Flow cytometric immunphenotyping of epithelial cancer cells in effusions—Technical considerations and pitfalls
Abstract
Background:
Data regarding the role of flow cytometry (FCM) in the characterization of malignant effusions are limited to date. In the present study, we optimized the conditions for FCM immunphenotyping of effusions using a four-color analysis and investigated aspects related to the advantages and limitations of this method in this setting.
Methods:
FCM analysis optimization for the study of epithelial cells was undertaken using five carcinoma cell lines, and subsequently applied to malignant pleural and peritoneal effusions using antibodies against epithelial and mesothelial markers (Ber-EP4 and EMA), CD138, and integrin subunits. FCM of frozen versus fresh specimens and the performance of FCM compared to immunhistochemistry were evaluated.
Results:
FCM optimization was achieved and applied to clinical specimens, with resulting detection of epithelial markers and adhesion molecules on cancer cells. Frozen clinical specimens and cell lines showed reduced CD138 expression compared to fresh specimens, with conservation of the remaining epitopes. FCM generally showed comparable performance to immunhistochemistry.
Conclusions:
FCM is an effective method for characterization of cancer cells in clinical effusion specimens in both the diagnostic and research setting, and is comparable to immunhistochemistry in terms of sensitivity and specificity, with the additional advantage of providing quantitative data. The majority of epitopes are conserved in frozen cells, but a minority may be lost, suggesting that the thorough testing of each antibody in both conditions is mandatory. © 2007 Clinical Cytometry Society
Flow cytometry (FCM) is an established method for the characterization of cells of hematological origin, and is routinely used in the diagnosis of lymphoma and leukemia (1-3). The ability of FCM to detect several antigens simultaneously renders it an optimal tool for the study of other difficult biological and diagnostic issues. However, because of the different characteristics of cell populations of nonhematological lineage, a great deal of work needs to be done in way of optimization and calibration of this technique.
The body cavities are frequent sites of tumor metastasis, and are the site of origin of several tumors, including primary peritoneal carcinoma and malignant mesothelioma (MM). The cytological distinction between carcinoma cells, inflammatory cells (especially macrophages), and reactive or malignant mesothelial cells can at times be challenging. Immunocytochemistry (ICC) on cytospin specimens or formalin-fixed paraffin-embedded cell block sections is the most widely used ancillary method in this setting and has been shown to increase the overall diagnostic accuracy in many studies (4-7). However, the use of different protocols and different antibodies or clones may lead to variable results, and some antibodies are not suitable for analysis in formalin-fixed material.
FCM immunophenotyping is a rapid, reproducible, and sensitive method for detecting cellular (cytoplasmic, nuclear, and surface) antigens in cytological material. Many directly-conjugated fluorescent antibodies are now commercially available and can be used in analyses of fresh or frozen material. Multicolor FCM provides the opportunity to evaluate multiple antigens simultaneously, making it possible to characterize various cellular classes in a more precise manner. In effusion cytology, FCM has so far mainly been used to detect DNA-aneuploid cell populations (8-11), sometimes in combination with immunophenotyping of admixed lymphoid cells (12, 13). Recently, a few studies from our laboratory have demonstrated the possibility of immunophenotyping epithelial and mesothelial cells in effusions using two-color analysis for diagnostic and research purposes (14-16).
- 1
To adapt the instrument settings and to optimize the protocol for the study of epithelial cells using a four-color analysis. This was performed by using several epithelial cell lines of breast, lung, and ovary origin and normal peripheral blood leukocytes (PBL). In addition, antibodies were titrated to obtain an optimal concentration.
- 2
To compare the antigenic immunoreactivity in frozen and fresh material, with the aim of evaluating possible alterations induced by freezing of cells.
- 3
To compare the sensitivity of FCM and ICC.
MATERIALS AND METHODS
Cell Lines and Peripheral Blood Leukocytes
The five human cell lines used in the present study were MDA-MB-231, SK-Br-3 and T47-D (breast carcinoma), OVCAR-3 (ovarian carcinoma), and NCI-H520 (lung squamous cell carcinoma). All cell lines were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Cambrex Bio Science, Rockland, ME) supplemented with 10% fetal calf serum (FCS) (Serva, Heideberg, Germany). Cells were either analyzed fresh or after freezing in RPMI 1640 medium with 50% FCS and 20% dimethylsuloxide (DMSO) (Merck KGaA, Darmstadt, Germany). PBL were from healthy donors.
Effusions
Antigenic immunoreactivity of fresh and frozen specimens was analyzed in 10 fresh nonfixed peritoneal and pleural effusions (eight adenocarcinomas, one MM, and one benign) submitted to the Pathology Clinic, Radiumhospitalet-Rikshospitalet Medical Center, for routine diagnostic purposes. Specimens were centrifuged for 10 min at 2,000 rpm. The resulting pellet was used for the routine cytological diagnosis and evaluation of adequacy. The remaining material was divided for FCM analysis and for freezing at −70°C in RPMI 1640 medium with 50% FCS and 20% DMSO at ratio of 1:1.
For comparative analysis of FCM and ICC, the analyzed material consisted of 20 effusion specimens. The material was divided for freezing as described above and for cellblock preparation using the thrombin clot method. These 20 cases consisted of 16 adenocarcinomas and 4 MM.
Informed consent was obtained according to national and institutional guidelines.
FCM
FCM was undertaken using the FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA) equipped with a 15 mW argon–ion laser (488 nm) and 12 mW red diode laser (635 nm). Fluoroscein isothiocynate (FITC, FL1, BP 530/30 nm), phycoerithrin (PE, FL2, BP 585/42 nm), peridinin chlorophyll protein (PerCP, FL3, LP 670 nm), and allophycocyanin (APC, FL4, BP 661/16) measurements were collected in the logarithmic mode. For each measurement, data from 50,000 to 100,000 events was acquired.
Antibody titration.
The antibodies used in this study are detailed in Table 1. Prior to clinical specimen analysis, the EMA, CD15, CD138 antibodies, and the αV, α6, β1 (CD29), β3 (CD61) integrin subunit antibodies were titrated using the T47-D cell line at serial dilutions of 1:2.5, 1:5, 1:10, and 1:20, to determine optimal concentration. The Ber-EP4 antibody was used in the same conditions employed in our previous studies (14-16). The CD45 and CD14 antibodies were previously titrated for routine diagnosis of hematology specimens and the same concentration was used in this study.
| Antibody | Source | Clone |
|---|---|---|
| Ber-EP4 | Dako, Glostrup, Denmark | Ber-EP4 |
| EMA | Dako, Glostrup, Denmark | E-29 |
| β1 integrin subunit | Dako, Glostrup, Denmark | K-20 |
| CD15 | Becton Dickinson, San Jose, CA | MMA |
| β3 integrin subunit | Becton Dickinson, San Jose, CA | RUU-PL7F12 |
| CD138 | Serotec, Oxford, UK | B-B4 |
| αV integrin subunit | Santa Cruz Biotechnology, Santa Cruz, CA | P2W7 |
| α6 integrin subunit | Santa Cruz Biotechnology, Santa Cruz, CA | GOH3 |
| CD45 | Becton Dickinson, San Jose, CA | 2D1 |
| CD14 | Diatec, Oslo, Norway | 18D11 |
| IgG1 | Dako, Glostrup, Denmark | DAK-GO1 |
| IgG1 | Becton Dickinson, San Jose, CA | X40 |
The same instrument settings were used and the median fluorescence intensity (MFI) of different concentrations was first compared with the isotype-matched negative control cells to appreciate how well the signals were separated from background staining (signal-to-noise ratio; S/N) and to evaluate distributions of dot plots to ensure that compensation was optimal. The S/N ratio was calculated by dividing the MFI value for the positive cells by that for the negative cells.
Direct staining procedure.
Frozen material was thawed and 10 ml RPMI 1640 with 10% FCS was added. After centrifugation at 1,200 rpm in 10 min, the supernatant was decanted. The cells were blocked by the addition of 100 μl normal rabbit serum (1:5 dilution) (Dako, Glostrup, Denmark) for 25 min at room temperature, washed in 1 ml PBS, centrifuged for 8 min at 1,000 rpm, and subsequently divided into respective tubes for direct staining. The antibody combinations employed are detailed in Table 2A.
| FITC | PE | PerCP | APC | |
|---|---|---|---|---|
| 1 | β1 integrin | αV integrin | β3 integrin | CD45 |
| 10 μl (1:5) | 10 μl(1:5) | 10 μl (1:5) | 5 μl (1:10) | |
| 2 | β1 integrin | α6 integrin | CD45 | Isotype (IgG1) |
| 10 μl (1:5) | 10 μl (1:5) | 5 μl (1:10) | BD – 5 μl (1:10) | |
| 3 | Ber Ep4 | CD138 | CD45 | CD14 |
| 5 μl (1:10) | 10 μl (1:5) | 5 μl (1:10) | 10 μl (1:5) | |
| 4 | Ber Ep4 | αV integrin | CD45 | CD14 |
| 5 μl (1:10) | 10 μl (1:5) | 5 μl (1:10) | 10 μl (1:5) | |
| 5 | EMA | α6 integrin | CD45 | Isotype (IgG1) |
| 5 μl (1:10) | 10 μl (1:5) | 5 μl (1:10) | BD – 5 μl (1:10) | |
| 6 | CD15 | CD138 | CD45 | Isotype (IgG1) |
| 5 μl (1:10) | 10 μl (1:5) | 5 μl (1:10) | BD – 5 μl (1:10) | |
| 7 | Isotype (IgG1) | Isotype (IgG1) | Isotype(IgG1) | Isotype (IgG1) |
| DAKO – 5 μl (1:10) | DAKO – 5 μl (1:10) | BD – 5 μl (1:10) | BD – 5 μl (1:10) |
Prediluted and directly conjugated monoclonal antibodies were added to each tube. The cells were vortexed and incubated in the dark at room temperature for 25 min. One milliliter PBS was added and the cells centrifuged for 8 min at 1,000 rpm. After staining with membrane makers the cells in each tube were incubated with 5 μl of 7-amino-actinomycin D (7-AAD) ready-to-use solution (BD Biosciences Pharmingen, San Diego, CA) for 10 min at 4°C in the dark. The cells were then washed with 1 ml PBS and centrifuged for 8 min at 1,000 rpm. The supernatant was decanted and 200–500μl FACSFlow (Becton-Dickinson) added, followed by filtration of the samples through a 70 μm nylon filter (Becton-Dickinson). The samples were then put on ice until analysis within 1 h.
Instrument calibration/optimization.
Optimization and calibration of the instrument setting was achieved using the mixture of T47-D cells and PBL. The cells were divided into different tubes and stained as listed in Table 2B.
| FITC | PE | PerCP | APC | |
|---|---|---|---|---|
| 1 | Unstained | Unstained | Unstained | Unstained |
| 2 | Isotype (IgG1) | Isotype (IgG1) | Isotype (IgG1) | Isotype (IgG1) |
| DAKO – 5 μl (1:10) | DAKO – 5 μl (1:10) | BD – 5 μl (1:10) | BD – 5 μl (1:10) | |
| 3 | Ber Ep4 | Isotype (IgG1) | Isotype (IgG1) | Isotype (IgG1) |
| 5μl (1:10) | DAKO – 5 μl (1:10) | BD – 5 μl (1:10) | BD – 5 μl (1:10) | |
| 4 | Isotype (IgG1) | αV integrin | Isotype (IgG1) | Isotype (IgG1) |
| DAKO – 5 μl (1:10) | 10 μl (1:5) | BD – 5 μl (1:10) | BD – 5 μl (1:10) | |
| 5 | Isotype (IgG1) | Isotype (IgG1) | CD45 | Isotype (IgG1) |
| DAKO – 5 μL (1:10) | DAKO – 5 μl (1:10) | 5 μl (1:10) | BD – 5 μl (1:10) | |
| 6 | Isotype (IgG1) | Isotype (IgG1) | Isotype (IgG1) | CD3 |
| DAKO – 5 μl (1:10) | DAKO – 5 μl (1:10) | BD – 5 μl (1:10) | 5 μl (1:10) | |
| 7 | Ber Ep4 | CD19 | CD45 | CD3 |
| 5 μl (1:10) | 5 μl (1:10) | 5 μl (1:10) | 5 μl (1:10) |
Setting up the flow cytometer.
Control and time delay calibration was carried out using FACSComp software version 4.1 (Becton-Dickinson) and CaliBRITE beads (Becton-Dickinson) for four-color flow cytometer setup. FSC and SSC parameters were defined in linear amplification mode; all fluorescence parameters (FL1, FL2, FL3, and FL4) were defined in logarithmic amplification mode. Threshold was based on FSC as a primary parameter and all compensation settings were set to zero. During calibration FSC photodiode amplifier gain and SSC PMT settings were adjusted using the unstained cells such that most of T47-D/PBL cells fell on scale within the FSC and SSC windows of analysis. Threshold value was then set in channel 97 to eliminate debris following adjustment of PMTs (FL1, FL2, FL3, and FL4) using first unstained and then isotypic stained cells. Each PMT voltage was set high enough guaranteeing that the negative population was slightly off the axis in every channel and located in left lower quadrant. Compensation was carried out for each fluorochrome. The mean channel value of FL positive population and negative population was comparable (+/−10 channel2), as detailed in Table 2C (17). Finally, the instrument settings and compensation settings were verified/fine-tuned with cells stained with antibodies conjugated to four different fluorochromes. The obtained instrument settings were then stored.
| Staining | Parameter | Dotplot (x, y) | Criteria |
|---|---|---|---|
| Unstained cells | Detectors/amplifier | FSC, SSC | Adjustment of FSC amplifier gain and SSC, FL1, FL2, FL3, and FL4 detectors as described above |
| FL1, FL2 | |||
| FL2, FL3 | |||
| FL3, FL4 | |||
| Isotypic stained cells | Detectors/amplifier | FSC, SSC | Adjustment of FSC amplifier gain and SSC, FL1, FL2, FL3, and FL4 detectors as described above |
| FL1, FL2 | |||
| FL2, FL3 | |||
| FL3, FL4 | |||
| Ber-Ep4 FITC | FL2 – % FL1 | FL1, FL2 | FL2 mean Ber-Ep4 FITC positive = FL2 mean Ber-Ep4 negative(+/− 10 channel2) |
| αV integrin PE | FL1 – % FL2 | FL1, FL2 | FL1 mean αV integrin PE positive = FL1 mean αV integrin PE negative (+/− 10 channel2) |
| αV integrin PE | FL3 – % FL2 | FL2, FL3 | FL3 mean αV integrin PE positive = FL3 mean αV integrin PE negative (+/− 10 channel2) |
| CD45 PerCP | FL4 – % FL3 | FL3, FL4 | FL4 mean CD45 PerCP positive = FL4 mean CD45 PerCPnegative (+/− 10 channel2) |
| CD3 APC | FL3 – % FL4 | FL3, FL4 | FL3 mean CD3 APC positive = FL3 mean CD3 APC negative(+/− 10 channel2) |
Evaluation of FCM immunophenotyping.
This was undertaken in a standardized way using CellQuest Software version 3.4 (Becton Dickinson). A gating procedure was generated by combining side angle light scatter channel (SSC) versus 7-AAD/CD45 PerCP fluorescence (7-AAD was detected in FL3 channel), and a region was drawn around clear-cut populations having negative 7-AAD/CD45 PerCP fluorescence. Cells in this region were again viewed by generating a cytogram combining SSC versus FSC, and a gating procedure was used to exclude cell debris, by including only cells with relatively high SSC and FSC values. Quadrant cursors were set by using isotypic negative controls. Quadrant setting was undertaken so that in negative controls 99% of the cells were localized in the left lower quadrant. Cell populations were interpreted as immunoreactive for a given antibody only when unequivocal separation from the negative controls (lymphocytes in the case of epithelial markers, carcinoma cell lines in the case of CD45) could be demonstrated. Expression in <1% of cells was scored as negative. Staining intensity was not recorded.
ICC
Formalin-fixed paraffin-embedded cell block sections, 4-μm-thick, from 20 effusions were mounted onto silane-coated slides. Slides were air-dried at 60°C for 45 min and then at 37°C overnight. Slides were deparaffinized and rehydrated prior to staining. The pretreatment antigen unmasking conditions are detailed in Table 3. Staining was performed using the EnVision Peroxidase (DAB) kit (Dako). Appropriate positive and negative controls were used.
| Antibody | Source | Clone | Dilution | Antigen retrieval |
|---|---|---|---|---|
| Ber-EP4 (mouse) | Dako (Glostrup, Denmark) | Ber-EP4 | 1:20 | Microwave, 1× DAKO Target Retrieval Solution (pH 6.0) |
| EMA (mouse) | Dako (Glostrup, Denmark) | E-29 | 1:40 | Microwave, citrate buffer (pH 6.0)(Sigma, Saint Louis, MO) |
| CD138 (mouse) | Serotec (Oxford, UK) | B-B4 | 1:20 | Microwave, 1× DAKO Target Retrieval Solution (pH 6.0) |
| αV integrin (mouse) | Santa Cruz Biotechnology (Santa Cruz, CA) | P2W7 | 1:50 | Microwave, EDTA buffer (pH 8.0) (Sigma) |
| β3 integrin (mouse) | Immunotech (Marseille, France) | SZ21 | 1:50 | Microwave, 1× DAKO Target Retrieval Solution (pH 6.0) |
Staining intensity and extent (percentage of stained cells) were scored. Staining intensity was scored semiquantitatively as 0–3 corresponding to absent, weak, moderate, and strong staining pattern. Staining extent was determined as the average number of cells in each 40× field throughout the specimen.
Statistical Analysis
Statistical analysis was performed applying the SPSS-PC package (Chicago, IL). Probability of <0.05 was considered statistically significant. Evaluation of the association between FCM and ICC results in 20 effusions was executed using the Wilcoxon Signed Ranks Test.
RESULTS
Optimization/Calibration of the Instrument Settings
The instrument setting used in the diagnosis of hematological malignancies is not suitable for use in the characterization of cells of epithelial origin, due to differences in cell size, cell complexity, autofluorescence background level, etc. Thus, calibration of the instrument is a crucial step to obtain an optimal instrument setting suitable for analysis of epithelial cells.
Adjustment of FSC and SSC.
FSC photodiode amplifier gain and SSC PMT settings were adjusted so that most of T47-D cells and PBL fell on scale within the FSC and SSC windows of analysis as illustrated in Figures 1A and 1B. Figure 1B shows that optimal values for FSC and SSC were gain 1.10 and 331 volt (V), respectively.
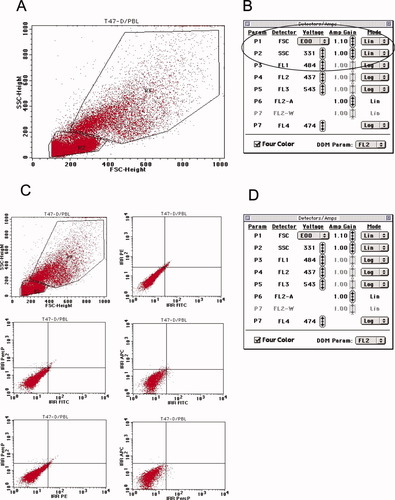
Optimization/calibration of the instrument settings: A, B: FSC photodiode amplifier gain and SSC PMT settings were adjusted so that T47-D cells and PBL are visible in the two regions R3 and R2, respectively; C, D: Background level of isotype stained T47-D/PBL cells. C: Six bivariate displays of FSC versus SSC, FL1 (FITC) versus FL2 (PE), FL1 versus FL3 (PerCP), FL1 versus FL4 (APC), FL2 versus FL3, and FL3 versus FL4. D: Window of detectors/Amps showing optimal values for FSC, SSC, FL1, FL2, FL3, and FL4. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
PMTs (FL1, FL2, FL3, and FL4) adjustment.
Each PMT was adjusted using first unstained and then isotypic stained mixture of T47-D cells and PBL. Each PMT voltage was set high enough to guarantee that the negative population was slightly off the axis in every channel (Figs. 1C and 1D). The values for FL1, FL2, FL3, and FL4 were 484V, 437V, 543V, and 474V, respectively. The background level of isotypic stained cells should not have exceeded 102 log decade.
Compensation.
Single-color stained mixture of T47-D and PBL cells (Table 2B) was acquired. The parameters for electronic compensation were adjusted so that each FL parameter was compensated. The mean channel value of FL positive population and negative population was comparable (+/− 10 channel2), as detailed in Table 2C. Figures 2A and 2B illustrate adjustments and pattern for compensation for Ber-Ep4 FITC and αV integrin subunit PE, respectively. Examples of compensation for FL3 (CD45 PerCP) and FL4 (CD3 APC) were based on PBL cells (data not shown). Finally, the instrument settings and compensation settings were confirmed and fine-tuned with cells stained with antibodies conjugated to four different fluorochromes (Fig. 2C).
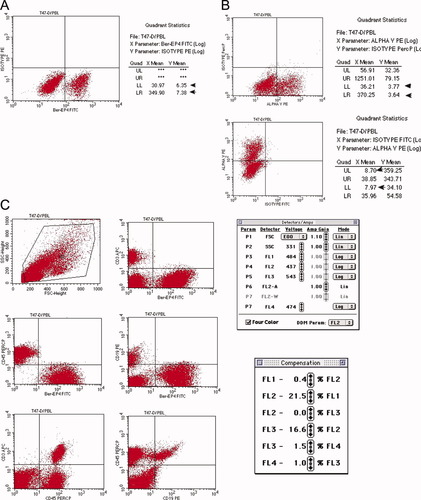
Compensation: A: The pattern obtained after single color compensation. The FL2 mean channel of Ber-Ep4-FITC positive cells and the FL2 mean channel of Ber-Ep4-FITC negative cells were 7.38 and 6.35, respectively (arrows); B: Illustration of proper compensation for the αV integrin subunit. Small amount of PE fluorescence signal leaks into the FL1 (top row) and FL3 (bottom row) channel and was collected by these filters. Appropriate adjustments were made to the compensation to obtain the correct pattern; C: Instrument and compensation settings were verified and readjusted with cells labeled with antibodies conjugated to four different fluorochromes, so that each population was located within the appropriate quadrant. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Antibody titration.
The β3 integrin subunit (CD61) was not expressed on T47-D cells. The concentration used in this study was therefore chosen based on previous titration for routine diagnosis of hematology specimens.
The S/N ratio of CD15 at concentrations 1:2.5 (20 μl), 1:5 (10 μl), 1:10 (5 μl), and 1:20 (2.5 μl) was 34.3, 54.5, 60.8, and 56.1, respectively. 1:10 (5 μl) dilution generated the highest ratio and provided the greatest discrimination between positive and negative cells (Fig. 3A). At 1:2.5 and 1:5 dilutions, the FITC fluorescence signals could not be sufficiently compensated as illustrated by the oblique and flattened distribution of the positive cells (Fig. 3B). These excessive concentrations also resulted in high background staining detected in the FL2, FL3, and FL4 channels (Fig. 3C). At 1:10 concentration, the compensation of FITC fluorescence from the FL2 channel was optimal and a strong fluorescence signal was maintained (Fig. 3B). Thus, this concentration was used throughout the study.
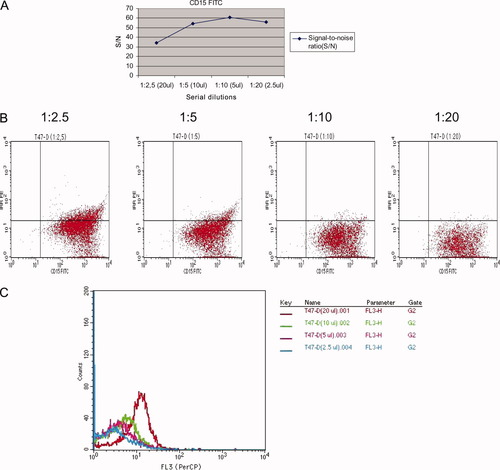
Antibody titration: A, B: Bivariate data displays of CD15 FITC titration at serial dilutions 1:2.5, 1:5, 1:10, and 1:20; C: An example of high background staining of CD15 positive cells detected in the FL3 (PerCP) channel. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The concentrations for EMA, CD15, CD138, αV, α6, β1 (CD29) integrin subunits shown in Table 2A were found to be optimal based on the aforementioned method of analysis. For EMA and CD15, using half the volume recommended by suppliers was appropriate to obtain good discrimination from negative control cells. The concentrations used for CD138 and the αV, α6, β1 (CD29) integrin subunits were as recommended by the suppliers.
Antigenic Immunoreactivity Comparison of Fresh and Frozen Material
The analyzed material consisted of the five cell lines described above and 10 fresh nonfixed peritoneal and pleural effusions submitted for diagnostic purposes. These were evaluated in the fresh and frozen state, using the antibodies listed in Table 1.
The β3 integrin subunit was not expressed in any of the five cell lines at both conditions. Ber-EP4, EMA, and CD15 were not expressed in MDA-MB-231 at both conditions. However, the expression of these markers was comparable in the other cell lines at both conditions, as was the expression of the αV, α6, and β1 integrin subunits (data not shown). In contrast, CD138 expression was either lost or significantly reduced in the frozen condition. CD138 expression was lost in MDA-MB-231 cells. OVCAR-3 had a slightly reduced expression in the frozen sample, whereas the SK-BR-3, T47-D, and NCI-H520 lines showed a reduction of 22, 44, and 33% compared to fresh samples, respectively (Fig. 4A). We repeated the experiment using another CD138 antibody (clone MI15, Dako) with similar results.
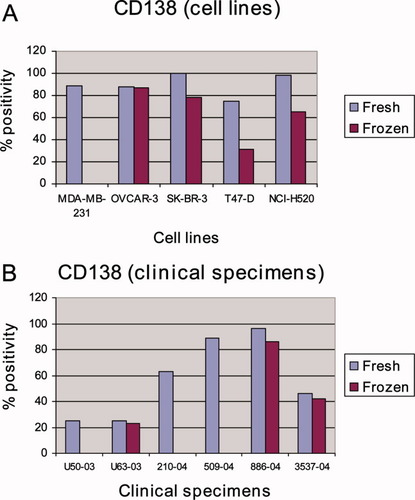
Antigenic immunoreactivity comparison of fresh and frozen material: A: Results for five cell lines stained for CD138 in the fresh and frozen state. The antigenic profile of CD138 was either lost or significantly reduced in frozen compared to fresh specimens; B: Results for 10 clinical specimens stained for CD138 in the fresh and frozen state. Four effusions did not express CD138 in either condition. Three effusions showed loss of CD138 and three remaining cases showed only mild reduction of CD138 in the frozen condition. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
As with cell lines, the β3 integrin subunit was not expressed in any of the clinical specimens in both conditions. Expression of Ber-EP4, EMA, CD15, and the αV, α6, β1 (CD29) integrin subunits correlated well between fresh and frozen specimens (results not shown). Four of ten cases did not express CD138 in either fresh or frozen samples. In three cases, the expression of CD138 was lost in the frozen effusion. Three specimens showed only mild reduction of CD138 expression in the frozen condition (Fig. 4B).
The loss of CD138 antigen was found both on viable and nonviable cells based on 7-AAD discrimination. The antigenic profile was also similar in both viable and nonviable cells in analysis of the other antibodies (data not shown).
Finally, a general observation was that the FSC and SSC properties of cells in frozen samples had changed and become more compressed and not as well distributed as in fresh samples. Nevertheless, this change in light scatter properties was not sufficient to modify or make the gating strategy difficult. Cases with long-term cryopreservation (dated back to 1998–1999) have shown a higher proportion of nonviable cells based on light scatter property observations and 7-AAD discrimination.
Comparison Between FCM and ICC
Formalin-fixed paraffin-embedded cell block sections from twenty specimens were stained using the Ber-Ep4, EMA, CD138, αV integrin, and β3 integrin antibodies. Four of these five antibodies were of similar clone and manufacturer as those used in FCM. The β3 integrin subunit used in FCM and ICC was from a different company (Becton-Dickinson and Immunotech, respectively). The β3 integrin subunit was absent in all cases using both methods. The αV integrin subunit was expressed in all 20 cases (100%) using both methods in a comparable percentage of cells (P > 0.05). CD138 was expressed in 7/20 (35%) and 15/20 (75%) specimens using FCM and ICC, respectively, with significantly higher percentage of cells using ICC (P = 0.01; Fig. 5A). Ber-EP4 was detected in 17/20 (85%) cases using both methods in a comparable percentage of cells (P > 0.05; Fig. 5B). EMA expression was seen in 19/20 (95%) samples using both methods. Statistically, the difference in the percentage of EMA expressing cells was marginally significant (P = 0.05) in favor of ICC.
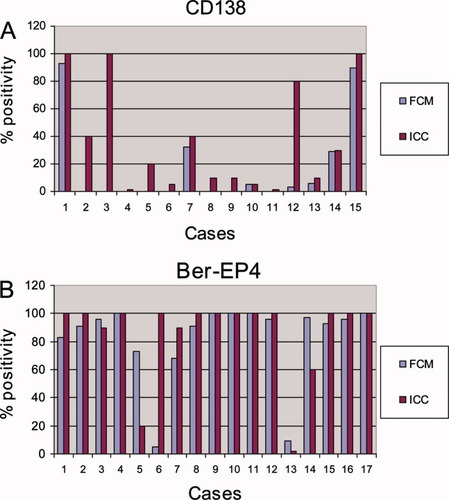
A: Results for 20 effusions stained for CD138 using FCM and ICC. CD138 was not detected in five samples using both methods. Eight specimens did not express CD138 using FCM. Statistically, the percentage of CD138 expression was significantly higher using ICC compared to FCM (P = 0.01, Wilcoxon Signed Ranks Test); B: Results for Ber-EP4. Negative staining was seen in three effusions using both methods. Ber-EP4 expression was significantly lower in one specimen using FCM. The percentage of positive cells in the other samples was comparable using both methods. Statistically, Ber-EP4 expression was comparable using ICC and FCM (P > 0.05, Wilcoxon Signed Ranks Test). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
FCM has a central role in immunophenotyping of hematological malignancies (1-3). In effusion cytology, FCM has previously been used mainly to detect DNA-aneuploid cellpopulations and/or for immunophenotyping of lymphoid cells (8-13). There are to date only rare reports regarding the use of FCM immunophenotyping as adjunct for the diagnosis and characterization of nonhematological cancer (14-16, 18, 19). Our studies have focused on several parameters related to FCM in metastatic carcinoma and MM.
Optimization/Calibration of the Instrument Settings
The instrument setting used in the diagnosis of hematological malignancies needs to be optimized to be used for characterization of cells of epithelial origin, because of differences in cell size, cell complexity, autofluorescence background level, etc. At present, most of the literature dealing with setting up and calibration of FCM for multicolor immunophenotyping is based on hematopoietic cell populations (17, 20, 21). Because of differences in cell properties and a higher level of autofluorescence background of epithelial cells, the values of FSC, SSC, and PMTs for FL1, FL2, FL3, and FL4 were lower compared to those used for hematological neoplasms.
Compensation is a very important process required for proper data analysis in FCM. The operators must be aware of the effects of compensation and recognize when data are improperly compensated. Some authors recommend the use of multiple-stained cells for compensation and assert that single-stained cells are inadequate for compensation (21). Others suggested that using single-stained cells for compensation setting would be optimal and adequate (17, 20). We use a single-stained mixture of T47-D/PBL cells to adjust the compensation settings for each FL parameter, and subsequently verify/fine-tune the compensation settings using multiply-stained cells. The obtained results are satisfactory concerning the epithelial cells, because the compensation is focusing on these cells. When using the compensation settings for epithelial cells on leukocytes, one can observe that the leukocytes, which are positive for CD45-PerCP, CD3-APC, and CD19-PE, were slightly over-compensated. In cases where the proper compensation settings were adjusted based on the leukocytes, epithelial cells may have been under-compensated (data not shown).
Most commercially available flow cytometers provide the possibility for compensating between many spectrally adjacent pairs of detectors: FL1 (FITC) – FL2 (PE), FL2 – FL3 (PerCP), and FL3 – FL4 (APC). For two-color analysis, such pair-wise compensation will be sufficient. However, by including a third or fourth fluorochrome, the potential for complex interactions exists and may be very difficult to correct by such pair-wise compensation. For example, when using a very bright FITC stain, some of the emission of FITC can be measured in FL3 detector. This overlap may be difficult to correct.
An additional complication to compensation is the use of tandem dyes, for example PE-Cy5, which are covalently linked combinations of the two fluorescent compounds PE (donor) and Cy5 (acceptor). The spectral characteristics of PE-Cy5 are variable due to the chemical composition that often differs between companies and between each batch of antibodies. Thus, compensation settings for a given PE-Cy5 conjugated monoclonal antibody (mAb) must be adjusted and verified when using other PE-Cy5 mAb (17, 20, 21). Another way to solve the compensation problems when using multicolor (more than four colors) analysis is to use software compensation. This compensation strategy is also suitable for easily solving the compensation problems associated with the variation in tandem fluorochromes conjugated to different antibodies. The compensation settings obtained in the present study were based on FITC-, PE-, PerCP-, and APC-labeled mAb and the spectral characteristics of these fluorochromes are quite similar. Therefore, the same compensation settings were used throughout the study.
Antibody Titration
This procedure is essential prior to immunophenotyping (20, 22, 23).
In the present study, some antibodies required only half the recommended volume. Using a higher concentration, cells expressing EMA, CD15, and β1 integrin, all conjugated to FITC, expressed strong signals that resulted in under-compensation. The lowest concentration (1:20) was associated with slight over-compensation of the positive cell populations.
CD138 and the αV, α6 integrin subunits were conjugated to PE and the spectral overlaps were detected in both the FL1 (FITC) and FL3 (PerCP) detectors. The spectral overlap of PE to FL1 detector was fully corrected at a 1:2.5 (20 μl) dilution. However, at the same concentration, the “spectral spillover” from PE to the FL3 detector was inappropriately compensated and led to under-compensation. Using higher concentrations of the antibodies led to increasing of nonspecific background staining.
These results demonstrate that one can use a lesser amount of some of the antibodies and still obtain good separation between positive and negative cell populations. Using the manufacturer's recommended amount of antibody may provide a false sense of security, since standards for antibody titrations may vary. Some companies offer antibodies at higher concentrations than stated and needed, whereas others may provide antibodies below titer. Other problems may arise when inappropriate target cells (fixed, frozen cells etc.) are used to determine the appropriate concentration of the antibody, since these may not be representative of the cells that are to be tested.
Antigenic Immunoreactivity Comparison in Fresh and Frozen Material
This experiment analyzed the comparative antigenic profiles for five cell lines and 10 fresh nonfixed peritoneal and pleural effusions in fresh and frozen condition. The current literature consists of several studies in which the impact of cryopreservation on hematological cell populations was investigated (24-27). However, the reported data are contradictory. Two groups have reported that the lymphocyte subset analysis using frozen-thawed whole blood yields comparable results to fresh samples and that no significant differences in cell surface expression of leukocyte markers, in efficiency of selection of peripheral blood mononuclear cells subpopulations, or in mitogen-induced proliferation are detected in freshly isolated versus cryopreserved cells (24, 25). However, other investigators reported that cryopreservation modifies the distribution of CD34 epitopes, the clonogenic capacity of peripheral blood progenitor cells from non-Hodgkin's lymphoma patients and CD34+ blasts in acute myeloid leukemia (26). In addition, cryopreservation was shown to modify B cell chronic lymphocytic leukemia phenotype, by decreasing CD5 and CD23 expression (27). To the best of our knowledge, the present study is the first to characterize phenotype alterations in fresh and frozen epithelial cell lines and in specimens from nonhematological malignancies.
Expression of β3 integrin subunit (CD61) was not detected in any of the cell lines or clinical specimens in both the fresh and frozen state. Good agreement was observed between the two conditions in the detection of Ber-EP4, EMA, CD15, and αV, α6, β1 (CD29) integrin subunits for both cell lines and clinical specimens. Expression results for Ber-EP4, EMA and the αV, α6, and β1 integrin subunits are in agreement with our unpublished pilot studies using several control cell lines, as well as with previously published results (14-16, 28-35). The data regarding CD15 expression on carcinoma cells in clinical specimens and cell lines support previously published expression data for human breast cancer cell lines (36) and adenocarcinoma specimens using immunohistochemistry (37, 38). The lack of CD15 expression on MDA-MB-231 breast cancer cells in the present study is in agreement with the report by Calvo et al. (36).
The loss of CD138 (syndecan-1) in MDA-MB-231 cells and in three clinical specimens and the significantly reduced expression in the SK-BR-3, T47-D, and NCI-H520 cell lines in frozen condition, suggest that the cryopreservation modifies the CD138 epitope and therefore calls for caution when analyzing this molecule in frozen samples. Our results regarding CD138 expression in fresh MDA-MB-231 cell line are in agreement with the finding by Beauvais et al. (39), and with additional studies documenting its expression in breast carcinoma, ovarian carcinoma, and malignant MM (39-41).
In view of the variable expression of CD138 in frozen cell lines and frozen clinical samples, another CD138 antibody (clone MI15, Dako) was tested on the aforementioned cell lines with similar results. However, preliminary work at our laboratory illustrated better CD138 antigenic preservation when cells were cryopreserved in liquid nitrogen. Regarding the other antibodies in the present study no significant differences was observed.
FCM Compared to ICC
The expression of the β3 integrin subunit (CD61) was, as observed with FCM, absent in all specimens employing ICC. This finding was unexpected, since the antibodies used for ICC and FCM were from a different manufacturer and of a different clone. In contrast, αV integrin subunit was frequently expressed, with good correlation between FCM and ICC.
Three samples, all diagnosed as malignant MM, did not express Ber-EP4 using FCM or ICC. It has been reported by several authors that Ber-EP4 positivity in MM varies considerably (5, 6, 16, 29, 42). Other studies of effusions using the Ber-EP4 antibody have shown essentially negative immunostaining in malignant and reactive mesothelial cells (28, 43). Our results are in agreement with the latter observation. Seventeen cases expressed Ber-EP4 using both ICC and FCM. One of the cases expressed significantly lower Ber-EP4 using FCM compared to ICC, whereas Ber-EP4 expression was significantly higher using FCM than ICC in another sample. This discrepancy may reflect different numbers of target cells in frozen specimens and in the paraffin block. The overall percentage of Ber-EP4 expressing cells in the other samples was comparable, and no statistically significant difference was found between the two methods, in agreement with our earlier reports (14-16).
EMA was not detected in 1/20 cases using both methods, in agreement with previously reported data showing that the expression of this marker ranges from 32 to 100% in adenocarcinomas and 42 to 100% in MM (6). Statistical analysis showed that the difference in the percentage of EMA-expressing cells using the two methods was marginally significant in favor of ICC. The results of the current study are in agreement with our previous studies, in which we have shown that both ICC and FCM are sensitive methods for the detection of malignant epithelial and mesothelial cells in effusions using EMA as epitope (16, 29).
CD138 expression was not detected in five cases employing both methods. In the remaining 15 specimens, FCM showed a low concordance with ICC: 7 and 15 cases expressed CD138 using FCM and ICC, respectively. CD138 expression was significantly different using these two methods. Our results in paraffin-embedded specimens using ICC are in agreement with the reports of other investigators (39-41). The discrepancy between fresh frozen and formalin-fixed material in the current study appears to reflect the variable degrees of loss or possible alterations of this antigen during cryopreservation and therefore calls for caution in interpreting results based on FCM analysis of frozen samples. Of note, the loss of CD138 expression in cases using FCM seems to be random and did not relate to any specific diagnostic category or to specimen site.
The diagnostic applications of our experience with effusion FCM are that the use of few effective antibodies enables one to diagnose this material with no major difficulties. This focuses on the use of Ber-EP4 as an epithelial marker and on the stronger intensity of EMA expression in MM compared to both adenocarcinoma and reactive mesothelial cells (44). The use of Ber-EP4, EMA, CD14, and CD45 panel is sufficient for diagnostic purposes. While integrin expression is of interest as a research tool, it has no major role in the diagnostic setting. Regarding research, we have recently applied FCM for further characterization of tumor cells in effusions using antibodies against chemokine receptors. We found that these are rarely expressed on ovarian carcinoma and MM cells, but are frequently present on both lymphocytes and macrophages (45, 46). In addition, chemokine receptor expression is upregulated in malignant compared to benign effusions (46).
In conclusion, the attempt to optimize and calibrate the instrument settings for four-color FCM analysis of epithelial cells in effusion was successfully achieved, and the compensation strategy employed is suitable for solving the compensation problems associated with the use of multicolor FCM. The results of antibody titration show that it is important to titrate the antibodies prior to use for immunophenotyping, since using an incorrect volume may lead to either over-compensation or under-compensation of the fluorescence signals. The comparison of antigenic profiles between fresh and frozen material and formalin-fixed paraffin-embedded samples has shown that all the antibodies, with the exception of CD138, were stable and no significant alteration of the antigenic profile was observed. Further research with different antibodies is necessary to resolve this issue.
Acknowledgements
We thank Ms. Elisabeth Emilsen for competent help in culturing the cell lines used in this study.




