Design considerations for array CGH to oligonucleotide arrays
Abstract
Background
Representational oligonucleotide microarray analysis has been developed for detection of single nucleotide polymorphisms and/or for genome copy number changes. In this process, the intensity of hybridization to oligonucleotides arrays is increased by hybridizing a polymerase chain reaction (PCR)–amplified representation of reduced genomic complexity. However, hybridization to some oligonucleotides is not sufficiently high to allow precise analysis of that portion of the genome.
Methods
In an effort to identify aspects of oligonucleotide hybridization affecting signal intensity, we explored the importance of the PCR product strand to which each oligonucleotide is homologous and the sequence of the array oligonucleotides. We accomplished this by hybridizing multiple PCR-amplified products to oligonucleotide arrays carrying two sense and two antisense 50-mer oligonucleotides for each PCR amplicon.
Results
In some cases, hybridization intensity depended more strongly on the PCR amplicon strand (i.e., sense vs. antisense) than on the detection oligonucleotide sequence. In other cases, the oligonucleotide sequence seemed to dominate.
Conclusion
Oligonucleotide arrays for analysis of DNA copy number or for single nucleotide polymorphism content should be designed to carry probes to sense and antisense strands of each PCR amplicon to ensure sufficient hybridization and signal intensity. © 2005 International Society for Analytical Cytology
Hybridization of genomic DNA to arrayed DNA probes is now widely used to assess genome copy number changes and/or allelotype throughout complex genomes. In mammalian analyses, initial arrays for copy number analysis carried relatively large DNA probes prepared from yeast artificial chromosome (YAC) (1) and bacterial artificial chromosome (BAC) (2, 3) clones. These array comparative genomic hybridization (array CGH) analyses provided sufficient measurement precision to detect gain or loss of single copies of a genomic segment in aneuploid cells or in populations contaminated by normal genomic DNA (3, 4). Early analyses provided approximately megabase resolution with a few thousand probes on each array (5). Higher resolution arrays were developed for limited regions of the genome by adding overlapping BACs that covered regions of interest (6). Most recently, BAC arrays have been developed carrying more than 30,000 BACs, thus providing near contiguous coverage over the entire human genome (7).
BAC arrays provide remarkable measurement precision and resolution. However, they are limited in several aspects. First, production of arrays carrying large numbers of BACs is labor intensive and clone management issues sometimes lead to probe identity errors. In addition, these arrays provide no information about allelotype and the results cannot be linked to specific genes because each BAC probe typically spans several genes. Higher resolution can be obtained by replacing BACs with cDNAs (8), but the array manufacture and clone management problems remain and these analyses also yield no information about allelotype.
All of these limitations can be overcome by substituting oligonucleotides for cDNAs or BACSs as array probes. The challenge in this approach is to achieve sufficiently high hybridization and signal intensity to allow precise measurements of allelotype or copy number. This has been overcome by reducing the complexity of the genomic DNA being hybridized. One approach, termed Representational Oligonucleotide Microarray Analysis (ROMA), reduces genomic complexity by preparing a polymerase chain reaction (PCR)–amplified representation of a fraction of the genome to be analyzed (9). This is accomplished by digesting with one or more restriction enzymes, ligating common PCR primer sequences to both ends of each restriction fragment, and amplifying the resulting complex mixture by PCR with the common primer sequence. Hybridization of this amplified mixture to an array carrying probes that match the amplified restriction fragments allows assessment of copy number (9) and allelotype (10). However, the hybridization efficiency and, hence, the measurement precision may vary considerably between probes so that some regions of the genome are analyzed with considerably more precision than others.
Our goal in this study was to develop a more robust oligonucleotide array CGH procedure by understanding some of the factors that influence signal intensity. We approached this by evaluating hybridization of reduced complexity genomic mixtures to arrays comprised of 50-mer oligonucleotides. We produced reduced complexity probes by multiplex PCR amplification of selected regions of the genome. Duplicates of four separate oligonucleotide probes were placed on the array for each PCR amplicon: two homologous to the sense strand and two complementary sequences homologous to the antisense strand. Hybridization intensities were measured for each of the four probes for each amplicon. We compared the performance of this approach to oligo-array CGH by analyzing X-chromosome copy number in cell lines with one to five copies of the X-chromosomes and by comparing oligo-array CGH to BAC array CGH for assessment of genome copy number abnormalities on chromosome 20 in a well-characterized breast cancer cell line.
MATERIALS AND METHODS
Oligonucleotide Arrays
We developed oligonucleotide arrays carrying four 50-mer detection oligonucleotide probes for each PCR amplicon as illustrated in Figure 1. Two of the four probes were homologous to the amplicon sense strand. The other two probes were complementary to the first two. Sequences for the oligonucleotide probes were selected using Primer3 software (11). They were 50 nucleotides in length, did not overlap with the PCR primers, had melting temperatures (Tm) between 73°C and 95°C (at 500 mM NaCl, calculated as described previously) (12), and had free energies greater than −2.5 kcal/mol for hairpin loop structure. Oligonucleotide arrays were printed on Motorola (Surmodics) 3D-Link slides as described by the manufacturer with the exception that oligonucleotides were resuspended at 30 μM in 10 mM sodium phosphate buffer (pH 8.5). Each oligonucleotide was printed in replicate spots (Fig. 1) at ∼150 μm center-to-center spacing and allowed to dry. The amino-modified 5′ ends of the printed oligonucleotides were covalently attached after printing by exposing the printed slides to 75% humidity. This humidification also serves to block inappropriate attachment of DNA in later steps.
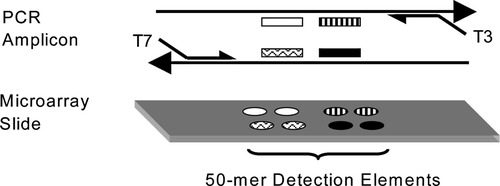
Method of multiplex and quantitative PCR. A schematic PCR amplicon illustrates the relative positions of primer pairs and detection oligonucleotides (probes), shown adjacent to their complementary sequences. The sequences of four probes per amplicon are flanked by those of composite PCR primers. The 5′-amino–modified probes were synthesized and printed as array elements onto 3D-Link (Surmodics) microscope slides. Genomic DNA was PCR amplified using a collection of 12 to 48 primer pairs that are comprised of sequence-specific portions in tandem with T3 or T7 universal primer sequences. These universal primers were used in a subsequent PCR step. Test and reference genomic samples were separately amplified. Fluorescent nucleotides were used to separately label the PCR products in a Klenow reaction using random hexamers. Labeled products representing the test and reference samples were cohybridized to the arrays.
Multiplex PCR Primers
The primer design strategy used for multiplex PCR amplification is illustrated in Figure 1. One PCR primer pair was prepared for each region of the genome to be interrogated. Each primer was comprised of a ∼25-nucleotide, unique 3′ sequence and a 23-nucleotide, universal 5′ sequence (13). The universal primer sequences were T3 (5′-TAATACGACTCACTATAGGGAGA) and T7 (5′-AATTAACCCTCACTAAAGGGAGA) sequences described by Wang et al. (14). Only coding sequences were interrogated in this study but this is not necessary. PCR primers were selected using Primer3 software (11) to produce PCR products from 170 to 350 bp in length and to have (a) ∼25-nucleotide locus-specific segments, (b) Tm between 60°C and 73°C at 100 mM NaCl (15), (c) differences in Tm between paired primers of lower than 5°C, and (d) free energies greater than 0 kcal/mol for 3′-end dimer formation and greater than −2.5 kcal/mol for hairpin loop structure.
Choice of Query Loci
Chromosome and array CGH analyses have shown that chromosome 20q is frequently amplified in ovarian and other cancers. For example, Suzuki et al. (16) showed that this region is present at increased copy number in ∼70% of ovarian cancers. Thus, we developed an oligonucleotide array and multiplex PCR strategy to assess 33 known or predicted genes in this region. This analysis system also interrogated 5 loci on the X chromosome and 10 loci on other chromosomes. The X-linked loci were used to evaluate and optimize the sensitivity and linearity of oligo-array CGH. The 10 loci on other chromosomes were used to normalize ratio data. Sequence data for each locus were taken from the National Center for Biotechnology Information (NCBI) databases (http://www.ncbi.nlm.nih.gov/), including dbEST, Unigene, dbSTS, nr, and htgs, and from the University of California, Santa Cruz (UCSC) Human Genome Project Working Draft (http://genome.ucsc.edu/). Sequences used in this work were blasted against several of these databases three times throughout the course of the work, to test for uniqueness within the genome as the databases were rebuilt.
Cell Lines
We obtained female human genomic DNA samples representing the normal or aneuploid genotypes 45XO, 46XX, 47XXX, 48XXXX, and 49XXXXX from The Coriell Institute for Medical Research (NIGMS Coriell Cell Repositories; Camden, NJ, USA). The breast cancer cell line MCF7 was obtained from ATCC (Rockville, MD, USA).
Multiplex PCR
Twelve to 48 loci were PCR amplified from normal, aneuploid, and tumor genomic DNAs in a two-step amplification protocol. In the first step, 10-μl volumes containing 2 μl of 5× modified RDA buffer (17) (340 mM TrisHCl, pH 8.8; 80 mM [NH4]2SO4, 20 mM MgCl2, 320 μM each dNTP; all from Sigma-Aldrich Corp., St. Louis, MO, USA; and 50 mM 2-mercaptoethanol from Promega, Madison, WI, USA), 0.038 U of AmpliTaq (Applied BioSystems, Foster City, CA, USA), 25 nM each primer, and 10 ng genomic DNA were compiled on ice in a cold (4°C) room. Template genomic DNA was pretreated by heat shearing (95°C for 5 min in 2 mM Tris-HCl, 0.2 mM ethylenediaminetetraacetic acid, pH 8.0) to enhance the PCR yield and uniformity. To minimize nonspecific amplification, the primer pools were heated to 65°C for 1 min and then snap-cooled on ice before their addition to the master mix. Primers and template genomic DNA were added in the cold room and the reactants were immediately inserted into a thermocycler (model 480, Perkin Elmer, Oak Brook, IL, USA) preheated to 80°C before the start of thermocycling. The parameters for amplification included an initial denaturation step of 95°C for 1 min, 14 to 22 cycles at 94°C for 30 s and at 62°C for 3 min, an extension step at 72°C for 7 min, and a 4°C soak. Three microliters of this reaction product was then added, in the cold room, to a second tube of PCR reactants that contained 6 μl of 5× modified RDA buffer, 0.12 U of AmpliTaq, and 500 nM each T3 and T7 primers (14) in a final volume of 30 μl. The second step of PCR was performed at 94°C for 30 s, 45°C for 1 min, and 72°C for 2 min, for 2 to 18 cycles. Primers used in this study can be obtained on request.
Successful PCR was assessed by 1.5% agarose gel electrophoresis in 0.5× Tris-Borate EDTA (TBE). Before electrophoresis, PCR products were purified using Qiagen PCR Purification Kit (Qiagen, Chatsworth, CA, USA). To 30 μl of PCR reaction, 500 μl of Qiagen “buffer PB” and 5 μl of 3 M sodium acetate (pH 5.4) were added before the first spin. The sodium acetate solution is added to adjust the pH to ∼7, to enable higher yield of the PCR product. After elution from the column using 0.5× Qiagen “buffer EB,” the concentrate was adjusted to 40 ng/μl and electrophoresed. Products of individual and pooled pairs of all primers were evaluated for yield and abundance relative to unwanted primer dimers.
PCR Product Labeling and Hybridization
Two-hundred nanograms of PCR product was labeled using a Klenow reaction, including 1 μl 10× Random Priming Buffer (Amersham Biosciences, Arlington Heights, IL, USA), 1 μg random hexamers (Invitrogen, La Jolla, CA, USA), 25 μM each dATP, dCTP, and dGTP, 10 μM dTTP (Invitrogen, Carlsbad, CA, USA), and 30 μM of Cy5-dUTP or Cy3-dUTP (Amersham Biosciences) in a total volume of 9.4 μl. The reactants are heated in a PCR machine to 94°C for 1 min to denature the template. Upon cooling to 37°C in normal maximum ramp time, 0.4 μl of Klenow Fragment (3′→5′ exo-; Amersham Biosciences) is added to each tube, and the reaction is incubated at 37°C for 1 to 2 h. The labeled test (Cy5) and reference (Cy3) products were combined, applied in a 50-μl volume (adjusted with nuclease-free water) to Microspin G50 columns (Amersham Biotech), and spun according to the manufacturer's protocol. The incorporation of Cy-dUTPs was demonstrated by electrophoresis on mini (22 × 22 mm) agarose gels poured on microscope slides. Briefly, 3.0% 3:1 NuSieve (Sigma-Aldrich) gels were formed under a coverslip with a controlled thickness of 1 mm. Lanes were loaded with a total of 3.0 μl containing 0.6 μl 30% glycerol in nuclease-free water, 1.4 μl labeled sample, and 1.0 μl 0.4% warm (40°C) agarose in exonuclease-free water. The mini gels were run at 150 V for 12 min and, after removing the coverslip, were dried for 15 min at 80°C on a heat block. These dried gels were then scanned on the laser scanner. The relative abundance of incorporated and unincorporated fluorophores could thus be qualitatively and quantitatively monitored. The remainder of the purified, labeled probe was combined with 25 nmol of each dNTP, 1.5 mmol of sodium pyrophosphate, 2.3 μl 20× saline standard citrate (SSC), and 1 μg salmon sperm DNA before concentrating the probe by lyophilization. After adjusting the volume to 15 μl, 0.38 μl of 10% sodium dodecylsulfate was added and the sample was denatured at 95°C for 2 min and allowed to cool gradually to room temperature for 5 min. After blocking the surfaces of the 3D-Link slides in accordance with the manufacturer's protocol, the denatured sample was spun to collect the condensation, mixed with a pipette, applied to each array, and covered with a 22- × 22-mm coverslip. Hybridization was carried out in a custom-built humidified chamber (Protein Design Labs Inc., Fremont, CA, USA) in a 64°C water bath for 1 to 2 h. Stringent washing done at room temperature included 2 min in 3× SSC, 0.03% sodium dodecylsulfate, 5 min in 1× SSC, and 5 min in 0.2× SSC. Slides were dried by centrifugation (3 min at 800 rpm) before imaging with a laser scanner.
Analysis of Array Hybridization Results
Slides were scanned at 532 nm and at 632 nm using a GenePix 4000B laser scanner (Axon Instruments, Inc., Union City, NJ, USA) and analyzed using the bundled GenePix Pro 3.0 segmentation software. Scanning was performed at a laser power setting of 30%, and the photomultiplier tube setting was adjusted independently for each laser channel and for each hybridization experiment, so that the brightest spots were 50% to 80% of saturation. Segmentation of spots was performed using the algorithms in this software, including alignment and spot-fitting features. Mean pixel intensity values were taken as the mean raw intensity of the population of the pixels in each segmented region containing a spot. We corrected for nonspecific background fluorescence by subtracting intensities of spots containing “null” oligonucleotides (expected not to hybridize to any sequences in the labeled probe). This subtraction of signal due to the nonspecific hybridization component of mean pixel intensity values gave null-subtracted intensity (NSI) values. If an NSI value was less than 50% greater than the median intensity of the null set, it was discarded as not informative. If informative, NSI values corresponding to a given locus from each of the fluorescence channels (where 635 nm represented the test samples and 532 nm represented the reference samples) were used to calculate a test/reference ratio describing the relative abundance for each locus. Ratios of NSI values were averaged among four replicate spots of detection oligonucleotides. Means and standard deviations of NSI ratios were compared between sets of replicate spots, alternate detection oligonucleotide sequences per locus, alternate sense and antisense strands for each detection oligonucleotide, and various loci.
RESULTS
Our main goal in this study was to explore aspects of oligo-array CGH that influenced hybridization intensity and linearity to guide development of improved strategies for genome copy number and single nucleotide polymorphism analyses. We assessed the relative importance of oligonucleotide probe sequence and PCR amplicon sequence on hybridization intensity during oligonucleotide array CGH analysis of several different genes. In addition, we assessed the linearity of oligo-array CGH during analysis of X-chromosome sequences in cell lines carrying 1, 2, 3, 4, or 5 copies of the X-chromosome. We compared oligo-array CGH and BAC array CGH for assessment of genome copy number along a region of chromosome 20 that is frequently amplified in breast and ovarian cancers.
We produced low complexity DNA for hybridization by multiplex PCR amplification of up to 48 different segments of the genome. We employed a two-step PCR procedure to balance representation among query sequences and to enhance linearity of the assay. The first PCR step involved a pool of 12 to 48 pairs of primers. This effectively amplified all of the PCR targets. However, because PCR primers tend to amplify with variable efficiency, complexity may become reduced with extended amplification. To prevent this, we limited the number of cycles in the first step and added a second step of PCR using modified T3 and T7 primers to produce sufficient material for labeling and hybridization.
In initial multiplex PCR experiments, primer-dimer formation to the exclusion of product of interest was a problem. We investigated the basis of primer-dimer formation by cloning and sequencing the primer-dimer products. These analyses revealed that primer dimers formed between primers with 3′-complementarity involving at least five nucleotides. Thus, some primers were redesigned so that none had 3′-complementarity of no more than four nucleotides. We also found that the number of cycles using the multiplex primer pool affected primer-dimer formation. We found that using 20-cycle amplification with sequence-specific primers, before switching to amplification using the universal primers, increased the yield of desired product (mainly ∼300- to 400-bp range) relative to that of a primer-dimer side product (∼90 to 100 bp).
We adjusted the number of cycles in the second step of the PCR amplification with T3 and T7 primers to allow linear, quantitative analysis. We tested the linearity of amplification and oligo-array CGH analysis by comparing copy number estimates at seven loci on chromosome 20 with five loci on the X chromosome for a cell line with four copies of the X chromosome and two copies of chromosome 20. Figure 2 summarizes results derived from various cycle numbers in the second step. Best linearity of detection was achieved using DNA prepared by second-step amplification of 6 to 10 cycles.
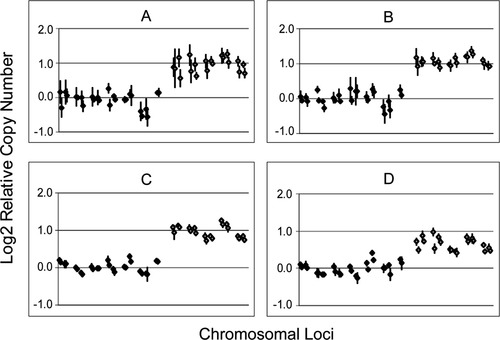
Optimizing the linearity of the method depends on the determination of appropriate number of PCR cycles. We tested the ability of our method to detect the difference between four copies and two copies of the X chromosome. These data are depicted as the log2 of ratios of Cy5/Cy3 fluorescence. Values represent the relative copy number of seven chromosome 20 and five chromosome X loci. Data points are averages of four replicate spots, with standard deviation bars. Each locus has four such averages, representing two different 50-mer probes, and two different locations on the microscope slide where replicate sets were printed. Results shown are the result of (A) 20 + 2 cycles, (B) 20 + 6 cycles, (C) 20 + 10 cycles, and (D) 20 + 14 cycles. Our results demonstrate that the capacity to detect twofold differences in copy number is optimal in the range of approximately 20 + 6 to 20 + 10 cycles of PCR (approximately equivalent to 23 to 27 cycles in total; see Materials and Methods).
We determined the extent of bias in hybridization and signal intensity due to the use of different dye-conjugated dUTPs by separately labeling two 200-ng aliquots of a multiplex PCR product with Cy5- or Cy3-dUTP. Labeled products were then mixed and cohybridized to arrayed oligonucleotides. We observed (data not shown) that fluorescent intensity variability associated with using alternate Cy3- and Cy5-dUTP conjugates was insignificant. We also observed some reaction-to-reaction variability during PCR as evidenced by starting with the same template and independently amplifying, labeling with only Cy3-dUTP, and hybridizing. Although this seemed to be a minor problem, we found that this could be diminished by pooling 10 separate multiplex PCR reactions before labeling. Because this pooling was deemed impractical, most of the data reported here were obtained without employing such pooling.
We investigated the extent to which hybridization and signal intensity varied between the sense and the complementary antisense oligonucleotide probes. In some cases, the signal intensity varied up to 16-fold between sense and antisense probes within one PCR amplicon, whereas the variability between alternate sense probes or alternate antisense probe sequences within a PCR amplicon was much less. Figure 3A, for example, shows this to be the case for PCR amplicons distributed along the gene, CDC25B. In other cases, hybridization intensities varied most between oligonucleotides complementary to the same strand of the PCR amplicon. Figure 3B shows this to be the case for sense and antisense probes for several amplicons distributed along the gene, ZNF217.

Variation in signal intensity between a sense and its complementary antisense probe can be substantial. Five genetic loci were used to assess the variability that exists when complementary oligonucleotides are used as probes during hybridization. Three different segments were analyzed per genetic locus, and each was hybridized with a multiplex PCR product labeled with only Cy3-dUTP. A, B: The results from two of these loci are depicted. The variation is quite large, up to a 16-fold difference in signal intensity between complementary probes.
We assessed the linearity of oligo-array CGH carried out as described above by analyzing cell lines carrying from one to five copies of the X chromosome and two copies of chromosome 20. A karyotypically normal, 46,XX cell line was used as the reference in these experiments. The results are summarized in Figure 4. Seven chromosome 20 and five X chromosome measurements were averaged for this analysis. These analyses were part of a study in which 48 separate loci were analyzed simultaneously, i.e., hybridization probe was prepared using 48-plex PCR amplification. The estimated chromosome X copy number measured in this study increased monotonically with the number of X chromosomes. However, the magnitude of the estimated copy number increase was somewhat lower than expected at higher X-chromosome copy numbers. The reasons for this attenuation are not fully understood.
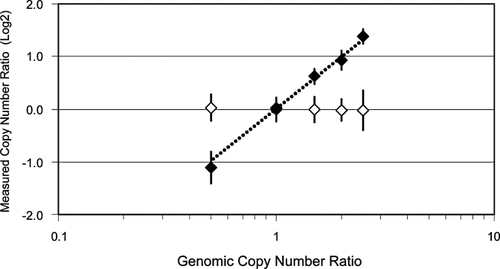
Copy number discrimination was determined using a series of aneuploid DNAs with one to five copies of chromosome X. These DNAs were amplified using 48 pairs of oligos, five of which amplified X loci, and by using 20 + 9 cycles of PCR. Fluorescence ratios were plotted as log2 values versus the ratio of the amount of chromosome X material to the amount of chromosome 20 material in each DNA sample (fluorescence ratios were adjusted by leave-one-out analysis). Data for autosomal loci are represented by open diamonds and chromosome X data by solid diamonds. Dashed line indicates where ideal chromosome X data might be plotted. Data points represent averages of locus averages (16 replicate spots per locus). There were 43 autosomal loci and five chromosome X loci. Error bars represent standard deviations of the averages of locus averages of replicate spots.
We compared the copy number measured using oligo-array CGH and BAC array CGH. BAC array CGH analyses were carried out as described previously (3). Figure 5 compares measurements of copy number at several loci along chromosome 20 for the breast cancer cell line, MCF7. This cell line was chosen for this comparison because chromosome 20 is highly amplified (18), and this amplicon is well characterized. Landmark gene loci are indicated for orientation. In general, oligo-array CGH and BAC array CGH estimates of copy number were concordant.

Comparison of oligo-array and BAC array CGH. This is a high-resolution analysis of the chromosome 20q amplicon assayed by both methods. In both assays, the same genomic DNA samples were analyzed, but in oligo CGH, the reference genome is represented by a pool of 10 replicate multiplex amplification reactions, using the 2× female DNA as template. Tester is MCF7 genomic DNA. BAC array data are indicated by solid diamonds and oligo-array data by open diamonds. Chromosome position was determined according to the December 2000 freeze of the UCSC compilation.
DISCUSSION
Array CGH using YACs (1), BACs (2, 3, 19), cDNAs (8), and cloned genomic sequences (20) as probes has been used successfully to detect genome copy number changes in several species including humans. In general, array CGH resolution is limited by the density and genomic extents of probes on the arrays. Typically, measurement precision is higher with larger probes because the hybridization signals are more intense. However, the ultimate genomic resolution is lower with large probes because the probes interrogate an extended portion of the genome. Lucito et al. (9, 20) demonstrated that signal intensity and, hence, measurement precision could be increased by decreasing the complexity of the DNA sample used for hybridization to the point where use of oligonucleotide arrays became possible. In their case, complexity was reduced by preparing a representation of the genome (e.g., by amplifying only fragments flanked by specific restriction sites). This increased signal intensity on oligonucleotide arrays enough that single copy number changes could be reliably detected. However, the signal intensity, measurement precision, and linearity varied along the genome. We assessed several aspects of probe and hybridization mixture design to optimize the process.
The most important influence on hybridization and signal intensity was revealed during analyses of hybridization to sense and antisense probes in the same PCR amplicon. In general, signal intensities among alternate sense or antisense probes were similar within a PCR amplicon. However, the signal sometimes varied dramatically between a sense probe and the complementary antisense probe within the same PCR amplicon. Because the concentration of hybridizing DNA is identical for all sense and antisense probes within one PCR amplicon, we conclude that the difference in hybridization and signal intensity between sense and antisense probes is due to differences in the conformation of the sense and antisense strands in the labeled DNA during hybridization. This is similar to the remarkable variation in hybridization intensity of RNA to oligonucleotides tiled along transcribed genes observed by Mir et al. (21). Our results suggest that oligonucleotide arrays should be designed to carry sense and antisense probes for each region of the genome for highest precision analyses of copy number or allelotype. This strategy will ensure that each locus will be interrogated with the highest precision. Of course, this comes at the expense of doubling the number of elements on the array but this becomes less of an issue as technologies are developed to increase the oligonucleotide densities. Further, oligonucleotides associated with suboptimal signal can be eliminated during second-generation array design.
Other aspects of oligo-array CGH can be improved in cases in which the number of loci interrogated is limited as was the case in the present study. For example, the problem of primer-dimer formation during PCR amplification influenced hybridization linearity and could be minimized by designing all primers with minimal 3′-end complementarity (fewer than five contiguous nucleotides) with any other primer in the pool. Use of a two-step PCR amplification strategy also helped linearity. This consisted of ∼20 cycles of amplification using a collection of all primer pairs, followed by 5 to 10 cycles using a pair of universal primers that amplified all PCR amplicons.
In conclusion, we have explored several aspects of oligo-array CGH that might influence copy number and/or single nucleotide polymorphism analyses. Conformational differences between sense and antisense strands of the PCR-amplified sequences during hybridization seem to have the largest effect so that sense and antisense probes should be tested for each PCR amplicon for best results during array design. When fully optimized, oligo-array CGH seems well suited to accurate assessment of genome copy number and/or allelotype across the genome (9, 10, 20) or in limited regions as described in the present report.




