EpCAM deficiency causes premature aging of intestinal epithelium via hyperactivating mTORC1 pathway
Zili Lei, Lei Chen, Yanyan Liu and Yanhong Yang contributed equally to this work.
We reported a new role of epithelial cell adhesion molecule (EpCAM) on maintaining the longevity of intestinal epithelial cells (IECs) via regulating the TSC1/mTORC1/Sirtuins pathway. EpCAM has been used as a cell surface marker of various cancer tissues and many kinds of stem cells,1-3 but its functions in cancers and stem cells were less studied. The deficiency of EpCAM causes the breakdown of the homeostasis of intestinal epithelium, which is called the congenital tufting enteropathy (CTE) in patients.4 However, the function of EpCAM in IECs was still unclear.
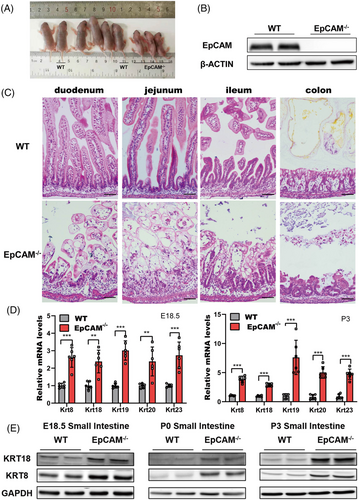
Intestines from E18.5 embryos and postnatal pups of wild type (WT) and EpCAM–/– mice were first compared. Similar to previously reported,1 the EpCAM–/– pups showed no bodyweight gain, serious diarrhoea and damaged intestines (Figures 1A–C, S1A–C). Genes for intestinal abundant keratins, including Krt8, Krt18, Krt19, Krt20 and Krt23, were remarkably upregulated in the small intestines of mutant embryos and pups (Figures 1D,E, S1F). These results confirmed that the homeostasis of intestinal epithelium was seriously affected in EpCAM–/– mice.
To investigate the function of EpCAM in IECs, the expression of telomerase reverse transcriptase (TERT) and telomerase RNA component (TERC), components of Telomerase,5 was tested. TERT was noticeably downregulated in the intestines of EpCAM–/– mice compared to the WT controls (Figures 2A–C, S2A–C). The transcription of Terc was only considerably reduced in the small intestines of mutant P0 pups (Figures 2A, S2A). Telomeres are bound by Shelterin which includes six subunits: TRF1, TRF2, POT1, TPP1, RAP1 and TIN2.5 TRF1 remarkably decreased in small intestines of mutant mice (Figures 2D, S2D). 53BP1, which can be recruited to the damaged telomeres,6 was upregulated in the small intestines from mutant mice at the posttranscriptional level (Figures 2D, S2D). EpCAM–/– Caco-2 cells were generated for in vitro exploration (Figure S1D,E). TERT and TRF1 were significantly reduced in EpCAM–/– Caco-2 cells (Figure S2G-J). However, the protein levels of TRF2 and 53BP1 were still normal in EpCAM–/– Caco-2 cells (Figure S2I-J). These results indicated that EpCAM mutation would affect the length of telomeres in IECs and induce premature aging of intestinal epithelium.
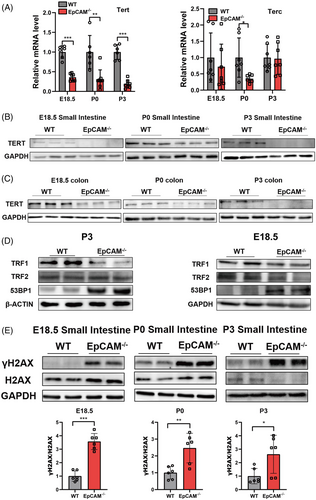
The accumulation of unrepaired DNA double-strand breaks (DSBs), marked by the increase of γH2AX,7 was assessed. At E18.5 and P0 stages, the ratio of γH2AX to H2AX was considerably increased in the small intestines of mutant mice (Figure 2E). For P3 mice, the expression level of H2AX became reduced in the small intestines of mutant pups, but γH2AX still significantly increased in them (Figure 2E). γH2AX also significantly increased in EpCAM–/– Caco-2 cells (Figure S3A). These results demonstrated that EpCAM mutation increased DNA damage in IECs both in vivo and in vitro. The accumulation of DNA damage is one aging hallmark.8
To explore the mechanisms of EpCAM deficiency on accelerating the aging of IECs, members of Sirtuin family8 were checked and they all downregulated in the small intestines from mutant mice at transcriptional or posttranscriptional levels, especially SIRT3, SIRT6 and SIRT7 (Figures 3A,B, S4A-B, S4E, S6A). SIRT1, SIRT5, SIRT6 and SIRT7 were also significantly downregulated in the EpCAM–/– Caco-2 cells (Figure S4C,D,F). After administration of MDL-800, activator of SIRT6,9 to EpCAM–/– pups, the expression of Sirtuins significantly increased in the small intestines of them (Figures 3C,D, S6B). MDL-800 could significantly upregulate the expression of Tert in the intestines of mutant pups (Figures 3E,F, S6C). γH2AX was remarkably decreased in the intestines of mutant pups administrated with MDL-800 (Figures 3G, S6D). These results demonstrated that the downregulation of the expression of Sirtuins might be one of the mechanisms of the premature aging of IECs from EpCAM–/– mice.

To study signal pathways related to the premature aging of IECs caused by EpCAM deficiency, the mTORC1 pathway10 was checked. TSC1, TSC2 and p-TSC2, but not p-TSC2/TSC2, were all considerably decreased in the small intestines of mutant pups (Figures 4A–C, S7A). The increase of p-mTOR, p-mTOR/mTOR, p-S6, p-S6/S6 and p-4EBP1 confirmed the hyperactivation of the mTORC1 pathway in the intestines of EpCAM–/– pups (Figures 4C, S7A). TSC1 was also significantly decreased in EpCAM–/– Caco-2 cells, and the ratios for p-mTOR/mTOR and p-S6/S6 remarkably increased in EpCAM–/– cells (Figures S5A,B, S8A). These results illustrated that the mTORC1 pathway was hyperactivated in EpCAM–/– IECs. Rapamycin was administrated to EpCAM–/– pups to reduce the hyperactivation of mTORC1 in IECs of them (Figures 4D,E, S7B). The small intestinal epithelium of P3 EpCAM–/– pups was slightly improved after administration of rapamycin (Figure 4F). The protein level of TERT increased and the level of γH2AX reduced in the intestines of rapamycin administrated P3 mutant mice (Figure 4G, S7C). The protein levels of SIRT1, SIRT4, SIRT5, SIRT6 and SIRT7 were also rescued in the intestines of P3 mutant mice by rapamycin (Figure 4G, S7C). Rapamycin treatment also significantly resumed the expression of Tert and Sirtuins and reduced the level of γH2AX in EpCAM–/– Caco-2 cells (Figures S5C–F, S8B–D). These results demonstrated that EpCAM deficiency caused the premature aging of IECs via hyperactivating the mTORC1 pathway both in vivo and in vitro. However, administration of MDL-800 could not inhibit the hyperactivation of mTORC1 pathway in the intestines of EpCAM–/– pups (Figures 4H, S7D), indicating that mTORC1 might be upstream of SIRTs in IECs.
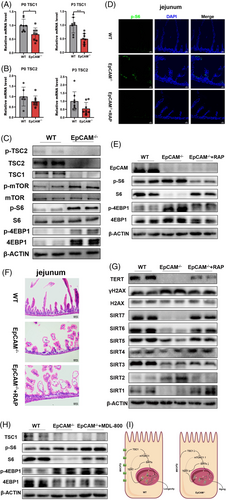
In summary, EpCAM deficiency causes the premature aging of IECs via TSC1/mTORC1/sirtuins pathway (Figure 4I). Therefore, mTORC1 and SIRT6 are potential targets for treating the premature aging of IECs of CTE patients. The highly expressed EpCAM might contribute to maintain the genomic stability and the length of telomeres in various stem cells and cancers.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (31671520, 82171855, 81830113, 81803912); National Key Research & Development Program of China (2018YFC1704200); the Guangdong Basic and Applied Basic Research Foundation (2019B030302005; 2021A1515012383); the Opening Foundation of the Key Laboratory of Regenerative Biology, Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences (KLRB201807); the Science and Technology Planning Project of Guangzhou City (201803010069); and the Science and Technology Project of Yue-Xiu District of Guangzhou (2018-WS-011).
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.




