Dysregulation of the CD147 complex confers defective placental development: A pathogenesis of early-onset preeclampsia
Cheuk-Lun Lee, Zhilong Chen and Qingqing Zhang contriuted equally to this work and should be considered cofirst authors.
Dear Editor,
Trophoblastic CD147, also known as basigin (BSG), regulates the differentiation and spiral artery remodelling functions of extravillous trophoblasts (EVCTs), and its deficiency contributes to the pathophysiology of preeclampsia (PE). The biological activities of CD147 are mediated by its interaction with integrin β1 and Wnt/β-catenin signalling. The reduced serum and villous CD147 levels in PE are worth further investigation in a clinical trial as an early biomarker of PE.
PE is a multifactorial gestational complication affecting 4.6% of pregnancies worldwide.1 It is the top cause of prenatal morbidity/mortality and is associated with a high incidence of maternal and perinatal complications, causing a heavy burden to the healthcare system.1 The aetiology of PE is associated with defective trophoblast differentiation and functions causing abnormal placental development, insufficient placental perfusion, and maternal–foetal exchange defects.1, 2 Our limited knowledge of the pathogenesis of the disease has hindered the development of a reliable approach for the prediction and treatment of PE.
CD147 is a component of several of the most abundant protein complexes in human and mouse placentas3 (Figure S1). It has been linked to the physiology and pathology of various reproductive processes.4 Systematic CD147 knockout leads to perinatal lethality before day 12.5 of gestation in mice.5 The observations could be due to deficiency of CD147 in the foetus and/or the placenta. To circumvent the limitation of the knockout model, we employed our nanoparticle model6 to specifically knockdown CD147 expression in mouse trophoblasts (Figure S2). Our results showed that trophoblastic CD147 knockdown caused PE-like symptoms in mice, including placental haemorrhage (Figure 1A), reduction of alive litter size (Figure 1B), reduction of body, placental and foetal weight (Figure 1C-E), and elevation of blood pressure (Figure 1F), urine protein/creatinine ratios (Figure 1G) and serum sFlt-1 (Figure 1H). Moreover, renal damage including glomerular capillary endotheliosis and glomerular erythropenia (Figure 1I) and deterioration of development in terms of placental diameter and thickness, biparietal diameter, crown-rump length and foetal heart rate (Figure 1J) were observed in the knockdown mice. Blockage of CD147 using a functional blocking antibody did not affect embryo implantation in mice (Figure S3).
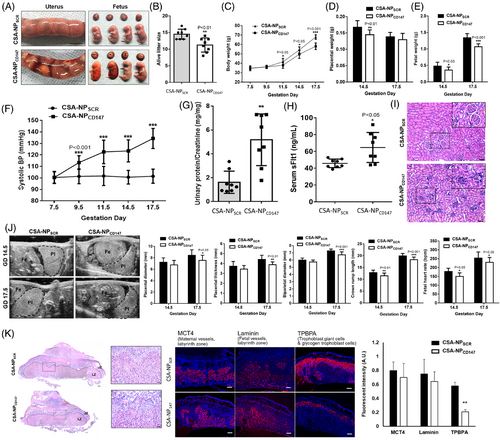
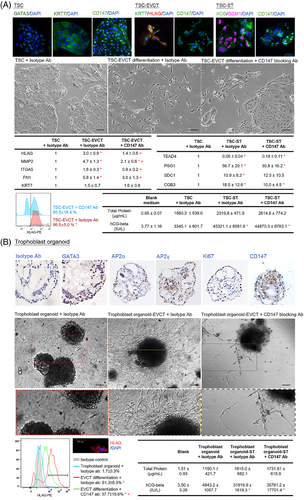
Dysregulated trophoblast differentiation contributes to placental dysfunction and PE.2 The trophoblastic CD147 knockdown mice exhibited a reduction in the area of the labyrinth and the number of trophoblast-specific protein α (TPBPA)+ invasive trophoblast giant cells in the placenta (Figure 1K), suggesting defective trophoblast differentiation. Ablation of TPBPA+ cells in mouse placenta is associated with defective remodelling of the maternal spiral arteries.7 Indeed, CD147 has been proposed to regulate trophoblast differentiation in humans.3, 4 Analysis of single-cell databases of the human cell landscape and human early maternal–foetal interface revealed high expression of CD147 in the cytotrophoblast (CT) and EVCT (Figure S4). Taken together, we hypothesised that CD147 regulated placental development in humans by modulating EVCT differentiation and functions. To test this hypothesis, we established human trophoblast stem cells (TSCs) from primary CT (Figure 2A) and trophoblast organoids from early placental villi (Figure 2B). Both models possessed molecular features of CT and functional features to differentiate into both EVCT and syncytiotrophoblast (ST) (Figure 2A,B). Our data showed that CD147 regulated the differentiation of EVCT but not ST cells in vitro (Figure 2A,B), consistent with the phenotypes of trophoblastic CD147 knockdown mice.
Vascular remodelling of the spiral arteries by EVCT transforms the spiral arteries into low-resistance and high-flow vessels crucial for providing sufficient blood supply to the foetal–maternal interface in early pregnancy. Failure in vascular remodelling would give rise to placenta-associated complications such as PE.1 We confirmed the expression of CD147 on primary EVCT and the EVCT-like cell line JEG-3 (Figure S1B). Suppression of CD147 functions by functional blocking antibody8 (Table S1) and siRNA (Figure S5) or stimulation of CD147 by ligation antibody9 (Table S1) showed that trophoblastic CD147 regulated characteristic features of the vascular remodelling process in early human pregnancy, including EVCT invasiveness and MMP2 expression/activity (Figure 3A,B), integration of EVCT into the endothelial network (Figure 3C), and endothelial cell angiogenesis (Figure 3D) and permeability (Figure 3E).
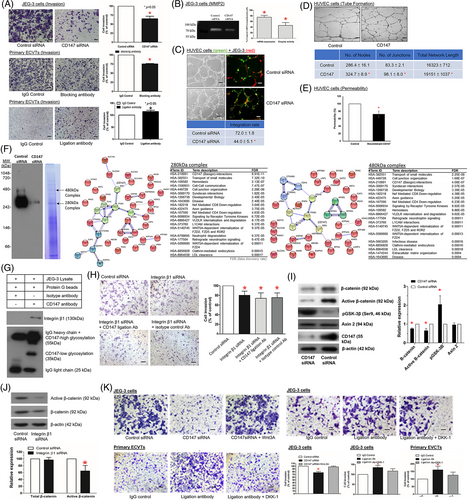
CD147 has an unusual transmembrane domain characterised by a single-charged glutamic acid in the hydrophobic transmembrane region, which promotes protein–protein interactions.4 CD147 is a component of two receptor complexes (Figure 3F; Table S2) on the plasma membrane of human EVCT. The CD147-integrin β1 interaction within the complex was confirmed by protein interaction analysis (Figure 3F), coimmunoprecipitation (Figure 3G) and their specific expression in EVCT (Figures S1C and S4). The interaction of CD147 with integrin-β1 (Figure 3H; Figure S5) and with Wnt/β-catenin signalling (Figure 3I-K) mediates the activities of CD147 on EVCT invasion.
CD147 has been applied as a predictive marker or therapeutic target of cancer.10 Based on the roles of CD147 in regulating placentation in vivo and EVCT functions in vitro, we speculated that deficiency of CD147 might contribute to the pathophysiology of early-onset PE. This was supported by our clinical data, which revealed downregulation of placental CD147 (Figure 4A) and serum soluble CD147 (Figure 4B) in early pregnant women who developed PE in late pregnancy when compared to normotensive controls (Table S3). Angiogenic factors including PlGF or sFlt-1:PlGF ratio, and novel markers such as cell-free RNA have been investigated as predictive biomarkers for PE during early pregnancy. Given the clinical heterogeneity of PE, there is a need to further develop an early prediction biomarker panel that reflects different pathophysiological processes of PE. The clinical significance of CD147 as a test for early prediction or prognostic marker of PE when combined with other established biomarkers is worth further investigation in larger clinical trials.
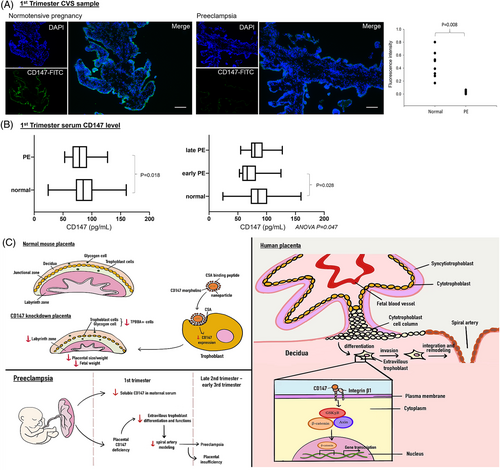
In summary, by studying the roles and mechanisms of CD147 in trophoblast differentiation and functions, we provide novel evidence that defective trophoblastic CD147 expression is a placental cause of PE (Figure 4C). Specifically, the defect affects the differentiation and vascular remodelling activities of EVCT and thereby disrupts normal placental development. Clinically, the results of this study indicate a possible research direction for the use of CD147 for the early prediction of PE.
ACKNOWLEDGEMENTS
This work has been supported in part by the National Natural Science Foundation of China (81971396), Health and Medical Research Fund, Hong Kong (09202056), High Level-Hospital Program, Health Commission of Guangdong Province, China (HKUSZH201902015), and HKU-SZH Fund for Shenzhen Key Medical Discipline (SZXK2020089). The authors are grateful to all the women who agreed to donate their tissue samples for this study. The authors are also grateful to Ms. Benancy PC Wong, and to the staffs at Faculty Core Facility, the University of Hong Kong, for their technical assistance in this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.




