KS). CAL-27 cells were cultivated in Dulbecco's modified Eagle's medium (ATCC, Manassas, VA) containing 10% FBS. SCC-4 cells were cultured in a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F12 medium (ATCC, Manassas, VA) plus 10% FBS. The cells were maintained in a 75 cm2 tissue culture flask (Corning, Acton, MA) and incubated at 37°C in 5% CO2. Cell number was determined using a hemocytometer, and cell viability was measured using a dye exclusion method (Invitrogen Corporation, Carlsbad, CA).
Buffy coats from healthy donors were obtained from American Red Cross, Central Ohio Region. RBCs were lysed with fresh lysing buffer (154 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) and incubated for 5 min at room temperature with occasional shaking. After 5 min of centrifugation at 300g, the cell pellet was washed twice using the labeling buffer (PBS supplemented with 2 mM EDTA and 0.5% bovine serum albumin) and resuspended in the buffer to obtain the PBL suspension. If necessary, the lysis protocol was repeated to remove more RBCs. The concentration of leukocytes was determined by hemocytometer using Unopette® Microcollection system (BD Biosciences, San Jose, CA).
Magnetic Labeling
To deplete the leukocytes, one-step and two-step immunomagnetic staining protocols were used to target the PBL for the immunomagnetic separation step, respectively. For the one-step magnetic labeling protocol, CD45-MACS (Cat No. 130-045-801, Miltenyi, Biotec, Auburn, CA) was used and FcR blocking reagent (Cat No. 130-059-901, Miltenyi Biotec, Auburn, CA) was also added to block potential nonspecific binding. Typically, the pellet of 80.0 × 106 cells was resuspended into 50 μl labeling buffer, 50 μl FcR blocking reagent, and 200 μl of anti-CD45 MACS placed in a 15-ml falcon tube. The cell suspension was incubated for 15 min in the dark at 4°C and then centrifuged at 300g for 5 min. After washing once, the cell pellet was resuspended in an appropriate volume of PBS labeling buffer for the subsequent separation.
For the double step immunomagnetic staining protocol, the primary antibody was a mouse anti CD45 PE (Cat No. IM 2078, Beckman Coulter, France) and the secondary antibody was anti-PE MACS microbeads (Cat No. 130-048-801, Miltenyi Biotec, Auburn, CA). As with the single step protocol, 80.0 × 106 cells were suspended in 50 μl PBS labeling buffer, 50 μl FcR blocking reagent, and 200 μl of anti-CD45 PE in a 15-ml falcon tube. The cell suspension was incubated for 15 min in the dark at 4°C and then washed once with PBS labeling buffer. Upon resuspenion in 50 μl PBS labeling buffer, 50 μl FcR blocking reagent and 200 μl of anti-PE MACS microbeads were added. After incubation at 4°C for 15 min in the dark, the cells were washed once with PBS labeling buffer and resuspended in 1 ml of PBS labeling buffer for further isolation.
The fluorescence analysis of samples was performed on a FACS Calibur Flow Cytometer (Becton Dickinson, Florida), and magnetophoretic mobilities of samples were measured by our previously developed and reported cell tracking velocimetry instrument (41).
Immunomagnetic Cell Separation Step
Figure 1 presents the design and construction of the QMS system described previously (42, 43). Before performing an actual separation, the QMS column was filled with degassed PBS labeling buffer to reduce the potential of bubble formation and entrapment with the device and tubing. Labeled cells were diluted to a final concentration of 5 × 106 cells/ml using PBS buffer before injection. To operate the QMS, total flow rates at the inlet and outlet remained constant using three syringe pumps. Two pumps were used at the inlet: the first pump injected the sample at the beginning of the operation until the sample has been injected into the column (a′ inlet). Upon completion of sample injection, flow entering the a′ inlet was switched to the second pump using a three-way valve which injected sheath fluid into the a′ inlet to flush remaining sample from the system and to maintain a constant flow rate at the inlet. A third pump was connected to the outlet a to collect sorted fractions.
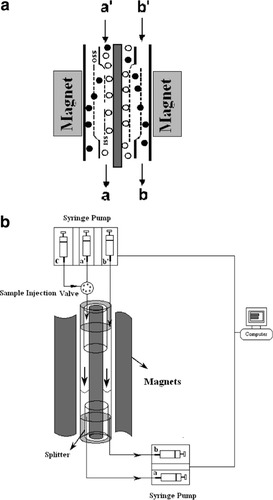
(a) Diagram of the channel within the magnetic energy gradient. (b) Schematic diagram of the quadrupole magnetic sorter (QMS) in operation. The syringe pumps used for injection and retrieval, as well as tubing connecting the system are also presented. The symbols ○ and • correspond to the nonmagnetic and magnetic cells, respectively.
Optimization of QMS Operation
Cell separation performance in the QMS is governed by several parameters including flow rate, magnetophoretic mobility of the labeled cells, cell feed concentration, properties of the sheath fluid, and the percentage of magnetic cells in the total cell suspension. In this study three adjustable, operational parameters were studied: (1) flow rate, Qt, (2) the magnetophoretic mobility of the labeled cells, and (3) the cell feed concentration. The flow rate is responsible for the residence time of the cell inside the column; therefore, Qt, is directly related to the radial displacement of an immunomagnetically labeled cell within the QMS channel. The magnetophoretic mobility of cells, in combination with the magnetic energy gradient, is responsible for the force which creates the radial movement of an immunomagnetically labeled cell. Since the magnetic energy gradient is fixed for the given system, the higher the magnetophoretic mobility of the cells, the faster the cells travel in the radial direction; correspondingly, the less time the cells need to be in the magnetic energy gradient for given radial displacement.
 (1)
(1)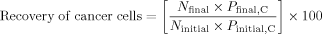 (2)
(2) (3)
(3)Detection/Analysis of Final, Enriched Samples
The enriched fraction from the nonmagnetic outlet, stream a (Fig. 1b), was evaluated in two ways: (a) ICCS and (b) RT-PCR assay. For all the experiments, two parallel runs of immunomagnetic cell separation were conducted under identical conditions, one run analyzed by ICCS, the second by RT-PCR. Specifically, to perform the analysis of ICCS, the nonmagnetic flow stream from the QMS sorter (stream a) was split into two samples, one of which was centrifuged and collected using a typical cytospin protocol, and then stained by anticytokeratin (CK3-6H5)-FITC (Cat No. 130-080-101, Miltenyi Biotec, Auburn, CA) and Hoechst 33342 (Invitrogen Corporation, Carlsbad, CA). The other fraction was used to measure the number of remaining leukocytes by hemocytometer using the Unopette® microcollection system.
The actual cytospin staining protocol was performed as follows: The enriched fraction from the QMS outlet a was centrifuged for 5 min at 300g and resuspended in 100 μl of PBS buffer to which 100 μl of 3.7% formaldehyde was added for fixation. After 15 min incubation at room temperature, the cells were washed once using PBS buffer and then resuspended in 200 μl of PBS buffer. An appropriate cell suspension, the cell number of which did not exceed 106 total cells, was added to the cytospin funnel and centrifuged for 4 min at 1,000 rpm. After spinning, 50 μl of Hoechst 33342 dye (working solution 2 μg/ml) was applied on the cell coated slide and incubated for 10 min at room temperature. The slide was washed three times (at least 5 min each) with PBS. Finally, cytokeratin–FITC solution was diluted with 0.5% Triton X-100 in a ratio of 1:10. One hundred microlitres of this diluted cytokeratin–FITC solution was dropped on the slide and incubated for 15 min at room temperature. The slide was washed three times (at least 5 min each) with PBS. After air-drying, the slide was prepared with mounting medium (Tissue-Tek® Sakura Finetek) and covered with a coverslip for observation and preservation. Only cells positive for Hoechst 33342 and cytokeratin (i.e. double positive) were identified as the cancer cells.
RT-PCR Assay
The expression of EGFR in the HNSCC cells, Detroit-562, SCC-4, and CAL-27, was analyzed by RT-PCR. Total RNA was extracted from cell suspension of interest using Trizol reagent kit (Invitrogen Corporation, Carlsbad, CA). The RNA concentration was determined by spectrophotometry (Amershan Biosciences, Sweden), and its integrity checked electrophoretically.
The RT-PCR assay was performed using the Stratascript first-strand synthesis system (Stratagene, La Jolla, CA). Briefly, 1 μg of RNA was incubated with 1.5 μl of oligodT and 1.5 μl of random primers for 5 min at 65°C in a total volume of 20.5 μl; subsequently the reaction samples were cooled down to room temperature to allow the primers to anneal to the RNA. To synthesize first-strand cDNA, 4.5 μl of master mix containing 2.5 μl RT buffer, 1.0 μl dNTPs, 0.5 μl RNase block reagent, and 0.5 μl reverse transcriptase (Stratagene, La Jolla, CA) was added to the reaction samples, and the samples incubated in a PCR instrument (Gen Amp® PCR System 9700, Applied Biosystems, Foster City, CA) at 42°C for 60 min. To amplify the target cDNA, 2 μl of RT-reaction sample was added in a final volume of 25 μl including 5 μl of 5× buffer, 5 μl of GC melt, 0.5 μl of dNTPs, 1 μl of EGFR primers, 0.5 μl of GC polymerase, and 11 μl of DEPC-treated water (Advantage™ GC cDNA PCR Kit, BD Biosciences, San Jose, CA). The PCR reaction was performed as follows: one cycle of denaturing at 94°C for 1 min; 32 cycles of 94°C for 30 s, 60°C for 1 min, and 72°C for 2 min; and extension incubation at 72°C for 5 min.
The sequences of the EGFR primers are GGGAGCAGCGATGCGA and CTCCACTGTGTTGAGGGCAAT that produced a 301-bp PCR product. As a control for cDNA quality, the PCR was performed simultaneously using the HPRT primers (GTAATGACCAGTCAACAGGGGAC and TGGTCAAGGTCGCAAGCTTGCTTG), which yielded a 177-bp PCR product. The PCR products were analyzed by 1.5% agarose gel electrophoresis.
Sensitivity of RT-PCR by EGFR for HNSCC Cells
To evaluate the sensitivity of the RT-PCR assay, we suspended 101, 102, 103, 104, and 105 HNSCC cells into 107 PBL from a healthy donor. Then, total RNA was extracted and RT-PCR performed. In addition, the detection sensitivity (i.e. how many micrograms of RNA was needed for detection) of EGFR in a HNSCC cell line by RT-PCR was further determined in a serial dilution study from 10−8 to 10−2 μg of RNA derived from only the HNSCC cells.
Statistical Analysis
Unless specially stated, the data shown are medians in the text. Statistical analyses were performed using JMP software (SAS Institute, Cary, NC), which has an option that allows multiple least squares analysis. With this multiple least squares analysis function, a screening analysis for the effects of the influencing factors on QMS performance was performed with a significance level set at 0.05. The variables chosen in this multiple parameter analysis followed the effect hypothesis tests, which is part of the Fit Model command routine in the software.
RESULTS
Labeling Saturation Studies
Figure 2a is a representative histogram, in log format, of the magnetophoretic mobility of unlabeled PBL and PBL labeled with anti-CD45 MACS (single step protocol) obtained by CTV analysis. Figure 2b presents a saturation curve of the mean magnetophoretic mobility of the PBL as a function of the concentration of CD45–MACS reagent (μl/μl total solution). Vertical bars represent the standard deviation of the mean magnetophoretic mobility at the given labeling condition (n = 3). Figure 2c corresponds to the normalized fluorescence intensity as a function of the concentration of the primary, anti-CD45 PE antibody (μg of antibody per ml of cell suspension with a total of 1.0 × 106 cells), while Figure 2d is a saturation curve of the mean magnetophoretic mobility of the PBL, previously labeled with anti-CD45 PE (15 μg/ml), as a function of the secondary antibody concentration, in units of microlitres of MACS reagent per total microlitre of suspension (note that Miltenyi Biotec does not provide concentration of their reagents in units of micrograms).

(a) Representative log plot of the magnetophoretic mobility of unlabeled and labeled PBL. A single step labeling was used for this specific example. (b) Saturation plot of the mean magnetophoretic mobility of the PBL cells labeled with the single step anti-CD45 MACS antibody, as a function of the number of microlitres of CD45 MACS reagent per total microlitre of suspension. A total of 10 × 106 cells were used in a total suspension volume of 300 μl. (c) Saturation curve of normalized fluorescence intensity of the PBL CD45 surface receptor labeled with an anti-CD45-PE conjugate. The x-axis corresponds to the concentration of the antibody (μg of Ab/ml). (d) Saturation plot of the mean magnetophoretic mobility of the PBL cells, previously labeled with anti-CD45 PE (15 μg/ml), as a function of the number of microlitres of MACS reagent per total microlitre of suspension.
The potential of nonspecific binding of the anti-CD45 antibody to HNSCC cell lines was tested by flow cytometric analysis. No nonspecific binding was detected for Detroit-562 and SCC-4 cells, while 10% of CAL-27 cells were found to be bound with anti-CD45 antibody. Thus, Detroit-562 cells were chosen for spiking studies in the QMS.
Assay Sensitivity
In the preliminary experiments, RT-PCR using EGFR was performed on the RNA of PBL from healthy donors using 30, 32, 35, and 38 cycles (Data not shown). A cycle number of 32 for RT-PCR was chosen as the highest level which presented no false positives for any of the samples tested.
Figure 3 presents representative examples of photographs of gel analysis of RT-PCR assays targeting the mRNA of EGFR from several different cell sources. Specifically, RT-PCR of mRNA from Detroit-562, from PBL of healthy donors, and primary tumor cells from head and neck cancer patients are presented in lanes 2–5, respectively. As can be observed, no EGFR gene expression was detected in samples from the PBL of healthy donors; in contrast, significant bands can be observed in the samples from the Detroit-562 cells and the cells from head and neck cancer patients. This band, at ∼300 bp, is the expected size of RT-PCR amplification product for EGFR.
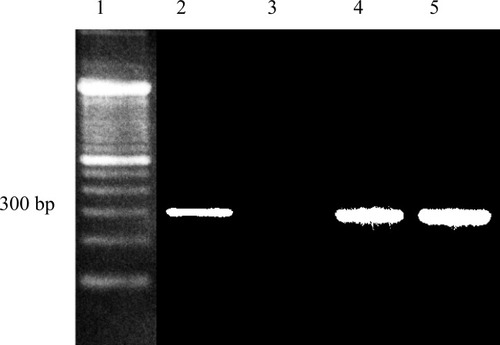
Representative examples of RT-PCR targeting the mRNA of EGFR. Each lane corresponds to samples from the following: 1, DNA ladder 100 bp difference per band (Invitrogen, Carlsbad, CA); 2, Detroit-562 cell line; 3, PBL; and lanes 4 and 5 are from frozen tumor cells from head and neck cancer patients.
Figure 4a–e present representative photographs of agarose gel analysis of RT-PCR amplification of various samples. Specifically, Figure 4a illustrates the sensitivity of detection of EGFR mRNA from Detroit-562 cells diluted in PBL. A visual band can begin to be observed at a dilution of one Detroit-562 cell per 104 PBL (lane 5). In contrast, the concentration of SCC-4 needs to be higher, one cancer cell in 103 PBL (Fig. 4b, lane 5), while the detection concentration is the lowest for the CAL-27 cells, i.e. one cancer cell in 105 PBL (Fig. 4c, lane 3). Figures 4d and 4e demonstrate the amount of RNA needed, in micrograms, to detect the EGFR mRNA in Detroit-562 and SCC-4 cells, respectively.
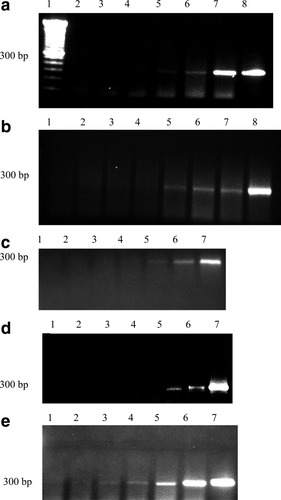
Representative photographs of agarose gel analysis of RT-PCR amplification of various samples. (a) The sensitivity of detection of EGFR mRNA from Detroit-562 cells diluted in PBL. Lane 1 corresponds to a DNA ladder, lane 2 pure PBL, lanes 3 through 8 to dilutions of cancer cell to PBL of 10−6, 10−5, 10−4, 10−3, and 10−2, and lane 8 corresponds to pure Detroit-562 cells. (b) Equivalent to (a) except for the use of SCC-4 cells and the omission of the DNA ladder in lane 1 which was left blank. (c) Equivalent to (a) except for the use of CAL-27 cells and omission of DNA ladder which was left blank. (d) and (e) present the sensitivity of detection of EGFR mRNA of Detroit-562 and SCC-4 cells, respectively, as a function of total amounts of RNA, ranging from 10−7 to 10−2 μg in (d) and 10−7 to 10−2 μg in (e). In both (d) and (e), the last lane corresponds to 1 μg of RNA sample.
QMS Optimization Studies
To determine the optimal operational parameters, a series of experiments using four bags of buffy coats from healthy donors was conducted, and was subsequently analyzed using JMP software. A significantly higher level of spiked cancer cell concentration (3%, Detroit-562 cells per leukocytes) than would normally appear in patient blood was used to better quantify the enrichment performance in the optimization studies. At a feed concentration of cancer cells of 3%, an ideal separation process which resulted in the complete removal of all PBL would yield an enrichment rate of 33. Consequently, in the figures that follow, the enrichment rate, presented on the right y-axis, ranges from 0 to 33.
To illustrate the changing performance with the operational parameters, some results of these studies are presented in three figures where one of the three variables studied was varied holding the other variables constant. Figure 5a presents an example of the effect that the magnetophoretic mobility, ranging from 0.8 × 10−4 to 3.5 × 10−4 mm3/(T A s), had on the separation performance, while holding the flow rate fixed at 10 ml/min and the feed concentration at 1 × 106 cells/ml. A number of salient features can be observed. First, a significant increase in recovery of the cancer cells, up to 80%, was obtained in the outlet a as the magnetophoretic mobility of the PBL decreases. Second, the enrichment rate of the cancer cells increased as the mobility of PBL increased.
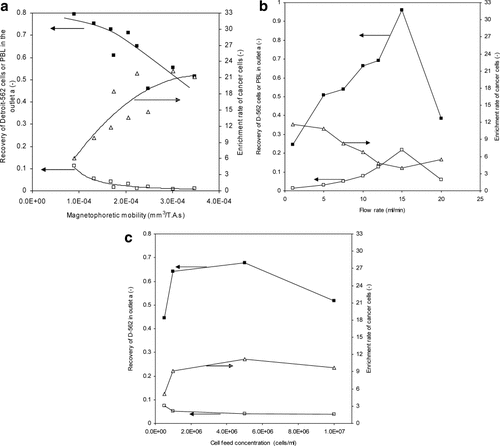
Representative examples of the effect of operational parameters on the separation performance of QMS. (a) Effect of magnetophoretic mobility on the enrichment performance, cell feed concentration is 1.0 × 106 cells/ml at a flow rate of 10 ml/min; (b) Effect of flow rate on the enrichment performance, cell feed concentration of 1.0 × 106 cells/ml with the magnetophoretic mobility of 1.42 × 10−4 mm3/(T A s) by a two-step immunomagnetic labeling; (c) Effect of cell feed concentration on the enrichment performance, the flow rate is fixed at 10 ml/min and the magnetophoretic mobility of cells is 1.2 × 10−4 mm3/(T A s). The recovery of Detroit-562 cells (▪), PBL (□), and the enrichment rate of cancer cell (▵) in the nonmagnetic outlet a of QMS were shown in the figures, respectively. In all of the above experiments, a ratio between cancer cell and PBL is 0.03.
Figure 5b presents a representative of the effect that changing the flow rate has on separation performance, while holding the magnetophoretic mobility constant at 2.14 × 10−4 mm3/(T A s) and the feed concentration constant at 1 × 106 cells/ml. In contrast to varying the magnetophoretic mobility, overall, varying the flow rate had less effect on the system performance. It is not known why there is a peak and then drop in the recovery of the Detroit-562 cells with increasing flow rate.
Figure 5c presents an example of the effect that changing the cell concentration has on separation performance, while holding the magnetophoretic mobility constant at 1.2 × 10−4 mm3/(T A s) and the feed rate at 10 ml/min. Again, relatively minor changes in performance were observed with changes in concentration. The initial increase in performance with increase in concentration could reflect an increase in accuracy of analysis as the total sample size and concentration increases.
Statistical Analysis for Optimization Studies
In the process of fitting the statistical model, three variables (the magnetophoretic mobility of the labeled cells, flow rate, and cell feed concentration) and one numerical variable (buffy coat) were chosen. These three variables were evaluated relative to the three response variables of QMS performance (recovery of PBL, recovery of cancer cells, and enrichment of cancer cells) at a 95% confidence limit.
The statistical package used in this analysis provides P values of the fit of the data. Table 2 presents the value of the P factor for the recovery of PBL, cancer cells, and the enrichment rate of cancer cells, as a function of the five variables: magnetophoretic mobility, flow rate, flow rate∧2, cell feed concentration, and specific buffy coat. The magnetophoretic mobility of the labeled cells is the most significant factor on the performance of the QMS separation (P = 0.0207 for the recovery of PBL, P = 0.062 for the recovery of cancer cells, and P < 0.0001 for the enrichment of cancer cells). The flow rate is another significant factor for the performance (P = 0.0088 for the recovery of PBL, P = 0.0506 for the recovery of cancer cells, and P = 0.0138 for the enrichment of cancer cells). Additionally, the second power of flow rate is also significant for the recoveries of PBL (P = 0.0141) and cancer cells (P = 0.0006), which demonstrates that the relationship to the flow rate is nonlinear. This phenomenon is also observed in Figure 5b. Note that no significant influences on the separation performance of QMS were observed for the cell feed concentration and buffy coat in the studied range.
| Influencing factor | P value | ||
|---|---|---|---|
| Recovery of PBL | Recovery of cancer cells | Enrichment rate of cancer cells | |
| Magnetophoretic mobility | 0.0207 | 0.062 | <0.0001 |
| Flow rate | 0.0088 | 0.0506 | 0.0138 |
| Flow rate∧2 | 0.0141 | 0.0006 | 0.1905 |
| Cell feed concentration | 0.655 | 0.2688 | 0.9032 |
| Buffy coat | 0.7222 | 0.1246 | 0.6076 |
- Twenty-eight experimental samples were run in reduplicate with the different operational conditions.
The above discussion indicates that three measures of performance: recovery of PBL, cancer cells, and the enrichment rate of cancer cells, are functions of three primary operational parameters of the QMS. Due to the contradictory impact of these operational parameters on QMS performance, the weight of each measure for the performance must be considered in search of the optimized values of the operational parameters. Generally, the enrichment of cancer cells and the recovery of cancer cells are most desirable in our studies. To simplify the prediction, the cell feed concentration was set to be 5 × 106 cells/ml. Therefore, the ranges of optimized values for the other operation parameters were determined as follows: flow rate in the range from 3 to 5 ml/min and the magnetophoretic mobility in the range of 1.5 × 10−4 – 2.5 × 10−4 mm3/(T A s).
To validate the feasibility of the above assumption, a preliminary experiment was performed using the above optimized parameters, i.e. a flow rate of 3 ml/min, a magnetophoretic mobility of 1.8 × 10−4 mm3/(T A s) obtained using the one-step labeling protocol, and a cell feed concentration of 5 × 106 cells/ml. With these conditions, a 61.6% recovery of initial spiked cancer cells and an enrichment rate of 98.7. (Note, for this run, the initial cancer cell concentration was one cancer cell in 103 total PBL).
Detection of CTCs by ICCS
Figure 6a–d illustrate a set of representative photographs showing the ICCS techniques used for visual, microscopic observations to identify the presence and number of cancer cells before and after the enrichment experiments. All four photos are of the same slide, which was stained with an anticytokeratin–FITC conjugate and Hochest 33342 (nuclei stain) stain. The larger, Detroit-562 cells are clearly visible in Figures 6a, 6c, and 6d and are the only cells stained “green” in Figure 6a. Figure 6b is a filtered photo for Hochest 33342 where cell nuclei appear blue, while Figure 6c is a combination of Figures 6a and 6b. As a point of reference, Figure 6d is a bright-field image. In subsequent analysis, to be considered a cancer cell, the cell must be doubled stained, i.e. green and blue, while to be a PBL it must be stained only blue, and any other object is considered a red blood cell or cell debris.
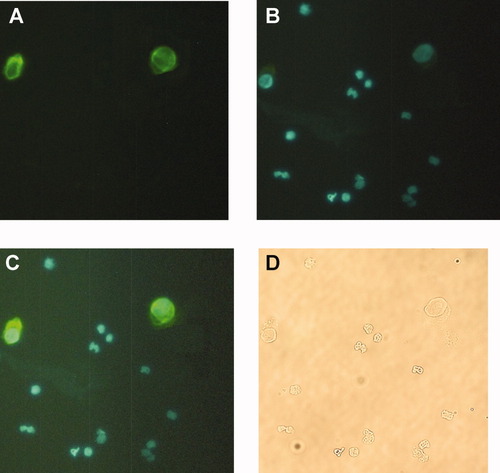
Various wavelength filtered, photographs of the same slide to which PBL and Detroit-562 cells were attached. The slide was stained with anti-cytokeratin-FITC (green) and Hochest 33342 (blue) labels. (a) Filtered photograph of FITC stained cells. (b) Filtered photograph of Hochest 33342 stained cells. (c) Filtered image combining (a) and (b), and (d) Bright-field image of the same slides.
Enrichment Model of CTC Suspension
To mimic the potential concentration of CTCs in clinical samples, Detroit-562 cells were spiked at a ratio of one cell to 105 total PBL. Based on the results of the preliminary optimization studies, two sets of experiments were conducted to enrich spiked cancer cells in human PBL: one set using the two-step and the other set using the one-step immunomagnetic labeling, respectively. The results of those two sets of enrichment experiments are listed in Tables 3 and 4.
| Run | Average | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Flow rate (ml/min) | 10 | 5 | 5 | 4 | 5 | |
| Cell throughput rate (cells/s) | 8.3 × 105 | 4.2 × 105 | 4.2 × 105 | 3.3 × 105 | 4.2 × 105 | |
| Magnetophoretic mobility (mm3/T.A.s) | 2.4 × 10−4 | 1.96 × 10−4 | 1.3 × 10−4 | 1.89 × 10−4 | 1.46 × 10−4 | |
| Feedstock | ||||||
| Number of PBL used | 8.0 × 107 | 7.0 × 107 | 8.0 × 107 | 8.0 × 107 | 8.0 × 107 | |
| Number of total Detroit-562 used | 800 | 700 | 800 | 800 | 800 | |
| Detroit-562 concentration (cells/PBL) | 1.0 × 10−5 | 1.0 × 10−5 | 1.0 × 10−5 | 1.0 × 10−5 | 1.0 × 10−5 | 1.0 × 10−5 |
| Cell numbers after QMS | ||||||
| Number of PBL recovered in the outlet a | 3.4 × 106 | 5.35 × 106 | 1.3 × 106 | 1.35 × 106 | 4.4 × 106 | |
| Number of Detroit-562 recovered in the outlet a | 560 | 450 | 278 | 242 | 300 | |
| Final Detroit-562 purity (cells/total cells) | 1.65 × 10−4 | 8.41 × 10−5 | 2.14 × 10−4 | 1.79 × 10−4 | 6.82 × 10−5 | 1.4 2± 0.63 × 10−4 |
| Detroit-562 recovery in the outlet a (%) | 70.0 | 64.4 | 34.8 | 30.3 | 37.5 | 47.4 ± 18.4 |
| Enrichment (–) | 16.50 | 8.50 | 21.20 | 17.93 | 6.82 | 14.2 ± 6.2 |
| Run | Average | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Flow rate (ml/min) | 5 | 5 | 3 | 5 | 3 | 3 | |
| Cell throughput rate (cells/s) | 4.2 × 105 | 4.2 × 105 | 2.5 × 105 | 4.2 × 105 | 2.5 × 105 | 2.5 × 105 | |
| Magnetophoretic mobility [mm3/(T A s)] | 1.77 × 10−4 | 1.58 × 10−4 | 1.58 × 10−4 | 2.51 × 10−4 | 2.51 × 10−4 | 2.26 × 10−4 | |
| Feedstock | |||||||
| Number of PBL used | 8.0 × 107 | 8.0 × 107 | 8.0 × 107 | 8.0 × 107 | 8.0 × 107 | 9.0 × 107 | |
| Number of total Detroit-562 used | 800 | 800 | 800 | 800 | 800 | 900 | |
| Detroit-562 concentration (cells/PBL) | 1.0 × 10−5 | 1.0 × 10−5 | 1.0 × 10−5 | 1.0 × 10−5 | 1.0 × 10−5 | 1.0 × 10−5 | 1.0 × 10−5 |
| Cell numbers after QMS | |||||||
| Number of PBL recovered in the outlet a | 3.6 × 106 | 2.04 × 106 | 6.5 × 105 | 1.09 × 106 | 7.8 × 105 | 1.17 × 106 | |
| Number of Detroit-562 recovered in the outlet a | 540 | 640 | 640 | 700 | 600 | 696 | |
| Final Detroit-562 purity (cells/total cells) | 1.50 × 10−4 | 3.14 × 10−4 | 9.85 × 10−4 | 6.45 × 10−4 | 7.69 × 10−4 | 5.95 × 10−4 | 5.76 ± 3.03 × 10−4 |
| Detroit-562 recovery in the outlet a (%) | 67.5 | 80.0 | 80.0 | 87.5 | 75.0 | 76.9 | 77.8 ± 6.6 |
| Enrichment (–) | 15.0 | 31.4 | 98.5 | 64.5 | 76.9 | 59.5 | 57.6 ± 30.3 |
In those spiked cell studies, the final, optimized process produced a final enrichment of the rare cancer cells of 14.2 ± 6.2 with a final recovery of (47.4 ± 18.4)% using the two-step immunomagnetic labeling, as shown in Table 3. Summarizing the data obtained in the enrichment experiments using the one-step immunomagnetic labeling (Table 4), the rare cancer cells could be enriched to an average enrichment rate of 57.6 ± 30.3 with a final recovery of (77.8 ± 6.6)%.
Detection of CTCs by RT-PCR
Detection of EGFR positive cancer cells in the enriched fraction using RT-PCR in addition to ICCS was also performed, and representative examples are presented in Figure 7. Figure 7a indicates that the EGFR-specific band was detected in all of the enriched samples, yet could not be detected in the unspiked PBL, and the spiked but not enriched PBL (lanes 1 and 2 of Fig. 7a). Furthermore, significantly brighter bands were obtained in the samples from the single-step immunomagnetic labeling protocol (lanes 5 and 6), which is consistent with the fourfold higher purity that results from the single step labeling protocol. It should be noted that there was no significant difference in the expression level of ubiquitously expressed control gene HPRT among the tested samples (Fig. 7b).
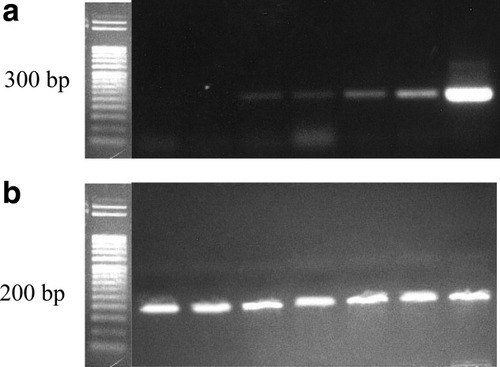
PCR detection of the enriched sample by EGFR and HPRT, respectively. Upper panel: 1, PBL; 2, initial cell suspension; 3, sample 3 in Table 3; 4, sample 5 in Table 3; 5, sample 5 in Table 4; 6, sample 6 in Table 4; 7, Detroit-562 cells. Lower panel: The same samples were analyzed with RT-PCR for HPRT primers. DNA ladder was purchase from Sigma, 50 bp per band.
DISCUSSION
The primary goal of this study was to optimize an enrichment process for rare cancer cells in human blood to a sufficient extent that RT-PCR analysis can be performed on the final sample in a reliable manner. Previously reported work in our laboratory has indicated that a negative depletion mode of enrichment using our QMS system produced reproducible, significantly enriched samples (34). To properly determine the level of enrichment needed, the sensitivity of RT-PCR for EGFR in three HNSCC lines was also investigated.
Semiquantitative observations of the gel electrophoresis of RT-PCR product indicated significant difference in both the final purity of cancer cells in the total cell suspension needed for detection, as well as significant differences in the total amount of RNA needed from the three different HNSCC lines. Table 5 attempts to quantify these observations. Specifically, for PBL and the three HNSCC lines, the amount of RNA, the amount of RNA per cell, and the calculated number of cells needed for RT-PCR detection is listed. In addition, the purity needed (number of cancer cells to number of total cells) for detection is also presented. While care should be taken in the use of these numbers, on an order of magnitude basis, one can observe a significant range in the number of cancer cells needed for detection with RT-PCR as well as a significant range in the purity needed. Also note the consistency in that, the purity needs to be higher in the low EGFR expressing cells relative to the high expressing cells. Such ranges in both the total amount of RNA needed as well as the purity for the same mRNA marker helps to explain variability reported in the literature for cancer cell detection in human peripheral blood.
| Cell line | Sensitivity limit of RNA mass for EGFR (μg) | RNA mass per cell (μg) | Cells needed for RT-PCR detection |
|---|---|---|---|
| PBL | – | 2.4 ± 0.6 ×10−6 | – |
| Detroit-562 | 1.0 × 0−4 | 14.2 ± 2.0 ×10−6 | 7.1 ± 1.0 |
| CAL-27 | 1.0 × 10−5 | 12.8 ± 2.9 ×10−6 | 0.8 ± 0.2 |
| SCC-4 | 1.0 × 10−3 | 35.3 ± 5.8 ×10−6 | 28.3 ± 4.7 |
While a 100% recovery of tumor cells without any unwanted blood cells in the final enrichment suspension is ideal, it is well known in the chemical process community that the overall recovery of a target product in a multistep process is the product of the recovery of each step. For example, in the enrichment for a rare cancer cell in a blood sample, one of the steps involves removal of the RBCs. According to our previous publication, a density-gradient centrifuge will lead to a 30% loss of spiked tumor cells while a red cell lysis procedure will result in a 10% loss in tumor cells (34). In the current studies, since the focus of the work was to optimize the magnetic depletion step for RT-PCR analysis, our starting blood sample was a buffy coat in which we had also performed a red cell lysis procedure, and to which we subsequently spiked the tumor cells.
A further example of the loss of cells in processing steps is observed in comparing the final recovery of spiked cancer cells using a single step versus a two step labeling protocol, which results in a 78 versus 47% recovery, respectively (Tables 3 and 4). We have previously reported that an average recovery of ∼93% per centrifuge step was obtained for washing the free antibody from the cell suspension. Consequently, it is highly likely that the increased recovery, and corresponding purity, is at least partially the result of less processing steps.
Our previous studies have demonstrated that the magnetophoretic mobility of an immunomagnetically labeled cell significantly affects the magnetic separation performance of the QMS system as well as other magnetic separation systems (34, 36, 44, 45). Our previous studies have also demonstrated that a “drafting” phenomena exists in which magnetically labeled cells call “pull” unlabeled cells in the direction that the magnetically labeled cells move as a result of the imposed magnetic energy gradient (46).
In addition to increasing the recovery, to have a 100% enrichment one needs to remove all the unwanted cells. Comparison of this current study to Lara et al. (34) indicates that despite targeting the same surface marker, CD45, having similar magnetophoretic mobilities, and separating similar number of total cells, the enrichment of cancer cells 171.9 ± 151.0 was three times better in the Lara et al.'s (34) study than the current one of 57.6 ± 30.3. The most obvious reason for this difference is the age of the blood sample. In the Lara et al. (34) study, the blood sample used was fresh (under 2 h after being drawn from the person) whereas the blood used in this study was at least 24 h old. Tanner et al. (47) indicated the significant changes in the relative expression of several genes for cytokine, chemokines, and CD surface markers in blood because of storage at room temperature. Although Schmidtke et al. (48) reported there is no obvious change of CD45 expression for monocytes and lymphocytes at 4 and 25°C during 3-day storage, the upregulation or downregulation of several gene expressions would result in the activation of phagocytes, such as neutropils, macrophages, and natural killer cells. Also, while on average, the expression level of a particular cell might not change significantly if the distribution was to “broaden”, with a significant increase in the low expressing, yet positive, CD45+ cells, the probability of separating these lower expressing cells would decrease.
All these speculations indicate the importance of using fresh blood. Other studies of 4-day-old blood (data not shown) further confirm the need for the rapid whole blood analysis. In conclusion, despite incomplete enrichment of the rare cells, the enrichment is sufficient to allow RT-PCR detection of only a few spiked cancer cells into a human. Ongoing studies are currently focused on improving the final purity (improve the enrichment) as well as identification of other relevant mRNA targets, and testing of the system on the peripheral blood of cancer patients.




