Expression of inhibitory receptor ILT3 on neoplastic B cells is associated with lymphoid tissue involvement in chronic lymphocytic leukemia
Abstract
T cell responses against leukemia-associated antigens have been reported in chronic lymphocytic leukemia (CLL). However, the relentless accumulation of CLL B cells in some patients indicates that anti-tumor immune responses are inefficient. Inhibitory receptors from the Ig-like transcript (ILT) family, such as ILT3 and ILT4, are crucial to the tolerogenic activity of antigen presenting cells. In this study, we examined the expression of ILT3 on CD5+ B cells obtained from 47 patients with CLL. Using flow cytometry and RT-PCR, we found that B CLL cells from 23 of 47 patients expressed ILT3 protein and mature ILT3 mRNA. ILT3 protein and mRNA were not found in normal B cells obtained from donors without CLL. Expression of ILT4 in normal and B CLL cells showed a pattern similar to ILT3. The frequency of ILT3 positive CLL B cells was higher in patients with lymphoid tissue involvement, suggesting that ILT3 may have prognostic value in CLL. Our findings indicate that expression of ILT3 and ILT4 on CLL B cells represents a phenotypic abnormality that may play a role in tolerization of tumor-specific T cells. © 2007 Clinical Cytometry Society
Chronic lymphocytic leukemia (CLL) is characterized by the accumulation of malignant B cells in bone marrow, peripheral blood, and lymphoid tissues (1, 2). When predominantly involving lymphoid tissues, the term small lymphocytic lymphoma (SLL) is used. CLL has a heterogeneous clinical course. In some patients CLL manifests as a slowly progressing disease with no treatment required, while in others it follows an aggressive course, which requires treatment and is associated with relatively short survival. Studies of B cell receptor genes expressed by CLL cells have demonstrated that the presence of somatic hypermutations in the variable region of the immunoglobulin heavy chain (IgV(H)) of the CLL B cells is associated with a favorable prognosis, whereas the absence of mutations is correlated with an aggressive clinical course (3, 4).
Although knowledge of IgV(H) mutational status is useful in assessing the prognosis of CLL, identification of IgV(H) sequences is currently too costly and time consuming to become part of the standard diagnostic workup for CLL. Therefore, the search for surrogate markers with prognostic value for patients with CLL has continued even after the correlation between the IgV(H) mutational status and disease progression had been established. Several surrogate markers have been proposed as prognostic factors in CLL, although with limited success (5). CD38, a cell-surface activation marker, has been linked to disease heterogeneity (4, 6) as it is expressed at higher levels by B-CLL cells from patients with poor prognosis. However, CD38 expression is largely independent of IgV(H) mutational status and, therefore, its prognostic value remains controversial (7).
Immunophenotypic characterization of CLL has indicated that B-CLL cells carry the features of antigen-experienced B cells (8), a finding confirmed by gene expression analysis (9, 10). Although the gene expression signature of CLL is mostly independent of the Ig mutational status, a small set of genes are differentially expressed in CLL with unmutated (compared to mutated) IgV(H) genes. The differentially expressed genes include Zap-70, a protein tyrosine kinase preferentially expressed in CLL with unmutated IgV(H) genes. Zap-70 participates in signal transduction of the BCR, and its overexpression by CLL B cells was shown to be associated with poor prognosis (11, 12).
CLL is characterized by impaired humoral as well as cellular immune responses (13, 14). Despite knowledge of these manifestations, the relationship between reactive T cells and neoplastic B cells is poorly understood. One noteworthy aspect of this relationship is the failure of tumor-specific T cells to respond against leukemic cells. The existence of tumor-specific T cells that recognize autologous CLL cells has been previously demonstrated (15-17). However, the continuous accumulation of neoplastic cells in progressive disease indicates that anti-tumor T cell responses are inefficient in many patients. In fact, evidence has been provided that T cell function is profoundly altered in CLL (14, 18). Conceivably, this may be due to abnormal expression of cell surface molecules involved in T-B cell interaction. We have previously demonstrated the crucial tolerogenic role played by certain inhibitory receptors, which belong to the immunoglobulin-like transcript (ILT) family (19, 20). This family of immunoreceptor genes includes the killing inhibitory receptors (KIRs), and is part of the leukocyte receptor cluster located on chromosome 19 (21). Some of these receptors, such as ILT3 and ILT4, are structurally and functionally related to KIRs, and contain immunoreceptor tyrosine based inhibitory motifs (ITIMs) (22, 23). Ligation of these receptors results in the phosphorylation of the ITIMs, which recruit SH2-containing protein tyrosine phosphatase 1 (SHP-1) that downregulates cell activation.
Previous studies have identified HLA-Class I as the ligand for ILT4 receptors (23), whereas the ligand of ILT3 remains unknown. ILT3 and ILT4 are expressed on monocytes, dendritic, and endothelial cells, but not on T or B lymphocytes (22, 23). We have shown that upregulation of ILT3 and ILT4 receptors on professional and non-professional antigen presenting cells (APC) results in reduced expression of costimulatory molecules and induction of anergy in CD4+ T helper cells, which recognize MHC/peptide complexes on the surface of these APCs (19, 20). Membrane and soluble ILT3 were further shown to elicit in vitro differentiation of CD8+ T suppressor cells in mixed lymphocyte cultures (24).
These findings prompted us to explore the hypothesis that certain B-cell lymphomas, such as CLL, express ILT3 and ILT4 inhibitory receptors. We now report that ILT3 and ILT4 are expressed on neoplastic B-lymphocytes in CLL, and that increased expression of ILT3 protein is associated with lymphoid tissue involvement.
MATERIALS AND METHODS
Human Specimens
A total of 56 consecutive specimens were obtained from 47 adult patients with CLL followed at Columbia University Medical Center between May 2003 and September 2005. Patient samples comprised 44 samples of peripheral blood, 9 samples of bone marrow, and 3 lymph nodes. Serial peripheral blood and bone marrow specimens were obtained from 10 patients. CLL was diagnosed based on morphology, complete immunophenotyping by flow cytometry, and cytogenetic studies including karyotyping and fluorescent in situ hybridization (FISH). Clinical parameters including age, gender, disease stage, involvement of lymphoid areas (lymph nodes, spleen, and/or liver), chromosomal abnormalities, hemoglobin concentration, platelet, and white blood cell count, lactate dehydrogenase (LDH) levels, and treatment history were available for most patients.
The following specimens were included as controls in this study: 12 samples of peripheral blood collected from healthy volunteers, 7 bone marrow samples obtained from patients with no evidence of bone marrow involvement by lymphoma (1 aplastic anemia, 2 gastric lymphoma, 2 acute leukemia in remission, and 2 viral infection cases), and 6 normal spleens obtained from deceased transplant donors. Patients and normal volunteers were enrolled in this study in accordance to an existing IRB protocol.
Flow Cytometry
Immunophenotypic characterization of lymphocytes obtained from CLL patients or normal volunteers was performed by three- or four-color flow cytometry using the following monoclonal antibodies: anti-CD45, −CD19, −CD20, −CD5, −CD23, −CD11c, −κ, and −λ Ig light chains, −CD38, −CD79a (provided by BD Biosciences, San Jose, CA), −FMC7 (Beckman Coulter, Miami, FL), and −Zap−70 (Caltag, Burlingame, CA). Cell surface staining of ILT3 protein was performed using anti-ILT3 PC5 mAb (Beckman Coulter) in combination with anti-CD19 FITC and −CD5 PE antibodies. Staining of ILT4 was performed using a rat anti-ILT4 IgG mAb (generous gift from Dr. Marco Colonna) conjugated with FITC. Samples were considered positive for cell surface ILT3 or ILT4 expression if more than 10% of the CD5+ B cells expressed the receptor. All specimens were tested within 48 h of collection.
Staining of lymphocytes from peripheral blood, bone marrow, and lymph nodes was performed according to the manufacturer's instructions. Briefly, cell surface staining was performed by incubating 500,000 mononucleated cells with recommended amounts of fluorescent antibodies for 15 min at room temperature. After red cell lysing using FACSLyse (BD Biosciences) solution, cell suspensions were washed two times with PBS supplemented with 2% fetal calf serum and 0.1% azide, and resuspended in 1% paraformaldehyde in PBS. For intracellular staining of CD79a and zap-70 proteins, cells were permeabilized using Fix-and-Perm reagents (Caltag), and then stained with anti-CD79a PE or -zap-70 PE mAb. Cell viability, as determined by 7-AAD staining, was higher than 80% in all the specimens included in the study. ILT3 expression was tested on fresh specimens in all cases. Cells were run and analyzed on a FACSCalibur (BD Biosciences), using CellQuestPro software.
RT-PCR
Normal and leukemic B cells were purified from PBMC using B Cell Isolation Kit II (Miltenyi Biotech, Auburn, CA). The purity of isolated B cells was in all cases higher than 95%. Total cellular RNA was extracted from purified B cells using RNeasy kit (QIAGEN, Valencia, CA), according to the manufacturer's instructions. RNA samples were treated with DNase I in order to remove residual DNA. For the synthesis of cDNA, 1 ug of RNA was reverse transcribed into first-strand cDNA using oligo-(dT) primer and CMV-reverse transcriptase (First Strand cDNA Synthesis Kit, Roche, Nutley, NJ). Semi-quantitative PCR for ILT3 and ILT4 transcripts was performed as previously described (19, 25). To exclude genomic DNA contamination, control samples containing RNA without reverse transcriptase were run for all specimens. The following primers were used: ILT3: 5′-ACG TAT GCC AAG GTG AAA CAC T and 3′-CAT TGT GAA TTG AGA GGT CTG C, ILT4: 5′-GCA TCT TGG TGG CCG TCG TCC TAC-3′ and 3′-CCC AAA GTT CCC AGC ATC TCC TCA-3′, and GAPDH: 5′-CGG AGT CAA CGG ATT TGG TCG TAT and 3′-AGC CTT CTC CAT GGT GGT GAA GAC.
Statistical Analysis
Statistical analysis was performed using Student's t-test of significance and two-tailed Fisher exact test. Bonferroni criteria were applied.
RESULTS
Cell Surface Expression of ILT-3 Protein on Normal and CLL B Cells
Flow cytometric analysis of human peripheral blood obtained from healthy volunteers and bone marrow obtained from non-CLL patients showing no evidence of medullary involvement indicated that ILT3 was strongly positive on monocytes, negative or weakly positive on granulocytes, and negative on lymphocytes [Fig. 1, (panels 1A and 1B)]. Since B cells represent a small fraction of all nucleated cells in specimens of normal blood and marrow, the possibility exists that ILT3 expression was not detected on B cell populations due to a dilution effect. However, this possibility is unlikely since flow cytometry analysis of spleen B cells in suspensions containing on average 45–55% B cells indicated that ILT3 was not expressed on B cells from either CD5+ or CD5- subset (Fig. 1C).

Cell surface expression of ILT3 protein in normal B cells. Peripheral blood obtained from normal volunteers (A), bone marrow obtained from non-CLL patients with no evidence of medullary involvement (B), and spleen obtained from deceased transplant donors (C) were analyzed by flow cytometry. Histograms obtained with mouse anti-human ILT3 PC5 antibody (solid lines) or mouse IgG1 PC5 isotype control (dotted lines) are depicted. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
To investigate the level of ILT3 expression on CD5+ CLL B cells, 56 specimens obtained from 47 patients with documented CLL were studied by flow cytometry (Table 1). These specimens included peripheral blood, bone marrow and lymph node samples. The frequency of CD5+ B cells in these samples ranged from 21 to 95% of the total lymphocytes. Among CD5+ B cells, the frequency of ILT3 positive B cells ranged from <1–72%, with a mean of 19%. Forty-eight percent of peripheral blood, 33% of bone marrow, and three out of 3 lymph node samples were positive for ILT3, displaying this receptor on more than 10% of the CD5+ B cells. Overall, ILT3 was positive in 23 of 47 patients. In four patients, peripheral blood as well as bone marrow specimens were available for flow cytometric analysis. As illustrated in Figure 2 (panels 2A and 2B), the levels of ILT3 expression were similar in blood and bone marrow samples obtained from the same patient.
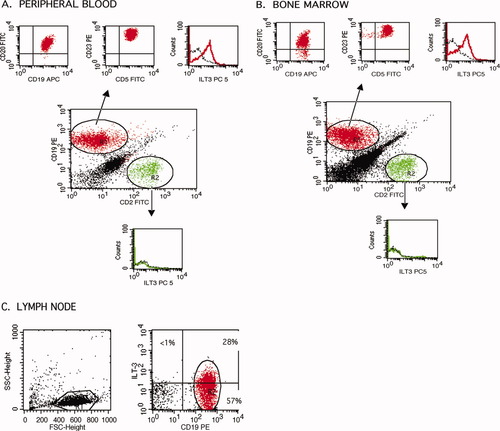
Cell surface expression of ILT3 protein in CLL B cells. Flow cytometry analysis of peripheral blood (A), bone marrow (B), and lymph node (C) specimens obtained from patients with CLL is depicted. Histograms obtained with mouse anti-human ILT3 PC5 antibody (solid lines) or mouse IgG1 PC5 isotype control (dotted lines) are shown. Results are representative for ILT3 positive CLL specimens. The peripheral blood and bone marrow samples shown here were obtained from the same patient (patient E in Fig. 3). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
| Group variable | All patients | ILT3 positive patients (>10% of CD19+CD5+ cells) | ILT3 negative patients (<10% of CD19+CD5+ cells) | P-value |
|---|---|---|---|---|
| No. of patients | 47 | 23 | 24 | |
| Patient age (n = 47) | 69 | 68 | 70 | NS |
| No. of male patients | 31 | 18 | 13 | NS |
| No. of female patients | 16 | 5 | 11 | |
| Rai stage at diagnosis (n = 33) | ||||
| 0 | 9 | 3 | 6 | NS |
| I-II | 12 | 9 | 3 | |
| III-IV | 12 | 6 | 6 | |
| Binet stage at diagnosis (n = 45) | ||||
| A | 20 | 11 | 9 | NS |
| B | 15 | 8 | 7 | |
| C | 10 | 5 | 5 | |
| Lymphoid tissue involvement (n = 23) | ||||
| Yes | 15 | 11 | 4 | P = 0.009 |
| No | 8 | 1 | 7 | |
| Hemoglobin, g/dl (n = 46) | 12 ± 3 | 12 ± 2 | 11 ± 3 | NS |
| Platelets/10−9 L (n = 46) | 186 ± 113 | 193 ± 80 | 162 ± 79 | NS |
| WBC/10−9 L (n = 46) | 56 ± 87 | 61 ± 85 | 40 ± 39 | NS |
| LDH, U/L (n = 32) | 244 ± 137 | 234 ± 103 | 257 ± 175 | NS |
| LDT, months (n = 25) | 47 ± 45 | 38 ± 38 | 56 ± 52 | NS |
| CD38 expression, % of B cells (n = 46) | 30 ± 31 | 33 ± 31 | 23 ± 31 | NS |
| zap70 expression, % of B cells (n = 29) | 29 ± 27 | 30 ± 32 | 26 ± 22 | NS |
Lymph nodes from 3 CLL patients were available for flow cytometry. Peripheral blood or bone marrow samples from these patients were not available for immunophenotyping. Analysis of the lymphocytes isolated from lymph nodes confirmed the biopsy findings regarding the presence of neoplastic B cells in all three samples. The frequency of CD5+ B cells ranged from 73 to 84% of the lymphocytes. In all three specimens, the frequency of ILT3+ cells among CD5+ B cells was higher than 10%, ranging from 35 to 70%, as illustrated in Figure 2C.
ILT-3 Transcription in CLL B Cells
Since ILT3 protein was detected in some but not all CLL cases, i.e. in 23 out of 47 patients, we used RT-PCR to examine ILT3 transcripts in CLL B cells. As indicated in Figure 3, no ILT3 mRNA message was detected in peripheral blood B cells obtained from healthy donors (n = 12). However, an mRNA transcript of 488nt, corresponding to the size of the mature ILT3 mRNA fragment (25), was present in all samples positive for ILT3 by flow cytometry. A weak or no mRNA signal corresponding to 488nt was detected in samples that showed no cell surface expression of the ILT3 protein. In previous studies, we have demonstrated that a 600nt precursor ILT3 mRNA was constitutively expressed in endothelial cells (25). Exposure of endothelial cells to tolerogenic stimuli, such as IL-10, IFN-α or HLA Class I specific CD8+ T suppressor cells, induced the expression of mature ILT3 mRNA (488nt) and production of ILT3 protein. Interestingly, the results of the present study indicate that, in contrast to endothelial cells, normal B cells showed no constitutive expression of ILT3. Tolerogenic stimuli such as IL-10 or IFN-α did not induce ILT3 expression on normal B cells (data not shown). Furthermore, CLL B cells expressed the mature ILT3 mRNA (488nt) yet were negative for precursor ILT3 mRNA (600nt). These results indicate that expression of the ILT3 gene is differently regulated in B lymphocytes and endothelial cells, and suggest that ILT3 expression on CLL B cells is an abnormal immunophenotypic feature of neoplastic B cells.

Expression of ILT3 and ILT4 mRNA in normal and CLL B cells. ILT3 and ILT4 transcripts were detected by RT-PCR using primers as indicated in Material and Method. The PCR products were analyzed on 1.2% agarose gel, stained with ethidium bromide.
ILT-4 Expression in CLL B Cells
ILT4 expression was examined by flow cytometry in blood samples obtained from 12 normal volunteers and 11 patients with CLL. As illustrated in Figure 4A, ILT4 was not detected on lymphocytes from healthy individuals. However, ILT4 was expressed by CLL lymphocytes in six out of 11 patients tested (Fig. 4B). ILT4 positive patients were also positive for ILT3, while the remaining five patients were negative for both ILT3 and ILT4. RT-PCR indicated that ILT4 mRNA was absent in purified B cells obtained from healthy individuals or ILT3 negative CLL patients (Fig. 3). However, ILT4 mRNA was detected in CLL B cells that also displayed ILT3 mRNA.

Cell surface expression of ILT4 protein. ILT4 expression on white blood cells from normal volunteers (A) or CLL patients (B) was studied by flow cytometry. Histograms obtained with rat anti-human ILT4 FITC antibody (solid lines) or rat IgG isotype control (dotted lines) are shown. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Clinical Parameters and ILT3 Expression in Patients with CLL
The clinical parameters studied in our patient population included age, gender, Rai/Binet staging of the disease, involvement of lymphoid areas (lymph nodes, liver, and/or spleen), cytogenetics findings, hemoglobin levels, platelet, and white blood count, LDH levels, lymphocyte doubling time (LDT), and expression of prognosis indicators, such as zap-70 and CD38 (Table 1). Chromosomal abnormalities were identified in 11 patients, and included trisomy 12 (six cases), 13q deletion (four patients), and 6q deletion (one patient). Statistical analysis using Bonferroni criteria indicated that there was no statistically significant correlation between the expression of ILT3 protein and the patient's age, gender, stage of the disease, hemoglobin or LDH levels, platelet or white blood count, lymphocyte doubling time (LDT), or expression of prognosis indicators, such as zap-70 and CD38 (Table 1). However, the patients with ILT3 positive B cells tended to have higher white blood counts, higher frequency of CD38 expressing cells, and shorter LDT.
The relationship between expression of ILT3 and expression of CD38 on CLL B cells was also evaluated by comparing the number of ILT3 positive and ILT3 negative cases within CD38 positive and negative groups, based on the previously reported thresholds of the frequency of CD38 positive cells (30, 20, and 7%) (4-7). This comparison did not reveal a statistically significant correlation between ILT3 and CD38 markers (P > 0.05). In a similar comparison, ILT3 did not correlate with zap-70 (P > 0.05), using a threshold of 20% for zap-70 positive B-CLL cells. However, the relationship between ILT3 and zap-70 may be difficult to assess since anti-zap-70 antibody conjugated with the PE fluorochrome (Caltag) was not validated in large studies as of yet. In our study, a statistically significant correlation was obtained between CD38 and zap-70 markers (P = 0.02; n = 29), suggesting that flow cytometric analysis using anti-zap-70 PE antibody (Caltag) provides reliable data on the level of zap-70 expression.
In 23 patients, histological and/or radiological studies were performed in order to assess the involvement of lymphoid area (lymph node groups, spleen, and liver). Involvement of at least one lymphoid area was documented in 15 patients. Among these, 11 patients had ILT3 positive CLL B cells in peripheral blood, bone marrow, or lymph nodes. In contrast, only one of 8 patients showing no evidence of lymphoid area involvement had ILT3 positive B cells in peripheral blood (P < 0.009, Table 1). Hence, there was a strong association between increased frequency of ILT3 positive B cells and involvement of lymphoid areas in CLL patients.
At the time of ILT3 testing, only three out of 47 patients had received prior treatment for CLL. The treatment was administered 7–60 months prior to characterization of ILT3 expression and consisted of cytarabine/ fludarabine/rituximab (one patient), cyclophosphamide/fludarabine (one patient), and fludarabine/rituximab (one patient). Of these 3 patients, two were ILT3 positive, while one was ILT3 negative. ILT3 expression did not significantly vary in the treated versus not-treated group (P > 0.05), although it should be noted that the group of previously treated patients was small (n = 3). An additional seven patients received therapy after the first determination of ILT3 expression was completed. Four of these patients received rituximab/fludarabine, one patient received Campath in addition to rituximab/fludarabine, one patient received only rituximab, and one received only chlorambucil. Serial flow cytometric determinations performed in four of these patients showed virtually no changes in the level of ILT3 expression on CLL B cells before and after treatment. Two of these patients were ILT3 negative and other two were ILT3 positive before treatment, and maintained that status after treatment. Taken together, these findings indicate that the anti-leukemic treatment had no impact on ILT3 expression.
DISCUSSION
Previous studies have shown that inhibitory receptors from the ILT family are predominantly expressed on the surface of myelomonocytic cells, such as macrophages and dendritic cells (21). More recently, we have reported that exposure of human endothelial cells to IL-10, IFN-α or allospecific CD8+CD28- T suppressor cells triggers processing of ILT3 precursor mRNA and results in expression of mature ILT3 transcript and production of ILT3 protein (20, 25). We have provided evidence that ILT3+ILT4+ endothelial cells become tolerogenic, and induce antigen-specific unresponsiveness of CD4+ T helper cells, in a similar manner to ILT3+ILT4+ tolerogenic dendritic cells (19, 20). Furthermore, ILT3+ILT4+ endothelial cells elicit the differentiation of CD8+CD28-FOXP3+ T suppressor cells (24).
In the present study we report that malignant B cells from a subset of patients with CLL express ILT3 and ILT4 receptors. Since ILT3 and ILT4 were not detected in normal B cells, expression of these receptors in a subset of CLL patients represents a distinctive feature of CLL B cells, possibly acquired upon malignant transformation. The mechanism accounting for the transcriptional and translational activation of ILT3 and ILT4 in CLL B cells is currently unknown. One possibility is that mutations within the promoter regions and/or translocations affecting the ILT3 and ILT4 genes are responsible for their aberrant expression. Alternatively, the expression of these inhibitory receptors may reflect the activation status of CLL cells. Recent studies have shown that expression of zap-70, a widely accepted prognostic marker in CLL, is upregulated on B cells from a subset of CLL cases, but also on normal, activated B cells (26). In contrast to these findings, our study did not identify any normal B cell populations that express ILT3. Furthermore, treatment of normal B cells with IL-10 and IFN-α did not induce expression of ILT3, although these cytokines induced expression of the ILT3 protein in endothelial cells (25). However, we cannot exclude the possibility that induction of ILT3 in B CLL cells occurs as a result of the interaction between these cells and an unknown soluble factor or regulatory cells.
Expression of other inhibitory receptors from the ILT family has recently been described in certain pathologic conditions. For example, high expression of ILT2 was been documented in clonal expansions of T large granular lymphocytes (27) and cutaneous T cell lymphomas (28). Increased ILT2 expression on peripheral lymphocytes was also described in lung allograft recipients with cytomegalovirus disease (29), while the frequency of ILT4 expressing monocytes was increased in peripheral blood of patients with HIV (30). To our knowledge, the present study shows for the first time that neoplastic B cells from patients with CLL express ILT3 and ILT4 inhibitory receptors.
Since CLL patients were followed in this study for less than five years, the prognostic value of ILT3 cannot be assessed at this time. However, the increased expression of ILT3 on CLL cells from patients with lymphoid tissue involvement suggests that ILT3 may facilitate intra-nodal proliferation of CLL B cells by suppressing T cell mediated immune responses against tumor cells. We postulate that ILT3 expressing CLL B cells may present tumor specific antigens in a tolerogenic manner, inducing T helper cell anergy and inhibiting the differentiation of cytotoxic T cells (19, 24). Furthermore, ILT3 positive CLL cells may induce the differentiation and proliferation of CD4+CD25+ T regulatory cells, which may further inhibit anti-tumor immune responses. Indeed, increased frequencies of T regulatory have been reported in patients with CLL (31). We cannot exclude that ILT3 may be also involved in transendothelial migration of CLL cells, facilitating nodal dissemination of neoplastic B cells.
In conclusion, the presence of ILT3 and ILT4 inhibitory receptors on CLL B cells represents an abnormality of cell surface antigen expression displayed by these cells. ILT3 expression is increased in patients showing lymphoid area involvement, suggesting that this receptor may be involved in intra-nodal accumulation of malignant cells. Further studies are required to elucidate the role of ILT3 in the progression of CLL and ascertain its potential as a therapeutic target.




