Clone-specific anti-CD45 blocking factor in patient plasma
Abstract
We observed inhibition of anti-CD45, but not anti-CD3, -CD4, or -CD8 staining in the whole blood of an individual clinical specimen. Subsequent experiments demonstrated the presence of a plasma factor, which likewise blocked CD45 staining of third party lymphocytes. The blocking activity was anti-CD45 clone (2D1) specific. Experiments utilizing immunoabsorption, immunofluorescence, and commercial blocking reagents failed to provide evidence for identifying blocking factor as heterophilic antibody. Biochemical identification of blocking factor was not accomplished. © 2007 Clinical Cytometry Society.
Human heterophilic antibodies have long been recognized to bind to animal antibodies and thereby interfere with immunoassays of serum proteins (1, 2). To date such antibodies, or other blocking factors, have not been reported against CD—specific monoclonal antibodies used to detect leukocyte populations by flow cytometry. We annually perform over 2,500 CD4/CD8 enumerations using a two tube, three color commercial antibody cocktail of CD4+CD3+CD45 and CD8+CD3+CD45. We report here a clone-specific anti-CD45 blocking factor present in an individual specimen received for analysis.
METHODS
The patient was a 40 year old heterosexual female with a 14 year history of HIV infection. Her absolute CD4 count was decreased at 631 mm3 and HIV RNA was less than 400 copies per ml. Past medical history was significant for peripheral neuropathy, fibromyalgia, osteomalacia, arthritis, and depression, and past surgical history was significant for cholecystectomy. Medications included fosamprenavir 700 mg PO BID, ritonavir 100 mg PO BID, and depoprovera. WBC count was 5,200, absolute lymphocyte count 1,856, hemoglobin 11.7, and platelet count 160,000. CD4/CD8 enumeration via flow cytometry required further analysis to identify a clone-specific anti-CD45 blocking factor in the patient's serum.
Clinical specimens are stained in whole blood followed by lysis with ammonium chloride, a phosphate buffered saline wash (PBS), and fixation in 1% paraformaldehyde in PBS. In some experiments, specimens were first lysed then stained either in the presence or absence of patient plasma then fixed. Lymphocyte preparations from four nonpatient clinical specimens were used for these experiments. Analyses were performed on a Beckton Dickinson FACScan (San Jose, CA). Analysis of allophycocyanin (APC) conjugated antibody was performed on a MoFlo flow cytometer (Cytomation, Fort Collins, CO). Antibodies used in this study are listed in Table 1. All monoclonal antibodies are IgG1.
| Antibody | Clone | Inhibited by patient plasma | Manufacturer |
|---|---|---|---|
| CD45 PerCP | 2D1 | Yes | BD |
| CD45 FITC | 2D1 | Yes | BD |
| CD45 — | 2D1 | Yes | BD |
| CD19 PerCP | S525C1 | No | BD |
| CD45 FITC | T29/33 | No | Dako |
| CD45PE | J33 | No | Immunotec |
| CD45APC | J33 | No | Immunotec |
| Goat antimouse | FITC | – | BD |
- Leukocytes from a non-patient clinical specimen were prepared by lysis, then stained in the presence of patient plasma. The inhibitory effect of patient plasma, when present, reduced the fluorescent levels to those of isotype controls.
RESULTS
Our initial observation is presented in Figures 1, 2, and 3. CD45, but not CD3, CD4, or CD8 staining of patient cells was blocked in the presence of autologous plasma either in unfractionated whole blood Figure 1B or with red cells lysed and patient plasma added back Figure 3B during stain incubation. Patient lymphocytes stained normally with CD45 in the absence of patient plasma Figure 2B.
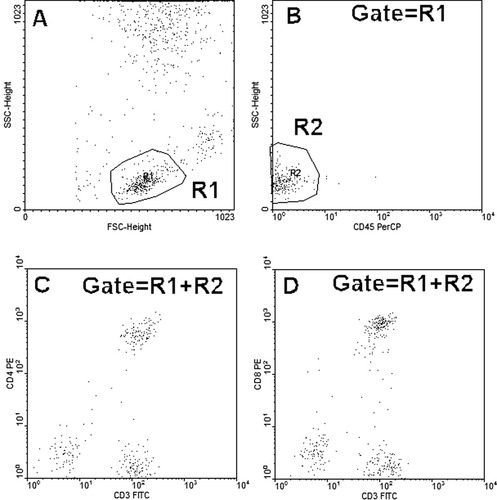
Patient whole blood was stained with Tube no 1: CD45+CD3+CD4 and Tube no 2: CD45+CD3+CD8 and analyzed as indicated.
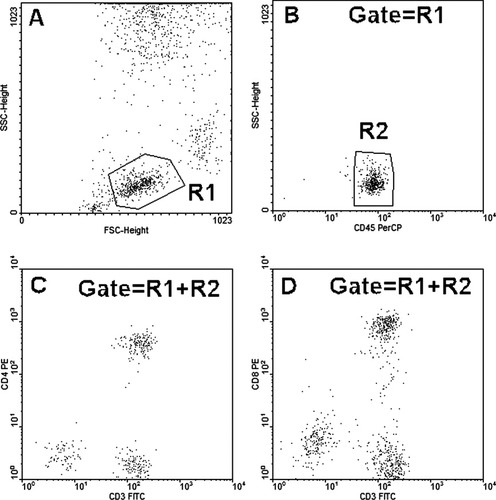
Plasma-free leukocytes were prepared by hypotonic lysis from patient blood then stained with Tube no 1: CD45+CD3+ CD4 and Tube no 2: CD45+CD3+CD8 and analyzed as indicated.

Patient leukocytes were prepared by hypotonic lysis, reconstituted with patient plasma, and then stained with Tube no 1: CD45+CD3+CD4 and Tube no 2: CD45+CD3+CD8 and analyzed as indicated.
The relative concentration of blocking factor was determined in a titration experiment of patient plasma against lymphocytes of an unrelated clinical specimen. Patient plasma was serially diluted in buffer then added to aliquots of third party lymphocyte prior to the addition of antibody stain. Results presented in Figure 4 demonstrated appreciable patient plasma blockage of CD45 staining of lymphocytes at a 1:4 dilution.
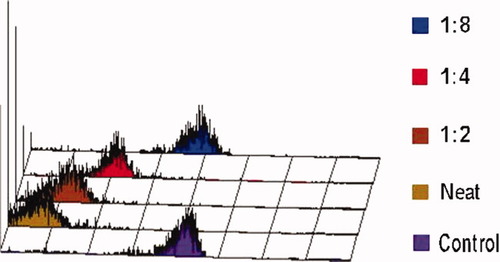
Leukocytes from a nonpatient clinical specimen were prepared by hypotonic lysis, reconstituted with indicated dilutions of patient plasma then stained with CD45 PerCP clone 2D1 (Becton Dickinson). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
A series of experiments were undertaken in order to define the specificity of the CD45 blocking factor in which patient plasma was tested against lymphocyte staining of other nonpatient clinical specimens. The results, tabulated in Table 1, demonstrate specificity for clone 2D1 CD45 antibody and furthermore that blockage is not directed against fluorochromes.
We next sought to identify the nature of blocking factor. Indirect immunofluorescence experiments in which cells were first treated with unconjugated clone 2D1 antiCD45 antibody then patient plasma followed by fluorochrome-conjugated antihuman immunoglobulin failed to identify the blocking factor as immunoglobulin. Immunoabsorption experiments using either a commercial heterophilic antibody blocking reagent (Scantibodies, Heterophile Blocking Tube, Scantibody Laboratory, Santee, CA) or antimouse immunoglobulin beads (BD Combeads, Becton Dickinson Biosciences, San Juan Capistrano, CA) coated with clone 2D1 anti-CD45 antibody failed to absorb inhibitory factor. The data is not shown for these experiments.
DISCUSSION
Heterophilic antibodies are a significant source of interference in clinical chemistry assays for soluble ligands in serum and can block therapeutically administered mouse monoclonal antibodies (3). We report here the presence of a blocking factor in the plasma of an individual with a clinical history associated with predisposing the development of heterophilic antibody. However, further experimentation failed to provide data to identify this blocking factor as heterophilic antibody and therefore its biochemical nature is not defined. A review of the literature failed to reveal analogous reports of plasma factors, such as heterophilic antibody or otherwise that similarly interfered with fluorochrome-conjugated monoclonal antibody staining and flow cytometric analyses of human leukocytes.
We readily recognized the presence of a technical issue in this clinical sample because the other antibodies in the pre-mixed antibody cocktail functioned properly and nonmalignant lymphocytes are expected to densely express CD45. The presence of a plasma blocking factor was confirmed and corrected for by applying lysis and wash steps prior to antibody stain. The major clinical pitfall of such a blocking factor would be in whole blood staining of hematopoietic malignancies in which CD expression is unknown and therefore could result in ‘false-negative’ result for a specific CD marker. In our laboratory the CD phenotype of hematopoietic malignancy is only determined on leukocyte preparations free of erythrocytes and plasma. We further suggest that a blocking factor directed against antibody could interfere with soluble ligand detection in patient plasma using antibody-coupled bead technology, which would be a more difficult problem to resolve.
Our experiments failed to provide data to identify blocking factor as an immunoglobulin and therefore a heterophilic antibody. Although an inconsistent observation, blocking factor was specific and of low titer, which may explain our failure to identify it. For example, purified mouse immunoglobulin from non-immune animals would not be expected to contain much, if any, epitope specific to the 2D1 anti-CD45 antibody clone and therefore fail to immunoabsorb blocking factor. Failure of immunoabsorption with 2D1 anti-CD45 attached to beads is more difficult to explain but may simply be the result of non-optimal experimental kinetic parameters. Indirect immunofluorescence most likely was insufficiently sensitive. Blocking factor is not specific for the CD45 epitope expressed on leukocytes that is recognized by the 2D1 anti-CD45 clone since it was removed with a buffer wash. Also, blocking factor inhibited clone 2D1 ant-CD45 staining of four different third party lymphocyte preparations used in these experiments.
Blocking factor was not specific for any of the fluorochromes, which we use in CD4/CD8 enumeration.
Detection of analyte blocking factors is the responsibility of the clinical laboratorian which, due to their apparent rarity, presents a considerable challenge in the case of factors that block flow cytometric analysis of leukocytes as presented in this study.
Acknowledgements
We thank Lou Ann Eskildsen and Kevin Radford for graph and manuscript preparation. Christopher Worth for operation and analysis on the MoFlo flow cytometer. Donald M. Miller M.D., Ph.D., Director of the James Graham Brown Cancer Center and Alvin W. Martin M.D., Medical Director of Flow Cytometry, for their support. Kristen Elmer of Becton Dickinson for reagent procurement. This study is approved by the University of Louisville IRB #222.05.




