A high-throughput 3-parameter flow cytometry-based cell death assay
Abstract
Apoptosis plays a role in many disease states, and the evaluation of novel therapeutics that alters the apoptotic cascade is an area of intense investigation. However, many generally available methods to evaluate cell death are either time consuming, imprecise, or both. We report a system that permits simultaneous evaluation of three apoptotic markers (cell membrane integrity, mitochondrial membrane potential, and cell cycle progression) with minimal technical manipulation. This system is particularly well-suited for toxicologic evaluation of novel compounds and profiling of new apoptosis-inducing agents. © 2007 International Society for Analytical Cytology
The regulation of apoptosis is an area of intense investigation. The opportunity to pharmacologically inhibit apoptosis (1, 2) has implications for disorders such as stroke (3, 4) and viral infection (5, 6), while the opportunity to induce apoptosis has implications for diseases such as cancer (7). Because of the broad implications surrounding the regulation of cell death, novel agents that alter the death pathway have potential therapeutic applications in multiple disease states. Thus, it is not surprising that both combinatorial libraries and natural product libraries have been employed to identify novel therapeutic leads that alter apoptosis (8). Regardless of the source of these therapeutic leads, high-throughput screening serves as the basis for the examination of the efficacy of these compounds (9).
While the signaling pathways involved in cell death are still being elucidated and debated (10, 11), there is a general consensus regarding the involvement of certain organelles, such as mitochondria, in apoptosis. The mitochondria are central summation points in apoptosis (12), and generally there is a distinction drawn between mitochondria-dependent and mitochondria-independent cell death (13). Because of this central role in the apoptotic cascade, the ability to measure the mitochondrial membrane potential (ΔΨm) provides an important clue to the mechanism of cell death (14). Similarly, evaluation of mitochondrial membrane potential is important in profiling the toxicology of novel compounds (15).
High-throughput screening of cell viability is an essential tool in the drug discovery process (9). Because of the large number of samples that require processing, even slight improvements in assay efficiency and precision represent substantial advances. For example, stably-expressed GFP in Jurkat cells permits the assessment of cell death by measuring the loss of GFP fluorescence (16). While this system is an inexpensive option, it provides scant insight into the mechanisms responsible for the cell death. For example, such a system provides no information regarding mitochondrial involvement (14). In certain contexts, such as the examination of closely related combinatorial chemistry products or the examination of the toxicological profile of a compound, more information regarding the death process is necessary and requires more extensive evaluation (17).
Our group identified a novel antibiotic (18) in a 400-year old historic herbal text (9, 19). While profiling the invitro toxicology of this compound (15, 20), we found many of the methods laborious and requiring multiple experiments. In this report, we describe an assay system that allows for simultaneous measurement of cell membrane integrity, mitochondrial membrane potential, and cell cycle progression directly from the experimental 96-well plate, with little technical manipulation. This straightforward flow cytometry-based assay provides information that previously required two or three separate experimental procedures to obtain.
MATERIALS AND METHODS
Cell Culture
Jurkat T lymphocytes (ATCC; Manassas, VA) were maintained in RPMI-1640 (Cellgro; Herndon, VA). Medium was supplemented with 10% heat-inactivated fetal bovine serum (Cellgro) and 1% penicillin and streptomycin (Gibco; Grand Island, NY) at 37°C in 5% CO2. Cells were maintained between 0.1 × 106 and 1 × 106 cells/ml. All experiments were performed with cells at a concentration of 1 × 106 cells/ml. Where appropriate, the ethanol extract of Atuna racemosa was used to induce apoptosis (20).
High-Throughput Assay Protocol
This assay was performed in a 96-well U-bottom plate (Fig. 1A). In fresh media, Jurkat T cells were seeded at a density of 1 × 106 cells/ml in 100 μl/well (Fig. 1B). Aliquots of the compound to be evaluated were created as 100× solutions in dimethyl sulfoxide (DMSO; Sigma; St. Louis, MO). For each well, 1 μl of aliquoted stock was added (Fig. 1C) and incubated for 18 h.
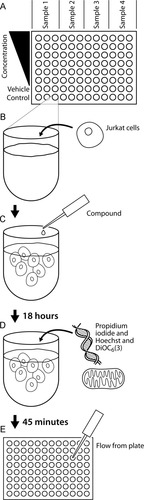
Schematic diagram of the assay. (A) This assay was developed using a 96-well U-bottom plate. (B) Jurkat T cells were seeded at a density of 1 × 106 cells/ml in 100 μl/well. (C) One microliter of aliquoted compound stock was added to each well and incubated for 18 h. (D) Each well was treated with 10 μl/well of dye stock. (E) The samples were analyzed by flow cytometry.
To measure cell death, each well was treated with 10 μl/well of dye stock (Fig. 1D). This stock was made in PBS (Cellgro) with Hoechst 33342 (Molecular Probes; Eugene, OR) at 100 μg/ml, propidium iodide (PI; Sigma) at 100 ng/ml, and DiOC6 (a kind gift from Dr. Joel Weaver, University of Ottawa, Ontario, Canada) at 100 nM. The cells and dye were incubated in a tissue culture incubator for 45 min. The plate was then analyzed with a LSR II flow cytometer (Becton Dickinson; San Jose, CA) using a high-throughput sampler (HTS; Becton Dickinson; Fig. 1E).
Evaluation by Microscopy
Concomitant with analysis by cytometry, aliquots of each treatment group were removed and adhered to poly-L-lysine (Polysciences; Warrington, PA) treated slides. Slides were mounted with a coverslip in PBS, examined by microscopy using an Olympus AX70 fluorescent microscope (Olympus; Melville, NY), and images captured with an Olympus DP70 camera (Olympus).
RESULTS
Confirmation of Appropriate Staining Profiles
Cells were mounted on slides and examined by microscopy for appropriate staining profiles (Fig. 2). As expected, DiOC6 (Fig. 2; green) localized to the area between the nucleus and the cell membrane in live cells (Fig. 2), while Hoechst 33342 (Fig. 2; blue) localized to the nucleus and propidium iodide (Fig. 2; red) in labeled dead cells (Fig. 2). Importantly, nearly all cells were either propidium iodide positive (dead) or DiOC6 positive (live), but not both.
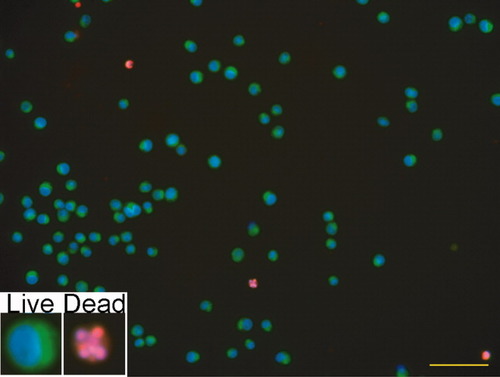
Microscopic evaluation of cell staining profiles. Cells were mounted on charged slides and examined by microscopy. DiOC6 (green) localized to the area between the nucleus and the cell membrane; Hoechst 33342 localized to the nucleus (blue); and propidium iodide labeled dead cells (red). These propidium iodide positive cells were confirmed to be dead by observation of nuclear fragmentation with the Hoechst 33342 staining. Thus, live cells stained blue and green and dead cells stained blue and red. Importantly, cells were generally either propidium iodide positive (dead) or DiOC6 positive (live), but not both. Scale bar equals 100 μm.
Evaluation of Apoptosis
Concurrent with examinations by microscopy, the cells were examined by flow cytometry. Examination of cells induced to undergo apoptosis showed an expected pattern of staining that was segregated into three distinct groups (Fig. 3). These three groups of cells represent distinct stages along the apoptotic cascade or necrotic cells (Table 1). Thus, this assay system permitted the determination of both live/dead ratio (by PI− and PI+) and early/late stage apoptosis ratio (early = PI− and DiOC6−; late= PI+ and DiOC6−).
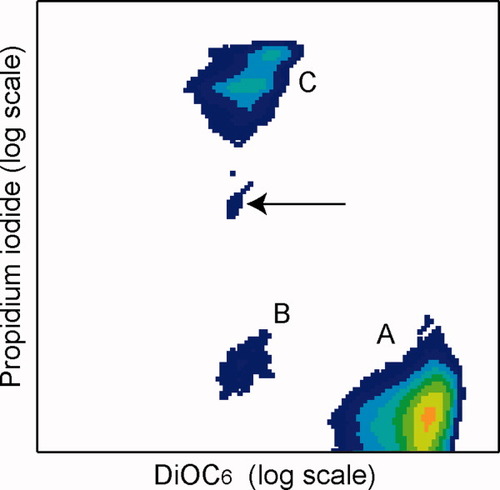
Flow cytometry-based evaluation of apoptosis. A typical plot of cells treated with A. racemosa extract undergoing apoptosis shows three populations. (A) Live cells; (B) early stage apoptosis; and (C) late stage apoptosis/necrosis. It is also possible to identify the small population of cells that have lost external membrane integrity but still support an intact nuclear membrane (arrow).
| Viability | Designation | Cell membrane permeability (PI) | Mitochondrial membrane potential (DiOC6) |
|---|---|---|---|
| Live | A | − | + |
| Early apoptosis | B | − | − |
| Late apoptosis/necrosis | C | + | − |
Cell Cycle Analysis
Hoechst 33342 dye was used for cell cycle analysis (Fig. 4). Since Hoechst 33342 intercalates exclusively in the cellular DNA, not both the DNA and RNA as propidium iodide does, RNAse treatment is not necessary (20). Additionally, since Hoechst 33342 is cell permeable, permeabilization of the cell membrane is not required. Using this method, we were able to observe the cycling of healthy cells and a significant sub-G1 population in cells induced to die through treatment with A. racemosa extract (20).
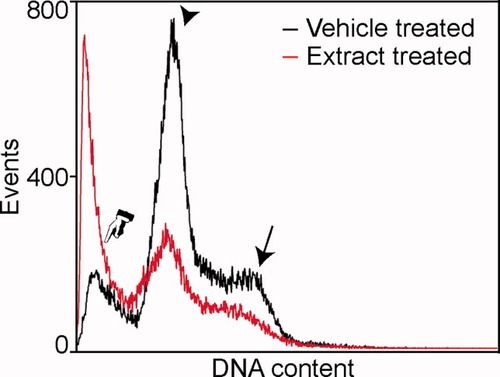
Cell cycle analysis. Examination of Hoechst 33342 staining revealed typical cell cycle profiles. It was possible to identify both a G0/G1 peak (arrowhead), a G2/M (arrow) peak, and to identify dead cells as a sub-G1 population (hand). The black trace shows vehicle-treated cells and the red trace shows cells induced to die through treatment with A. racemosa extract.
DISCUSSION
The process of apoptosis is important in numerous disease states. Therefore, it is not surprising that a number of evaluation methods have been developed to measure various cell death parameters. However, many of the existing evaluation methods are laborious, such as terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), or lack precision, such as the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. To our knowledge, high-throughput assay systems that permit examination of cell membrane potential, mitochondrial membrane potential, and cell cycle progression in small volumes without laborious sample washing and technical manipulation have not been previously reported.
The methodology described herein has allowed our group to screen novel plant-based compounds for apoptosis-inducing properties in a streamlined, cost-efficient manner, thereby speeding the agent discovery process. However, it is important to note that this system is best suited for particular situations. Because the system is restricted to examining cells in suspension, certain frequently used cell lines, such as MCF-7, are not readily compatible with this technique. Furthermore, only active compounds can be evaluated. Compounds requiring phase I or phase II metabolism would remain inactive under these experimental conditions. Also, this technique is limited to agents (and cell lines) that undergo mitochondrial-dependent death.
Nonetheless, for certain applications, such as generating toxicology profiles, this system allows for the rapid assessment of numerous parameters implicated in apoptosis and also affords additional advantages. First, there is little technical manipulation required. Second, this assay employs live cells, not a cell-free system. This is particularly important since there are reports of compounds that exhibit activity in cell-free systems that ultimately are ineffective when used in a cell-based model (21). Third, this assay is quick and cost-efficient. Fourth, if desired and appropriate, it is possible to create a dose-response toxicity curve for four compounds in a single experiment.
Because of the number of disease states involving apoptosis, the ability to pharmacologically regulate cell death is a therapeutic option for many disorders. Here we have shown that in particular contexts where information regarding the type of death induced is desirable, it is possible to employ a high-throughput system that provides information regarding cell membrane integrity, mitochondrial membrane potential, and cell cycle progression. Taken together, data regarding these three factors can provide information as to the mechanism of action for novel pharmaceutical and nutraceutical compounds.
Acknowledgements
We thank Holly Lamb and Colleen Moe, Mayo Clinic College of Medicine, Rochester, MN, for their expert assistance with developing this assay. Also we thank Ruth Stricker and Bruce Dayton for their continuing advice and support.