Defective α-tectorin may involve tectorial membrane in familial Meniere disease
Dear Editor
Although there have been considerable advances in recent years, the contribution of genetic factors to Meniere's disease (MD) is not yet fully understood. MD (OMIM 156000) is an inner ear disorder defined by episodes of vertigo associated with sensorineural hearing loss (SNHL) affecting low to medium frequencies, tinnitus, and aural fullness. Familial aggregation in MD has been described in 9–10% of European descendant population, showing an autosomal dominant inheritance pattern in most families.1 Despite familial MD displays extensive genetic heterogeneity,2 we recently observed multiple families carrying rare variants in genes encoding proteins involved in the structure of the hair cells stereocilia and their attachment to the tectorial membrane (TM): an enrichment of rare missense variants in the OTOG gene was found in 15 unrelated MD families3 and other 9 families showed rare heterozygous variants in the MYO7A gene.4 In this study, we have performed bioinformatic analyses in exome sequencing data obtained from patients of 77 families with MD to better understand the genetic underpinnings of the disease.
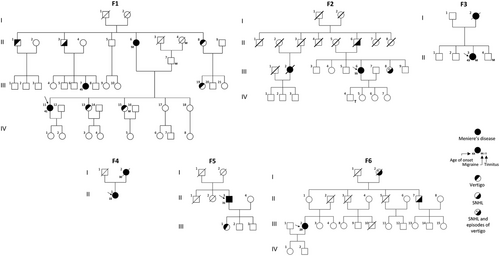
The results of the present study add new evidence to support the involvement of α-tectorin in the pathophysiology of MD. We report four multicase MD families carrying rare missense heterozygous variants (F1–F3) and a short deletion (F4) in the coding region of the TECTA gene. Variants in this gene were also found in two additional families with one MD patient and relatives with partial syndromes carrying a missense heterozygous variant (F5) and a short deletion (F6) (Table 1). A briefcase report was made of each family to assess the genotype-phenotype correlations (Supporting Results). Figure 1 and Supporting information Figure S1 shows the pedigrees and the pure-tone audiograms, respectively, of the six families included in this study. Supporting information Table S1 shows a summary of the clinical information of familial MD patients carrying variants in the TECTA gene.
| Location | Protein change | Info | Exon | Domain | MD family | gnomADNFE MAF | gnomADMAX MAF | ACMG |
|---|---|---|---|---|---|---|---|---|
| 11:121158016T > C | p.Val1494Ala | Missense | 14 | VWFD 4 | F1 & F2 | 8.8 × 10−5 | 9.7 × 10−5 (AFR) | VUS |
| 11:121152980G > C | p.Cys1402Ser | Missense | 13 | TIL | F3 | 1.5 × 10−5 | 4.8 × 10−4 (OTH) | VUS |
| 11:121157956AC > A | p.Asn1474LysfsTer91 | Deletion | 14 | – | F4 | Novel | Novel | LP |
| 11:121165368C > T | p.Pro1790Ser | Missense | 17 | – | F5 | Novel | Novel | VUS |
| 11:121189864GC > C | p.Gly2118ProfsTer22 | Deletion | 23 | – | F6 | 0 | 2.4 × 10−5 (AFR) | LP |
- Reference sequence: NM_005422.2 (TECTA); ACMG, American College of Medical Genetics and Genomics; LP, Likely pathogenic; MAF, Minor allele frequency; MAX, Highest MAF value in gnomAD populations; NFE, Non-Finish European; VUS, Variant of unknown significance.
The TECTA gene encodes α-tectorin, a large protein that contains 10 functional domains divided into three major regions: (1) an entactin-like (NIDO) region; (2) a larger middle region, the zonadhesin region containing a von Willebrand factor type C (VWFC) domain, four VWFD domains, and three trypsin inhibitor-like cysteine-rich (TIL) domains; and (3) the zona pellucida region.5 This protein is one of the main non-collagenous proteins of the TM, an extracellular matrix that lies over the stereocilia of the sensory hair cells and mediates the deflection of the stereocilia and the gating of mechanotransduction channels. Variants in this gene are known to cause phenotypes of non-syndromic autosomal hereditary hearing loss in humans.6 Furthermore, endolymphatic hydrops, a histopathological feature of MD, has been documented on TectaC1509G mouse model.7
The three variants in all multicase MD families were found clustered in the zonadhesin-like region, whereas the two variants observed in the two families with partial syndromes were found close to the zona pellucida (Figure 2). To predict the impact of these rare variants on protein stability, the α-tectorin domains were modelled using the AlphaFold2 method and assembled with the DEMO method (Figure 3 and Supporting Methods).
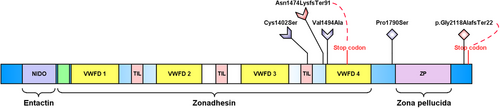
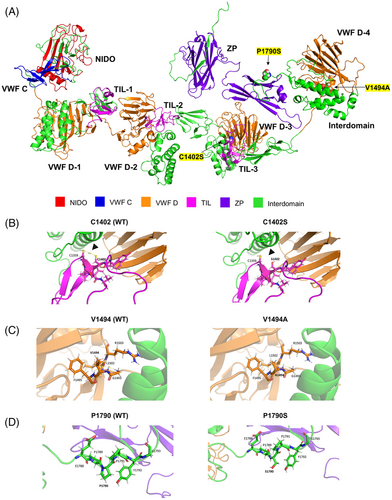
Of note, the variant p.Val1494Ala was found in F1, F2, and a sporadic case. The segregation of this variant was confirmed in F1, where it segregated in all three affected MD individuals and the father of III-7, who only suffered from hearing loss (Figure 1). The missense variant p.Cys1402Ser found in F3 was classified as a variant of unknown significance (VUS). According to the protein model, the change of cysteine to serine at residue 1402 can lead to the breaking of the Cys1359-Cys1402 disulphide bond with a destabilizing effect on α-tectorin (Figure 3). In addition, the novel frame shift deletion in p.Asn1474LysfsTer91 found in F4, classified as likely pathogenic, resulted in a premature stop codon after 91 amino acids that generates a truncated protein of 1594 residues instead of 2155.
The two additional variants observed in the families with partial syndromes were classified as VUS. The novel variant p.Pro1790Ser was found in F5 segregating in two patients, the father with MD and his daughter with only vestibular symptoms. Finally, the short deletion p.Gly2118ProfsTer22 observed in F6 causes a premature stop codon at position 2139, generating a slightly shorter protein with a modified C-terminal region and, in turn, an alteration of the glycosylphosphatidylinositol (GPI) anchorage signal located in this region. The α-tectorin suffers a post-translational modification in which it is tethered to the membrane via GPI.8 Thus, this frame shift deletion could be involved in an alteration of the TM during its formation by the modification of the GPI anchorage signal and leading to the clinical phenotype.
We hypothesized that these deletions and missense variants in the TECTA gene could change the TM micromechanics involved in the sound-evoked motion of stereocilia causing hearing fluctuation in familial MD (Supporting Discussion). On the other hand, and unlike the hearing loss phenotype, the association between variants in α-tectorin and vestibular dysfunction has not been established since most patients reported in the literature carrying variants in α-tectorin do not show a vestibular phenotype. Nevertheless, some of these patients reported episodic vertigo or showed vestibular hyporeflexia.9 Furthermore, the expression of α-tectorin in the vestibular system has been demonstrated in several mouse models, being detected in the saccule and the utricule.10 However, other genes or epigenetic factors that could modulate the vestibular phenotype in familial MD patients should be considered.
In conclusion, the results of this study seem to be in line with the results obtained in recent genetic studies about familial MD, where rare variants in MYO7A and OTOG gene were suggested to modify the stability or the interactions of different proteins in the apical surface of the sensory epithelia such as hair cells (stereocilia) or TM. In this study, the presence of rare missense variants and frame shift deletions in the TECTA gene in six unrelated families with MD suggests a role of this gene in the pathophysiology of the disease. However, because of the lack of a reliable association between α-tectorin and vestibular function, we consider that additional variants and genes may contribute to the vestibular phenotype in MD.
ACKNOWLEDGEMENTS
The authors appreciate the participation of all patients with MD and their families in this study. This project was partially funded by H2020-SC1-2019-848261 (UNITI), CECEU PY20-00303 (EPIMEN), European Regional Funds B-CTS-68-UGR20 (M3N-OMIC), and the Schmieder-Bohrisch Foundation (Geneva, Switzerland). AMPP is supported by a predoctoral grant from the Regional Ministry of Economic Transformation, Industry, Knowledge and Universities of Junta de Andalucía (Grant number PREDOC2021/00343). DB is supported by a national MD-PhD scholarship from the Swiss National Science Foundation (SNSF). AHE is supported by a career development grant (“Filling the Gap”) from the University of Zurich, Switzerland. Funding for open access charge: Universidad de Granada/CBUA.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.




