Production of inflammatory cytokines by peripheral blood monocytes in chronic alcoholism: Relationship with ethanol intake and liver disease
Abstract
Background:
Controversial results have been reported about the effects of alcoholism on the functionality of monocytes. In the present study we analyze the effects of chronic alcoholism on the intracellular production of inflammatory cytokines by peripheral blood (PB) monocytes.
Methods:
Spontaneous and in vitro-stimulated production of interleukin (IL) 1α (TNFα) by PB monocytes was analyzed at the single level by flow cytometry in chronic alcoholics without liver disease and active ethanol (EtOH) intake (AWLD group), as well as in patients with alcohol liver cirrhosis (ALC group), who were either actively drinking (ALCET group) or with alcohol withdrawal (ALCAW group).
Results:
A significantly increased spontaneous production of IL1β, IL6, IL12, and TNFα was observed on PB monocytes among AWLD individuals. Conversely, circulating monocytes form ALCET patients showed an abnormally low spontaneous and stimulated production of inflammatory cytokines. No significant changes were observed in ALCAW group as regards production of IL1β, IL6, IL12, and TNFα.
Conclusion:
Our results show an altered pattern of production of inflammatory cytokines in PB monocytes from chronic alcoholic patients, the exact abnormalities observed depending on both the status of EtOH intake and the existence of alcoholic liver disease. © 2007 Clinical Cytometry Society
For decades it is known that alcoholism is associated with an imbalanced immune response, alcoholic individuals frequently showing an immunodeficiency state which is associated with a higher risk of infection and cancer (1-3). In line with this, it has been reported that acute in vitro exposure to ethanol (EtOH) decreases production of inflammatory cytokines (i.e., interleukin [IL] 1, IL6, and tumor necrosis factor α [TNFα]) by human monocytes (4-8) and macrophages (i.e. TNFα) (9), these effects being mediated through toll-like receptors (TLR) (10). Conversely, prolonged in vivo exposure to alcohol has been related to a paradoxical activation of cytotoxic T and natural killer (NK) cells (11-21), results about the functionality of monocytes in chronic alcoholism being heterogeneous and even contradictory. Such discrepancies may be related to the methodological approach and type of samples used to evaluate the functional status of monocytes (i.e., animal models vs. human studies, in vitro vs. ex vivo analyses, or the use of different stimulatory conditions), the status of EtOH intake at the moment of the study (i.e., active alcoholism vs. withdrawal period), the nutritional status of the patients, and the presence or absence of alcohol liver disease (ALD) and the specific type of hepatic damage. In line with this, it has been shown that chronic EtOH ingestion by mice activates macrophages, increasing the expression of both the CD80 and CD86 co-stimulatory molecules as well as the secretion of inflammatory cytokines after stimulation with lipopolysaccharide (LPS) (22); in addition, prolonged in vitro exposure of human monocytes to EtOH has also been associated with increased secretion of TNFα after stimulation with LPS and phorbol myristate acetate (PMA) (7). By contrast, ex vivo stimulation of peripheral blood (PB) monocytes and macrophages from chronic alcoholic patients without liver disease with LPS has been related to an increased secretion of IL1β, down-regulation of both TNFα and IL12 production by PB monocytes, (23) and TNFα secretion by alveolar macrophages (24). Concerning ALD, both a spontaneous and stimulated increase in the secretion of TNFα by monocytes has been reported in patients with alcoholic hepatitis (25, 26), while in alcoholic liver cirrhosis (ALC) downregulation of IL1 and TNFα secretion by monocytes has been demonstrated (27).
In recent years, new methods have been developed that allow direct assessment of cytokine production at the cytoplasmatic level in individual cells through the use of multiple staining in which fluorochrome-conjugated monoclonal antibodies directed against specific cell subsets and the cytokines of interest are combined (28).
In the present study, we analyzed for the first time at the single cell level the pattern of spontaneous and stimulated ex vivo production of the IL1β, IL6, IL12, and TNFα inflammatory cytokines by PB monocytes in patients with chronic alcoholism, grouped according to the status of EtOH intake and the presence or absence of ALC at the moment of entering the study. Our results indicate that in the absence of liver disease, chronic alcoholism is associated with an increased intracellular production of inflammatory cytokines, while alcoholic cirrhosis patients with active EtOH intake show reduced production of these cytokines.
MATERIALS AND METHODS
Patients, Control Subjects and Samples
A total of 38 alcoholic male patients referred to the Alcoholism Unit of the University Hospital of Salamanca were included in this study. From them, 17 were alcoholics without liver disease (AWLD group) and 21 patients were diagnosed as having ALC. The most relevant clinical and biological data about both groups of patients at the time of entering the study are shown in Table 1. In parallel to the patients, 10 healthy male volunteers (control subjects) were also analyzed. Prior to entering the study, all individuals (patients and controls) gave their informed consent to participate, and the study was approved by the Ethics Committee of the University Hospital of Salamanca. For each case, EDTA- and heparin- anticoagulated PB samples were obtained between 9:00 and 10:00 a.m. under fasting conditions.
| Controls (n = 10) | AWLD (n = 17) | ALCAW (n = 10) | ALCET (n = 11) | |
|---|---|---|---|---|
| Mean age (yr) | 33 ± 6 | 49 ± 9 | 49 ± 8 | 54 ± 11 |
| Leucocytes (cells/mm3) | 6,298 ± 1,747 | 6,904 ± 1,804 | 5,666 ± 1,676 | 6,000 ± 2,091 |
| Monocytes (cells/mm3) | 517 ± 218 | 642 ± 213 | 507 ± 218 | 490 ± 261 |
| Bilirubin (mg/dl) | 0.7 ± 0.1 | 0.9 ± 0.2 | 1.9 ± 0.2 | 2.1 ± 0.1 |
| AST (IU/l) | 23 ± 6 | 38 ± 9 | 54 ± 5 | 59 ± 4 |
| GGT (IU/l) | 26 ± 13 | 112 ± 13 | 67 ± 9 | 148 ± 21 |
| Prothrombin time (%) | 98 ± 0.8 | 96 ± 1 | 74 ± 8 | 69 ± 10 |
| Albumin (g/l) | 51 ± 8 | 43 ± 7 | 35 ± 2 | 34 ± 3 |
| Hemoglobin (g/dl) | 15 ± 1 | 16 ± 1 | 13 ± 2 | 14 ± 2 |
| Child-Pugh score (A/B) | 10/0 | 8/3 |
- Results expressed as mean ± standard deviation. AST, aspartate aminotransferase; GGT, γ-glutamyl transferase; AWLD, alcoholics without liver disease; ALCAW, alcoholics with liver cirrhosis and at least one year of alcohol withdrawal; ALCET, alcoholics with liver cirrhosis and active ethanol intake.
Alcoholics without liver disease (AWLD group; n = 17).
All patients included in this group were actively drinking (≥90 g of EtOH/day) until the day of entering the study. Subjects who where polydrug abusers and those displaying physical stigmata of chronic liver disease (i.e., cutaneous signs, hepatosplenomegaly, gynecomastia, testicular atrophy, and/or muscle wasting) were excluded from the study. All patients had normal hemoglobin concentrations, prothrombin time, and albumin serum levels. Alanine aminotransferase and aspartate aminotransferase serum levels were required to be less than twice the upper normal limits (40 IU/l). Hepatitis B surface antigen (HbsAg) and antibodies to hepatitis C virus (HCV) and to human immunodeficiency virus (HIV) were constantly negative. As liver biopsy was not performed for ethical reasons, liver disease was excluded on the basis of ultrasonographic studies. To avoid enrolling patients with malnutrition, evaluation of the nutritional status was made using both laboratory parameters (serum albumin concentrations and transferrin levels) and anthropometric analyses (height, weight, triceps skin-fold, and midarm muscle circumference measurements); the results were compared with published criteria to derive percentages of standard values (29). Only subjects with anthropometric tests >90% of expected normal values were included in the study.
Alcoholics with liver cirrhosis (n = 21).
Depending on their status of alcoholic intake at the time of entering the study, this group of patients was subdivided into two categories: (1) alcoholics with liver cirrhosis and at least 1 year of alcohol withdrawal (ALCAW group; n = 10); and (2) alcoholics with liver cirrhosis and active EtOH intake at the moment of entering the study (ALCET group; n = 11). Histopathological examination of the liver in all 14 cirrhotic patients in whom it was performed revealed micronodular cirrhosis, but no signs of alcoholic hepatitis. In the remaining 7 patients, liver biopsy was not performed because of blood coagulation abnormalities. In these cases, diagnosis of ALC was established on the basis of the presence of physical stigmata of chronic liver disease, history of ascites, variceal bleeding, or hepatic encephalopathy, as well as on gastroscopic and/or ultrasonographic findings (30). All patients were HBsAg (−), HCV antibodies (−), and HIV antibodies (−), and none of them had malnutrition according to the above-mentioned criteria. None of the ALC patients had been diagnosed as having alcoholic hepatitis prior to the time of entering the study. None had ascites, jaundice, encephalopathy, or gastrointestinal bleeding in the year before the study, and none of them had received blood transfusions or was under treatment with steroids or immunosuppressive therapy.
Control subjects (n = 10).
Control subjects were males who reported to drink <15 g of EtOH/day. In these individuals, liver function tests, as well as routine hematological and biochemical tests, were within the normal ranges.
Analysis of Cytokine Production by PB Monocytes
Production of inflammatory cytokines by PB monocytes was analyzed at the single cell level using a well-established technique that combines four-color stainings for the specific identification of monocytes and the measurement of cytokine production on erythocyte-lysed whole blood samples (31, 32). Briefly, production of IL1β, IL6, IL12, and TNFα by PB monocytes was analyzed after a short term culture for 6h at 37°C in a 5% CO2 and 95% humidity, sterile environment, in the presence of 10 μg/ml of brefeldin A (Sigma, St Louis, MO) to block cytokine production. In addition, to an aliquot of each sample 100 ng/ml of LPS from Escherichia coli (serotype 055:B5; Sigma) plus 10 ng/ml of human recombinant interferon (IFN) γ (Promega, Madison, WI), were added as stimulatory agents. After this short-term culture, the sample was aliquoted in different tubes and stained with 10 μl of anti-CD14- fluorescein isothiocyanate (clone FWKW-1; Cytogonos SL, Salamanca, Spain), 10 μl of anti-HLADR-peridin chlorophyll protein (clone L243; Becton Dickinson Biosciences -BDB-, San Jose, CA), and 5 μl of anti-CD33-allo-phycocyanin (Leu-M9; BDB) to unequivocally identify the monocyte population. After an incubation period of 15 min at room temperature in the dark, cells were fixed, permeabilized and stained with monoclonal antibodies (MAb) directed against different human cytokines, using the Fix & Perm reagent kit (CALTAG Laboratories, San Francisco, CA), strictly following the recommendations of the manufacturer. The source and specificities of the MAb used to detect intracytoplasmic human cytokines were as follows: anti-IL1β-phycoerythrin (PE) (clone AS10; BDB), anti-IL6-PE (clone MQ2-6A3; PharMingen, San Diego, CA), anti-IL12-PE (clone C11.5; PharMingen), and antiTNFα-PE (clone Mab 11; PharMingen). All cytokine-directed MAb were used at saturating concentrations and conditions. As we have previously shown that under the culture conditions used in the present study the frequency of dead cells -monocytes or other cells- was irrelevant (31), the use of specific dyes to exclude dead cells were considered unnecessary.
Once stained, samples were acquired in two consecutive steps in a FACSCalibur flow cytometer (BDB), using the CellQUEST software (BDB). In the first step, information was collected about 5 × 104 events/test, corresponding to the whole sample cellularity; in the second step, information was stored exclusively for the HLADR+ events (where PB monocytes are included), acquired through an electronic “live-gate” (32), as shown in Figure 1. In this second step, information on a minimum of 3 × 105 HLADR+ cells was collected. Data analysis was performed using the Paint-A-Gate PRO software (BDB); monocytes were identified as HLADR+/ CD14hi/CD33+ events (Fig. 1), to clearly discriminate them from PB CD16+ dendritic cells, which show dim CD14 expression. For the evaluation of cytokine production, the percentage of cytokine positive monocytes was calculated. In addition, a ratio between the in vitro- stimulated and nonstimulated (spontaneous) ex vivo cytokine production was calculated for each sample.
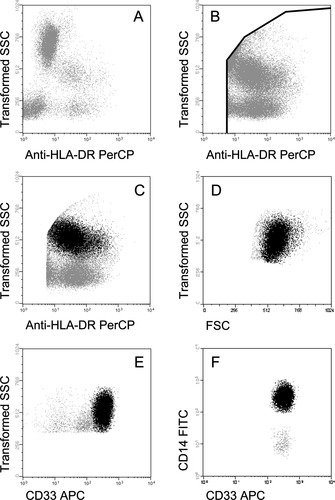
Bivariate dot plots illustrating the gating strategy used for both the acquisition and the analysis of PB monocytes from a representative chronic alcoholic patient. Data acquisition was performed in two-steps as illustrated in panels A and B. In the first step (panel A), information about around 5 × 104 events/test, corresponding to the whole sample cellularity, was stored; in the second step, information was collected only for those events included in an electronic “live-gate” drawn in the SSClo/int/HLA-DR+ fraction (panel B). The gating strategy to identify PB monocytes (black dots) is displayed in panels C to F; accordingly, monocytes were characterized as those events included in the HLA-DR+ fraction (panel C) showing intermediate light scatter values (FSC -forward scatter- and SSC -sideward scatter-) (Panels C and D), and high reactivity for both CD33 (panel E) and CD14 (panel F).
Statistical Methods
Mean values, and their standard deviations (SD), median, range, and the 25th and 75th percentiles were calculated for each variable under study using the SPSS software program (SPSS 12.0, Chicago, IL). The Mann–Whitney U and Kruskal–Wallis nonparametric tests for unpaired data were used for the evaluation of the statistical significance of the differences observed between two or more groups, respectively. In addition, the Friedman and Wilcoxon signed ranked tests were used for the comparison of paired variables between different groups of individuals. P-values <0.05 were considered to be associated with statistical significance.
RESULTS
Spontaneous Production of Inflammatory Cytokines by PB Monocytes
Patients with chronic alcoholism without liver disease (AWLD group) showed increased absolute numbers of circulating monocytes, statistically significant differences being detected with respect to ALC patients with active EtOH intake (ALCET group) (P < 0.05). From the functional point of view, AWLD patients showed a remarkably higher production of IL1β and TNFα in comparison to both control subjects and the other two ALC patients (P < 0.05) (Fig. 2). In addition, PB monocytes from AWLD patients also showed an increased ex vivo production of IL6 and IL12 with respect to both normal controls and ALCET patients (P < 0.05) (Fig. 2).
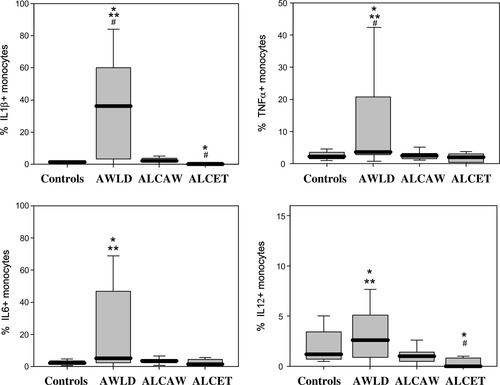
Spontaneous production of IL1β, TNFα, IL6, and IL12 by PB monocytes from chronic alcoholic patients and healthy controls. Results are expressed as percentage of cytokine-producing cells from all PB monocytes. Notched-boxes represent 25th and 75th percentile values; the line in the middle and the vertical lines correspond to the median value and both the 10th and 90th percentiles, respectively. *Statistically significantly different (P < 0.05) vs. controls. **: Statistically significantly different (P < 0.05) vs. ALCET group. #: Statistically significantly different (P < 0.05) vs. ALCAW group. AWLD: alcoholics without liver disease; ALCAW: alcoholics with liver cirrhosis and at least one year of alcohol withdrawal; ALCET: alcoholics with liver cirrhosis and active EtOH intake.
From all groups of individuals studied, ALCET patients showed the lowest spontaneous ex vivo production of inflammatory cytokines (Fig. 2). Accordingly, they showed reduced production of IL1β, IL12, and TNFα by PB monocytes, the levels of both IL1β and IL12 being significantly lower (P < 0.05) than those obtained in controls and ALCAW patients (Fig. 2). Of note, no statistically significant differences were detected between ALC patients with a Child-Pugh score A and those with a score B, with respect to the spontaneous ex vivo production of intracellular inflammatory cytokines by PB monocytes. Regarding ALCAW patients, similar patterns of production of IL1β, IL6, IL12, and TNFα were observed with respect to controls (Fig. 2).
Stimulated Production of Inflammatory Cytokines by PB Monocytes
In vitro stimulation of PB monocytes with LPS plus IFNγ induced a significantly (P < 0.05) increased production of inflammatory cytokines in all groups of individuals (patients and controls) analyzed (Fig. 3) with respect to non-stimulated PB monocytes (Fig. 2). Despite this, after stimulation, ALCET patients showed a significantly lower percentage of IL1β and IL12-producing monocytes with respect to the control group (P < 0.05) (Fig. 3). In addition, the ratio between the number of PB monocytes producing IL1β, IL12, and TNFα after in vitro stimulation with LPS plus IFNγ and the percentage of PB monocytes, which spontaneously produced the same cytokines, was significantly decreased (P < 0.05) among AWLD patients with respect to controls (IL1β: 52 ± 96 vs. 114 ± 88; IL12: 5 ± 4 vs. 18 ± 14; TNFα: 22 ± 19 vs. 53 ± 34).
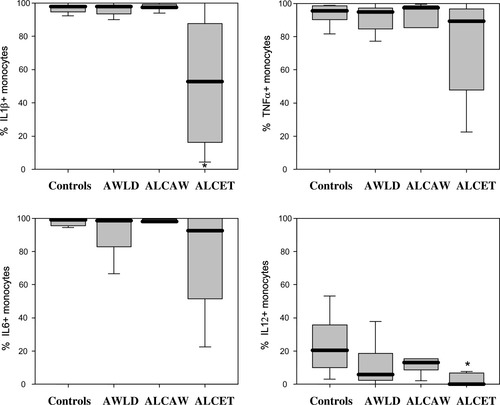
LPS-plus interferon γ-stimulated production of IL1β, TNFα, IL6, and IL12 by PB monocytes from chronic alcoholic patients and healthy controls. Results are expressed as percentage of cytokine-producing cells from all PB monocytes. Notched-boxes represent 25th and 75th percentile values; the line in the middle and the vertical lines correspond to the median value and both the 10th and 90th percentiles, respectively. *Statistically significantly different (P < 0.05) vs. controls. AWLD: alcoholics without liver disease; ALCAW: alcoholics with liver cirrhosis and at least 1 year of alcohol withdrawal; ALCET: alcoholics with liver cirrhosis and active EtOH intake.
DISCUSSION
Accumulating evidences indicate that secretion of inflammatory cytokines by monocytes and macrophages from chronic alcoholic patients could be altered. However, the results reported in the literature are highly variable and even frequently contradictory, depending, among other factors, on the status of EtOH intake and the presence or absence of ALD. In the present study, three groups of alcoholic patients (chronic active EtOH intake without hepatic damage, alcoholic individuals with liver cirrhosis and at least 1 year of alcohol withdrawal, and ALC patients with active alcoholism) were carefully selected, in order to allow for the evaluation of the effects of EtOH intake and the presence of ALC on both the spontaneous and the LPS plus IFNγ- stimulated intracelllular production of the IL1β, IL6, IL12, and TNFα inflammatory cytokines by PB monocytes. Overall, we found a significantly increased spontaneous ex vivo production of inflammatory cytokines in AWLD patients. These results support and extend on previous observations (7), suggesting the existence of an in vivo activation of human monocytes and macrophages in chronic alcoholic individuals. In the present study, the increased ex vivo production of inflammatory cytokines observed for individual PB monocytes among AWLD patients would even be enhanced because of the increased absolute number of circulating monocytes detected among this group of individuals.
As could be expected, in vitro stimulation of PB monocytes with LPS plus IFNγ induced a significantly increased production of all inflammatory cytokines analyzed in all groups of alcoholic patients and control subjects. Nevertheless, the ratio between the spontaneous and stimulated production of IL1β, IL12, and TNFα was significantly reduced in AWLD with respect to control subjects and ALCET patients, respectively. Such apparent functional defect of PB monocytes from AWLD patients could be related to the increased in vivo activation of PB monocytes specifically found in this group of individuals. In spite of our findings in AWLD patients, it should be noted that acute alcohol treatment has been shown to directly inhibit secretion of inflammatory cytokines (4-8). Altogether, these observations would support the notion that the effects of acute and chronic alcoholism on the production of inflammatory cytokines by PB monocytes could be paradoxically different.
The exact mechanisms involved in the activation of monocytes in chronic alcoholism remain unclear. At present it is well established that EtOH increases intestinal permeability and bacterial translocation, leading to entrance of endotoxin (LPS) into the circulation (33-35). Accordingly, while acute exposure to EtOH could potentially lead to a reduced synthesis of inflammatory cytokines by monocytes through the inhibition of TLR signaling by LPS (10, 36), an increased cellular sensitivity to the effects of LPS has been shown in mice chronically exposed to EtOH (37), which could also contribute to explain the increased production of inflammatory cytokines in chronic alcoholism. In addition, it has been reported that chronic EtOH exposure may also up-regulates the expression of the p75 and p55 TNF receptors on monocytes (38) associated with an increased TNFα production by macrophages (39), while acute exposure to EtOH has been shown to suppress LPS-induced TNFα secretion through the inhibition of its cleavage by the TNFα-converting enzyme (40). Interestingly, the increased production of IL12 by PB monocytes from AWLD patients found in the present study could also be related to priming of a T-helper (Th)-1 response (41), supporting the notion that active chronic EtOH intake in patients without liver injury is associated with a predominant Th-1 pattern of cytokine secretion by T cells (42). At present, it is well-established that both IL12 and Th-1 cytokines favor the activation and expansion of effector CD8+ cytotoxic T-lymphocytes (41, 43), a T-cell population that has been related to hepatic damage, presumably through the recognition of neoantigens such as acetaldehyde adducts (44) coexpressed together with HLA class I molecules on the surface of hepatocytes (45) from active chronic drinkers.
Altogether, our results suggest that, in the absence of ALD, chronic alcoholism is associated with an increased activation of monocytes, as reflected by an excessive production of inflammatory cytokines, which could represent a risk factor for the development of systemic inflammatory syndrome and sepsis in AWLD patients (46, 47). In addition, activation of an effector CD8+ T-cell-mediated cytotoxic response, favored by an increased secretion of IL12, could also play a role in the development of early alcohol-induced liver injury (19).
From all groups of chronic alcoholic individuals studied, ALCET patients showed the lowest production of inflammatory cytokines ex vivo, changes in both the spontaneous and LPS plus IFNγ stimulated production of IL1β and IL12 by PB monocytes being significantly decreased with respect to both control group and cirrhotic patients with at least 1 year of alcohol withdrawal (ALCAW group). By contrast, no significant changes were observed with respect to the control group as regards intracellular production of inflammatory cytokines among ALCAW patients. Previous reports have clearly shown the existence of an increased activation of monocytes among patients with alcoholic hepatitis, leading to a significant increase of spontaneous and LPS-stimulated release of TNFα by monocytes (25, 26). However, currently available information about the secretion and expression of inflammatory cytokines in alcoholic patients with liver cirrhosis remains controversial, and production of IL12 by monocytes from these individuals has not been analyzed so far in the literature. Reported studies show that ALC is associated with depressed secretion of IL1 (27, 48, 49) and TNFα (27, 49) by both LPS-stimulated PB monocytes (27) and macrophages (49), which is even more pronounced among patients with severe ALC and malnutrition (48, 49). In contrast, others have found an increased secretion of IL1 (50) and TNFα (50, 51) by LPS-stimulated PB monocytes from ALC patients. Additionally, a close relationship between severity of ALC (Child-Pough score B or C) and the elevation of the expression of both the TNF and TNF-receptor genes in PB mononuclear cells has been observed (52). Noteworthy, none of patients analyzed in the present study had malnutrition, and we failed to show differences in cytokine production between cirrhotic patients with different Child-Pugh scores. In turn, the overall decreased production of inflammatory cytokines by PB monocytes from ALCET patients found in the present study could be related, at least in part, to coexistence of altered immunoregulatory mechanisms. In line with this, previous studies have shown significantly increased numbers of CD4+/CD25+ regulatory T-lymphocytes in the circulation of chronic alcoholic patients (11, 14), which may exert a suppressive effect on antigen-presenting cells, including the monocytes. In any case, independently of the mechanisms involved in the reduced ability of PB monocytes from ALCET patients to produce inflammatory cytokines, it could contribute to explain the increased risk of infection in this group of chronic alcoholic patients.
In summary, our results confirm the existence of functional abnormalities on PB monocytes from chronic alcoholic patients, their nature depending on both the status of EtOH intake and the presence or absence of ALD. Accordingly, we show that chronic alcoholism in the absence of liver disease is associated with an increased production of inflammatory cytokines by PB monocytes, potentially representing a risk factor for the development of systemic inflammatory response syndrome/sepsis; in contrast, active EtOH intake in ALC patients (ALCET group) leads to a reduced production of these cytokines and a subsequent immunodeficiency state, associated with a higher risk of infection.




