Evaluation of a single-platform microcapillary flow cytometer for enumeration of absolute CD4+ T-lymphocyte counts in HIV-1 infected Thai patients† ‡
Part of this work was presented at the 5th Euroconference on Clinical Cell Analysis, September 22–24, 2005, Athens, Greece.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry.
Abstract
Background:
Various assays are used to enumerate peripheral blood absolute CD4+ T-lymphocytes. Flow cytometry is considered the gold standard for this purpose. However, the high cost of available flow cytometers and monoclonal antibody reagents make it difficult to implement such methods in the resource-poor settings. In this study, we evaluated a cheaper, recently developed single-platform microcapillary cytometer for CD4+ T-lymphocyte enumeration, the personal cell analyzer (PCA), from Guava® Technologies.
Methods:
CD4+ and CD8+ T-lymphocyte counts in whole blood samples from 250 HIV-1 infected Thais were determined, using a two-color reagent kit and the Guava PCA, and compared with the results obtained with two reference microbead-based methods from Becton Dickinson Biosciences: the three-color TruCOUNT™ tube method and the two-color FACSCount™ method. Statistical correlations and agreements were determined using linear correlation and Bland–Altman analysis.
Results:
Absolute CD4+ T-lymphocyte counts obtained using the Guava PCA method highly correlated with those obtained using TruCOUNT method (R2 = 0.95, mean bias +13.1 cells/μl, limit of agreement [LOA] −101.8 to +168.3 cells/μl). Absolute CD8+ T-lymphocyte counts obtained using the Guava PCA method also highly correlated with those obtained with the two reference methods (R2 = 0.92 and 0.88, respectively).
Conclusion:
This study shows that the enumeration of CD4+ T-lymphocytes using the Guava microcapillary cytometer PCA method performed well when compared with the two reference bead-based methods. However, like the two reference methods, this new method needs substantial technical expertise. © 2007 Clinical Cytometry Society.
The recent introduction of inexpensive and generic antiretroviral therapy (ART) has been an important step forward in the treatment of human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS) in resource-limited settings. In Thailand, over 1 million people are infected with HIV, and about 30,000 new infections are estimated to occur each year (1). Availability of affordable and reliable CD4 testing to allow decisions regarding initiation and monitoring of ART is a critical issue.
Flow cytometry (FCM) is the gold standard technology for CD4+ T-lymphocyte counting, because of its accuracy, precision, and reproducibility (2-5). Alternative nonflow cytometric technologies, although less expensive, have not been implemented widely due to their complexity, low throughput (1–10 samples/day), and poor quality control (6-9). There are two FCM methods commonly used today to determine absolute CD4+ T-lymphocyte counts: dual-platform (DP) and single-platform (SP) methods. DP method uses two instruments (a FCM and a second instrument, such as a hematology analyzer, to determine lymphocyte counts) to generate absolute CD4+ T-lymphocyte counts. SP methods use one instrument to generate absolute CD4+ T-lymphocyte counts, without the need for a hematology analyzer. There are two main drawbacks of DP method: problems with precision of the DP-derived CD4+ T-lymphocyte count, which are technically related to deriving the absolute lymphocyte count from the hematology analyzer (10-14) and the relatively high cost of DP methods (US$12–US$20/test). Therefore, various SP (15-17) approaches are being evaluated. Volumetric and bead-based SP are two currently available approaches. Examples of SP methods include the FACSCount™ from Becton Dickinson Biosciences (BDB, San Jose, CA), which is bead-based, and the CyFlow counter (Partec, Munster, Germany), which is based on a volumetric method. Although there have been several evaluations of the performance of new SP FCMs (18-20), the FACSCount is the only dedicated SP FCM that has been extensively validated and widely used (21). A study by the United Kingdom External Quality Assessment Scheme for Immune Monitoring comparing several SP and DP methods showed that SP methods had the lowest intralaboratory variation, making them the preferred choice (22). Nevertheless, SP bead-based flow cytometric methods are still limited by the high cost of the fluorescent beads added to the assay system.
The U.S. Centers for Disease Control and Prevention (CDC) recommend a three-color monoclonal antibody panel for staining whole blood (a three-tube method with CD3/CD4/CD45 for CD4+ T-lymphocytes, CD3/CD8/CD45 for CD8+ T-lymphocytes, and lyse-wash or lyse-no-wash steps) and is used for both DP and SP methods (3-6, 22). Additional cost saving can be obtained by use of fewer monoclonal antibody reagents in the assays. Two-color methods with CD3/CD4 or CD3/CD8 can potentially reduce costs in many developing countries including Thailand.
A new method to determine CD4+ T-lymphocyte counts has been developed by Guava Technologies. It uses a volumetric approach (a microcapillary cytometer, the Guava® personal cell analyzer (PCA)) and two-color staining (with the inexpensive Guava EasyCD4™ and EasyCD8™ reagent kit, costing less than US$4/test). The low cost of testing can increase access to CD4+ T-lymphocyte count determinations, particularly for people living in developing countries or in resource-limited settings.
The purpose of this study was to evaluate the performance of the Guava PCA method in determining absolute CD4+ T-lymphocyte counts from clinical samples. Values obtained with this method were compared with values obtained on the same samples using two standard SP bead-based methods from BDB: the TruCOUNT™ tube method using FACSCalibur™ FCM and the FACSCount method using FACSCount FCM. Since absolute CD4+ T-lymphocyte count values below or equal to 200 cells/μl are used for clinical decision making, data analysis will be separately performed for counts ≤200 and >200 cells/μl.
This study was performed as part of the WHO Health Technology and Pharmaceuticals, which supports the evaluation of alternative methodologies for enumerating absolute CD4+ T-lymphocytes.
MATERIALS AND METHODS
Patients and Blood Samples
Samples were obtained from 250 HIV-1 infected Thais at Siriraj Hospital, Bangkok, Thailand. HIV-1 infection was diagnosed serologically (AxSYM HIV-1/HIV-2, Abbott, Germany) with confirmation by two other different serologic tests (1 + 2 VITROS, Ortho-Clinical Diagnostics and SERODIA HIV, Fuji Rebio, Japan). For CD4+ T-lymphocyte determinations, 2 ml of blood was collected (by venipuncture) in K3EDTA-containing tubes and processed for immunophenotyping within 6 h. All samples used in these studies were leftover clinical specimens that were unlinked and anonymized at the Department of Immunology, Faculty of Medicine, Siriraj Hospital, Bangkok, Thailand. This study was approved by the Ethics Committee of the Faculty of Medicine, Siriraj Hospital, Mahidol University and was determined exempt from IRB ethical review by the U.S. CDC, since all samples were unlinked.
Method and Equipment Used in This Study
The FACSCalibur™ system (BDB) is a multicolor bench-top FCM equipped with a dual-laser set up that includes a 15 mW argon ion laser that operates at 488 nm and excites the three fluorescent parameters of fluorescein isothiocyanate (FITC), phycoerythrin (PE), and peridinin chlorophyll protein (PerCP). The second laser is a 635-nm red diode laser for excitation of allophycocyanin fluorochrome. This system is combined with three (FITC/PE/PerCP) fluorescence-conjugated TriTEST™ monoclonal antibody reagents of CD3/CD4/CD45 and CD3/CD8/CD45 (BDB), and the known density fluorescent-integrated TruCOUNT™ beads (BDB).
The FACSCount™ system (BDB) is an SP bench-top FCM equipped with a green laser. It is combined with a built-in software and two-color monoclonal reagents in a twin-tube containing calibrated beads, with additional control beads. The first tube in each pair consists of a mixture of monoclonal antibody reagents of CD4/CD3 conjugated to a PE and PE.Cychrome (PE.Cy5) fluorescence and a known density of fluorescent beads. The second tube contains CD8/CD3. The control set consists of fluorescent beads at four different densities: zero (0 beads/μl), low (50 beads/μl), medium (250 beads/μl), and high (1,000 beads/μl).
The Guava® PCA is a bench-top SP machine known as a microcapillary cytometer. The system is equipped with a 532-nm green diode laser with a forward scatter detector and detectors of orange (580 nm) and red fluorescence (675 nm). It is used in combination with the two-color Guava EasyCD4™ (CD4-PE/CD3-PE.Cy5) and EasyCD8™ (CD8-PE/CD3-PE.Cy5) monoclonal antibody reagents and a volumetric control system that allows a precise count of cell numbers and fluid volume, and is regulated by a variable-speed fluid (stepper motor syringe pump) that does not require sheath fluid.
Immunophenotypic Staining of Peripheral Blood
For the TruCOUNT method (performed at the Department of Immunology, Faculty of Medicine, Siriraj Hospital), 20 μl of TriTEST three-color monoclonal antibodies and 50 μl of EDTA-anticoagulant whole blood were added to a TruCOUNT tube containing a known bead concentration. The mixture was incubated for 20 min at room temperature in the dark before adding 450 μl of FACS™ lysing solution (BDB). After 15 min of incubation, the lyse-no-wash stained samples were analyzed by FACSCalibur FCM.
For the FACSCount method (performed at the Thailand Ministry of Public Health-U.S. CDC Collaboration laboratory), 50 μl whole blood was added to each of a pair of CD4/CD3 and CD8/CD3 reagent tubes by using electronic pipette. The tubes were vortexed for 5 s and incubated in the dark at room temperature for 60 min. After incubation, 50 μl of the fixative provided with the reagent kit was added to each tube, and they were incubated for 30 min in the dark at room temperature. After vortexing the tubes, the no-lyse stained samples were analyzed using the FACSCount FCM.
For the Guava PCA method (performed at the Department of Immunology, Faculty of Medicine, Siriraj Hospital), 10 μl of EDTA-anticoagulant whole blood and 10 μl of premixed monoclonal antibody solution (Guava EasyCD4 and EasyCD8 reagents consisting of 1 μl CD3, 1 μl CD4 or CD8, and 8 μl phosphate buffered saline) were added to 1.5-ml microfuge tubes. The mixtures were vortexed and incubated for 15 min at room temperature in the dark before adding 180 μl of lysing/fixing solution, making a total mixture volume of 200 μl. The mixture was incubated for an additional 15 min prior to running on the Guava PCA.
Flow Cytometric Analysis
For the TruCOUNT method, data from each sample stained with TriTEST reagents were acquired and analyzed using the MultiSET™ software (BDB) on the FACSCalibur machine. Cells stained with FITC-, PE-, and PerCP-conjugated monoclonal antibodies were detected using the logarithmic amplification of green, orange, and red fluorescences, respectively. The forward scatter (FSC-H) and side scatter (SSC-H) of cells were measured using a linear scale. After acquiring data on 15,000 cells, a region was automatically set on SSC-Hlow/CD45 PerCPhigh+ cells. Cells in this gate were considered to be lymphocytes, while cells outside this gate were considered to be monocytes (SSC-Hmedium/CD45 PerCPintermediate+ cells) and granulocytes (SSC-Hhigh/CD45low+) (Fig. 1A). Once this was established, the percentage and absolute counts ofCD3+/CD4+ (Fig. 1B) and CD3+/CD8+ T-lymphocytes (Fig. 1C) were then automatically generated by the profile obtained using CD3-FITC/CD4-PE and CD3-FITC/CD8-PE data and the software provided.
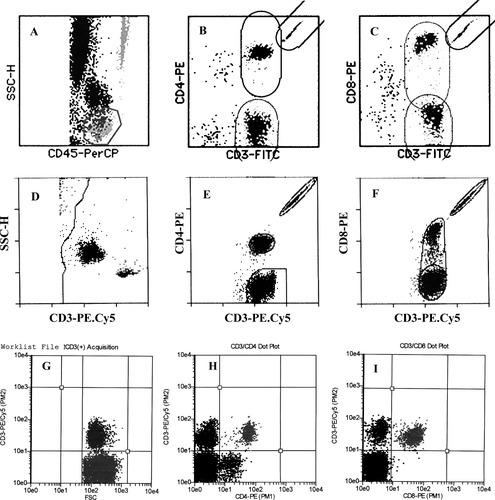
Representative flow cytometric dot-plots illustrating the software algorithm of the TriTEST/ TruCOUNT MultiSET method (A–C), the two-color FACSCount method (D–F), and the Guava PCA method (G–I).
For the FACSCount method, when a stained sample was introduced to the FACSCount, an elliptical region was automatically set around each cell population and the integrated beads by the built-in gating software, and 30,000 events were acquired. The ratio of fluorescent cells to beads multiplied by the known concentration of beads in the tube was automatically used by the built-in software to calculate the CD3+, CD4+, and CD8+ T-lymphocytes as absolute numbers of lymphocytes per microliter of blood (Figs. 1D, 1E, and 1F).
For Guava PCA analysis, a quadrant was set on the PE.Cy5-conjugated CD3+ T-lymphocytes and FSC dot plot (CD3-PE.Cy5/FSC) (Fig. 1G). After acquiring at least 2,000 CD3+ cells, the double positive CD4+/CD3+ (Fig. 1H) or CD8+/CD4+ T-lymphocytes (Fig. 1I) were then automatically obtained in another quadrant plot of CD3-PE.Cy5/CD4-PE or CD3-PE.Cy5/CD8-PE. To obtain an absolute CD4+ or CD8+ T-lymphocyte count, a dilution factor of 1:20 was directly calculated by using the Cytosoft™ software (Guava).
Quality Control and Assay Precision
To ensure quality control of the flow cytometric immunophenotyping regarding performance of personnel and instrument, the same batch of reagents was used throughout the study. In addition, all of the immunostaining procedures and the flow cytometric analyses were performed by the same operator for each instrument. Moreover, adequate training on the use of reverse pipetting technique and electronic pipette was also provided for each operator. In addition, the FCM photomultiplier tube voltage, sensitivity, and fluorescent compensation settings were optimized prior to sample acquisition and analysis using Calibrite™ beads (BDB), a control set of fluorochrome-integrated beads (BDB), FACSCount controls and Guava Check beads kit for the FACSCalibur, FACSCount and Guava PCA, respectively. To assess the precision of the Guava system, stabilized whole blood preparation from one CD-Check Plus CD4 Low (Streck, Omaha, NE), an internal quality control stabilized whole blood was used. Within-run and between-run variations, and, when applicable, the across-instrument pooled coefficient of variations (CVs) were calculated for absolute CD4+ and CD8+ T-lymphocyte counts.
Statistical Analysis
Comparison of CD4+ and CD8+ T-lymphocyte counts obtained by different assays was performed by linear correlation analysis using StatView™ (Brainpower, Calabasas, CA). In order to allow the determination of whether the two assays agreed sufficiently to be used interchangeably, the difference between each data pair of measurements (assay A − assay B) was graphically plotted on the vertical axis against the average of the pair (assay A + assay B)/2 on the horizontal axis as described by the Bland–Altman statistical bias method (23).
Bland–Altman statistical bias of each two assays was also separately performed on each group of absolute CD4+ T-lymphocyte values ≤200 and >200 cells/μl.
RESULTS
First, operational precision for both within-run and between-run variation of the Guava PCA system was determined using both the Guava Check bead kit and the Streck CD-Check Plus CD4 Low-stabilized whole blood samples. The mean %CVs of CD4+ and CD8+ T-lymphocyte counts derived from analyzing 10 replicate stabilized blood samples were less than 5%. The between-run reproducibility of five replicate stabilized blood samples analyzed over the period of study was also excellent with CVs less than 8%. The Guava PCA measurements from 10 acquisitions of 1,000 Guava Check beads/ml were acceptably precise with all values within ±10% of the expected value.
Absolute CD4+ and CD8+ T-lymphocyte counts of 250 HIV-1+ samples obtained with the Guava PCA were compared with the counts obtained with the two reference bead-based flow cytometeric methods (Fig. 2). Correlation coefficients for CD4+ T-lymphocyte counts from Guava PCA method were highly significant with the TruCOUNT FCM method (R = 0.97, coefficient of determination [R2] = 0.95; y = 16.63 + 0.99x, P < 0.0001, Fig. 2A), and with the FACSCount method (R = 0.97; R2 = 0.94;y = 20.49 + 1.04x, P < 0.0001, Fig. 2B). CD4+ T-lymphocyte counts determined by the two bead-based assays were also highly correlated (R = 0.99; R2 = 0.98; y = −1.32 + 0.95x; P < 0.001, Fig. 2C). For absolute CD8+ T-lymphocytes, data from the Guava PCA method was highly correlated with data from the two standard methods (R2 = 0.92; R2 = 0.88, P < 0.001, Figs. 2D and 2E), and data between the two standard methods was also highly correlated (Fig. 2F).

Correlation analysis of absolute CD4+ and CD8+ T-lymphocyte counts. Correlation plots for Guava PCA versus three-color TruCOUNT using FACSCalibur (A,D). Guava PCA versus FACSCount (B,E) and FACSCount versus TruCOUNT (C,F).
Bland–Altman bias plots were analyzed by plotting the difference between the absolute lymphocyte subset values generated using the Guava PCA method and the standard bead-based flow cytometric methods against the mean absolute values of the two methods (Fig. 3). As the mean CD4+ T-lymphocyte counts increased, there tended to be an increase in the bias. The mean bias was +13.1 cells/μl (limits of agreement [LOAs], −117.9 cells/μl to +144.1 cells/μl) when the Guava PCA method was compared with the TruCOUNT method (Fig. 3A) and +33.2 cells/μl (LOAs, −101.81 cells/μl to +168.3 cells/μl) when the Guava PCA method was compared with the FACSCount method (Fig. 3B). These findings indicate that the volumetric Guava PCA method yielded higher CD4+ T-lymphocyte counts than did the two bead-based methods. Of the two bead-based methods, the FACSCount method had lower CD4+ T-lymphocyte counts than the TruCOUNT FCM, since the mean bias of CD4+ + T-lymphocyte counts was −20.2 cells/μl (LOAs −86.4 cells/μl to +46.1 cells/μl, Fig. 3C).
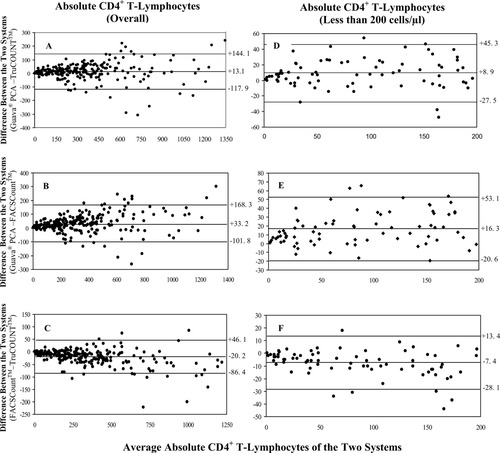
Bland–Altman bias plots showing CD4+ T-lymphocyte data for all values (A–C) and values that are less than 200 cells/μl. The differences between data from the Guava PCA and the three-color TruCOUNT using FACSCalibur (A,D), Guava PCA and FACSCount (B,E), and FACSCount and TruCOUNT (C,F) are shown. Lines represent means and LOAs. CD4+ counts are indicated on the X-axis.
The bias analysis was separately performed on the 76 HIV+ samples with absolute CD4+ T-lymphocyte counts ≤200 cells/μl and the remaining samples with count >200 cells/μl. In the samples with CD4+ T-lymphocyte counts ≤200 cells/μl, the higher values from the Guava PCA than the TruCOUNT and FACSCount methods were again observed. The Guava PCA method has a bias of +8.9 cells/μl (LOAs, −27.5 cells/μl to +45.3 cells/μl) compared with the TruCOUNT method (Fig. 3D) and a bias of +16.3 cells/μl (LOAs, −20.6 cells/μl to +53.1 cells/μl) compared with the FACSCount method (Fig. 3E). Same as observed in the overall data set, a bias toward lower CD4+ T-lymphocyte counts of −7.4 cells/μl (LOAs, −28.1 cells/μl to +13.4 cells/μl) was observed with the FACSCount compared with the TruCOUNT methods (Fig. 3F).
When subset of samples with CD4+ T-lymphocyte counts of >200 cells/μl were analyzed, the same trend of bias was observed with the Guava PCA method higher than the TruCOUNT method (+17.3 cells/μl; LOAs, −149.4 cells/μl to +184.1 cells/μl, Fig. 4A) and the FACSCount method (+44.9 cells/μl; LOAs, −122.6 cells/μl to +212.3 cells/μl, Fig. 4B). The FACSCount method also had a higher bias toward the CD4+ T-lymphocyte values >200 cells/μl (−27.5 cells/μl; LOAs −107.7 cells/μl to +52.6 cells/μl) than the TruCOUNT method (Fig. 4C).

Bland–Altman bias plots of absolute CD4+ T-lymphocyte counts of >200 cells/μl. The differences between Guava PCA and the three-color TruCOUNT using FACSCalibur (A), Guava PCA and FACSCount (B), and FACSCount and TruCOUNT (C) are shown. Lines represent means and LOAs. CD4+ counts are indicated on the X-axis.
Analysis of the Bland–Altman plots of the CD8+ T-lymphocyte data also showed good agreement between the data from the Guava PCA method and the two standard bead-based methods, although the bias was for lower values with the Guava PCA method than with the TruCOUNT and FACSCount methods. The mean difference in CD8+ T-lymphocyte counts between the Guava PCA method and the TruCOUNT method was −79.5 cells/μl (LOAs, −455.7 cells/μl to +296.8 cells/μl, Fig. 5A), and between the Guava PCA method and the FACSCount method was −52.3 cells/μl (LOAs, −370.7 cells/μl to +266.2 cells/μl, Fig. 5B). The difference between the two standard bead-based methods was −18.8 ells/μl (LOAs of −197.1 to +159.5 cells/μl, Fig. 5C).
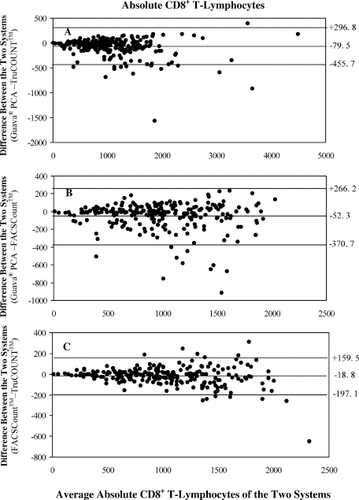
Bland–Altman bias plots of absolute CD8+ T-lymphocyte counts. The differences between Guava PCA and the three-color TruCOUNT using FACSCalubur (A), Guava PCA and FACSCount (B), and FACSCount and TruCOUNT (C) are shown. Lines represent means and LOAs. CD8+ counts are indicated on the X-axis.
DISCUSSION
Conventional flow cytometric methods to determine CD4+ T-lymphocyte counts are relatively complex, technically demanding, costly, and the equipment needs constant maintenance. Thus, these methods are difficult to apply for routine use in most laboratories with limited facilities and in resource-limited settings. An ideal CD4 testing method for such settings would be an SP approach that minimizes the sources of variation, and is simple to use, reliable, accurate, and less expensive. The Guava PCA method potentially satisfies most of these criteria and was the focus of this study. It was evaluated for its accuracy and compared to the TruCOUNT and FACSCount methods, since these are the SP systems currently in use in Thailand and many other countries.
Unique features of the Guava PCA method are the use of only two-color (instead of three-color) monoclonal antibody reagents and only 1/10 of volume of reagents used in the standard assays. Samples are stained with combinations of monoclonal antibodies against CD4, CD8, and CD3 T-lymphocytes, a method recommended by CDC for quality assured analysis of HIV samples (3, 6). In gating, a simple quadrant gate is set on CD4+/CD3+ or CD8+/CD3+ T-lymphocytes. This same strategy of identifying CD4+/CD3+ T-lymphocytes as a proportion of total CD3+ T-lymphocytes has been well described using the FACSCount system (21). Although a reliable absolute CD4+/CD3+ or CD8+/CD3+T-lymphocyte value can be obtained automatically by the Cytosoft™ software on the Guava PCA machine, it is important to periodically carefully analyze gate integrity of both FSC/CD3+ and CD4+/CD3+ or CD8+/CD3+ T-lymphocytes, to ensure that positive cells are clearly distinguished from the negative cells. Because a CD3+ T-lymphocyte count is performed in each tube, the reported CD3+ T-lymphocytes can also be used as a consistency check of the CD3+ T-lymphocytes results from the two tubes.
In this study, absolute CD4+/CD3+ and CD8+/CD3+ T-lymphocyte values obtained from the Guava PCA method correlated highly (R2 = 0.94) with those from standard bead-based three-color TruCOUNT using FACSCalibur and two-color FACSCount FCMs. The overall bias for absolute CD4+ values was +13.1 and +33.2 cells/μL when the Guava PCA method was compared with the standard TruCOUNT and FACSCount methods, respectively. If one considers HIV-infected individuals who need ART because their absolute CD4 counts are less than 200 cells/μL, CD4+/CD3+ T-lymphocyte counts were only +8.9 and +16.3 cells/μL higher with the Guava PCA method than the two predicate methods (Fig. 2). These biases will result in CD4 counts that are 5 and 8% higher when the Guava PCA method is used, and while this might influence clinical decision making, they are unlikely to affect monitoring as long as the methods are not used interchangeably.
Data from the Guava PCA method showed good correlation with TruCOUNT and FACSCount methods for CD4+ and CD8+ T-lymphocyte counts throughout the whole study range. However, there were some consistent biases, with higher values for absolute CD4+ T-lymphocyte counts and lower values for absolute CD8+ T-lymphocyte counts. These variations may be due to technical differences in the methods. All three assay systems require a high level of precision in the dispensing of reagents and at all the pipetting steps, with particular care regarding dilution steps (11, 24). In the two standard bead-based methods, since the beads used are preloaded and strictly prepared and controlled by the manufacturer; there should not be any biases attributable to the beads, in relation to pipetting or calculations. The Guava PCA method, on the other hand, gives absolute CD4+ T-lymphocyte counts by defining absolute CD4+ T-lymphocyte counts in a known final volume of cell suspension. Hence calculations may be affected by any factor influencing concentrations. Other factors that may influence the values obtained could include differences in the red blood cell lysing or fixing solutions used in the three assays. It is known that lysing reagents may affect leucocyte populations differently; some reagents may even cause significant loss of leucocytes, which may contribute to error especially in samples from HIV-infected patients whose cells may be more susceptible to lysis (11, 25). Artifacts such as cell aggregates, cell debris, background fluorescence and, more importantly, the interference by some monocytes contaminating in the lymphocyte boundary may further lead to lymphocyte impurities (11, 26) and influence CD4+ or CD8+ T-lymphocyte count accuracy.
This study demonstrated that the microcapillary Guava PCA method is a valid method of CD4 enumeration. Since this evaluation was performed in reference laboratories that fulfill all required FCM standards, a multicenter validation study should be initiated to determine whether the Guava PCA method can be interchanged with other methods of CD4 counting and before widespread implementation in resource-limited settings. Although the evaluation suggests that the assay is promising compared to the existing standard bead-based assays, there are some limitations of the method and challenges to implementation. Operation of the Guava PCA system still requires substantial technical expertise and maintenance. The small blood volume used in the assay may be problematic in samples from leucopenia patients or patients with very low levels (<17 CD3 T-lymphocytes cells/μl) of CD3+ T-lymphocyte counts in that acquiring the required 2,000 CD3+ T-lymphocytes in 4 min (for quality control in the FSC/CD3 plots) may be challenging and may generate statistical errors in determining accurate CD4+/CD3+ or CD8+/CD3+ T-lymphocyte counts. Moreover, setting gates to distinguish both CD3+ and CD4+/CD3+ or CD8+/CD3+ T-lymphocytes from non-CD3+ and non-CD4+/CD3+ or CD8+/CD3+ T-lymphocytes can be difficult, since the intensities of positive CD4-PE.Cy5/CD3-PE or CD8-PE/CD3+-PE staining in the Guava PCA method tend to be lower than that observed with the TriTEST/TruCOUNT and FACSCount methods (personal observations). Another critical issue is regular maintenance of the fluidic system (e.g., to prevent obstruction of the capillary flow cell), since the absence of a need for sheath fluid in the Guava PCA system means that the system's sampling precision depends solely on the integrity of the fluid pathway. An implementation challenge is that like FACSCount method and unlike the TriTEST/TruCOUNT method, the Guava PCA method is not suitable for pediatric blood samples, since it only provides absolute CD4+ and CD8+ T-lymphocyte counts, and not percentages. This is particularly important, since the percentages of CD4+ T-lymphocytes is generally used in pediatric clinical practice, and the WHO has recommended that the percentage of CD4+ T-lymphocytes of total lymphocytes be used for decision making to initiate ART in HIV-infected children under the age of 18 months (27). Another issue is the detection of double CD4+/CD8+ T-lymphocytes. Some HIV-infected patients can have high frequencies of these T-lymphocytes in their peripheral blood (28, 29), but the clinical significance of this subpopulation is not known. Neither the FACSCount method nor the Guava PCA method can accurately determine the proportion or the absolute number of this subpopulation. Should it become clinically relevant to evaluate these double positive T-lymphocytes, alternative strategies will need to be developed to identify them. One option includes labeling with CD3/CD4/CD8 monoclonal antibodies as in the TriTEST/TruCOUNT method and direct visualization using FACSCalibur FCM.
In conclusion, this new Guava PCA method, but like other two reference microbead-based methods, needs substantial technical expertise. However, this new method is simple and easy to use. The cytometry has a high throughput of 100 samples per day, which is compatible with the standard FACSCount system. The Guava PCA system is relatively cheap, and its compact size means it can easily be moved between laboratories. The smaller number and volume of reagents reduce CD4 testing costs by up to US$8–US$16, since the cost of a CD4 testing using the Guava PCA method is less than US$4 compared to US$12–US$20 for the standard bead-based methods. These cost saving will have a strong impact on access to CD4 testing for persons with HIV infection in resource-limited countries. The use of ART has dramatically reduced rates of mortality and morbidity, and increased the life expectancy of persons with HIV/AIDS. In Thailand, more than 80,000 HIV-infected persons per year are anticipated to need access to ART (1). This will result in a need for 200,000–300,000 CD4 tests per year. Available of this cheaper assay, the Guava PCA method, will make CD4 testing in Thailand and other resource-limited settings more affordable.
Acknowledgements
The authors thank Dr. Hla Shain for his valuable comments.




