Effect of neonatal seizure burden and etiology on the long-term outcome: data from a randomized, controlled trial
Abstract
Background
Neonatal seizures are common, but the impact of neonatal seizures on long-term neurologic outcomes remains unclear. We addressed this question by analyzing data from an early-phase controlled trial of bumetanide to treat neonatal seizures.
Methods
Neonatal seizure burden was calculated from continuous video-electroencephalogram data. Neurologic outcome was determined by standardized developmental tests and postneonatal seizure recurrence.
Results
Of 111 enrolled neonates, 43 were randomized to treatment or control groups. There were no differences in neurologic outcomes between treatment and control groups. A subgroup analysis was performed for 84 neonates with acute perinatal brain injury (57 hypoxic–ischemic encephalopathy [HIE], 18 stroke, 9 intracranial hemorrhage [ICH]), most of whom (70%) had neonatal seizures. There was a significant negative correlation between seizure burden and developmental scores (p < 0.01). Associations between seizure burden and developmental scores were stronger in HIE and stroke groups compared with ICH (p < 0.05).
Conclusion
Bumetanide showed no long-term beneficial or adverse effects, as expected based on treatment duration versus duration of neonatal seizures. For neonates with perinatal brain injury, higher neonatal seizure burden correlated significantly with the worse developmental outcome, particularly for ischemic versus hemorrhagic brain injury. These data highlight the need for further investigation of the long-term effects of both neonatal seizure severity and etiology.
Introduction
Seizures are common in neonates with acute perinatal brain injury, including hypoxic–ischemic encephalopathy (HIE), ischemic stroke, and intracranial hemorrhage (ICH), and seizures in this population are often prolonged and refractory to treatment.1, 2 Complicating clinical care, neonatal seizures can be difficult to detect, as clinical manifestations of the seizure may be subtle3, 4 and many seizures are electrographic only, especially after initial antiseizure medication (ASM) administration.5 Therefore, the American Clinical Neurophysiology Society guidelines recommend continuous video-electroencephalogram (cvEEG) monitoring for at-risk neonates to detect seizures.6
With increased prolonged cvEEG monitoring for critically ill neonates, an accumulating body of evidence, primarily in neonatal HIE, has shown a correlation between higher neonatal seizure burden and worse short-term1, 7, 8 and long-term9-15 outcome measures, including increased mortality and abnormal neurodevelopmental outcome. There is, however, a relative paucity of studies investigating the relationship between seizure burden and long-term outcomes in neonates with other types of acute perinatal brain injury, including stroke and ICH. Work in animal models has demonstrated that neuronal injury secondary to seizure activity is significantly worse in the setting of hypoxia–ischemia,16, 17 suggesting that the effect of neonatal seizure burden on long-term neurologic outcome may crucially depend on the seizure etiology, with worse outcomes in patients with hypoxic–ischemic injury. This hypothesis has yet to be carefully studied in the clinical setting.
Here we report the developmental outcome and rate of postneonatal seizure recurrence from a randomized, controlled, double-blind, early-phase trial of bumetanide as an add-on therapy to phenobarbital to treat neonatal seizures.18 Neonates enrolled in this study had cvEEG during the entire period of acute neonatal seizures with quantification of seizure burden, as well as follow-up evaluation with standardized developmental psychological measures. Since the trial included subjects with stroke and ICH as well as HIE, we could evaluate the effect of seizure etiology on the relationship between seizure burden and long-term neurologic outcome, in addition to evaluating any effect of bumetanide on neurologic outcome.
Methods
Study design and participants
The study population consisted of neonates enrolled between 2010 and 2017 in a randomized controlled double-blind trial of bumetanide as an add-on therapy to phenobarbital to treat seizures.18 In this early-phase trial, one of three escalating dosages (0.1–0.3 mg/kg) was administered and the acute pharmacokinetics and EEG responses were studied. The single dose of bumetanide was not expected to have a sustained effect on seizure control over the entire period (days) of susceptibility to seizures.18 This multicenter trial was conducted in four neonatal intensive care units (NICUs) in Boston, MA. All neonates were born at postmenstrual age 34–44 weeks and either had clinically suspected and/or EEG-confirmed seizures or were deemed high risk for seizures due to a diagnosis of HIE, focal stroke, ICH, acute meningoencephalitis, brain malformation, or a suspected/known genetic disorder. Exclusion criteria included seizures secondary to transient metabolic abnormalities, diagnosis of inborn errors of metabolism, prior administration of bumetanide, furosemide, phenytoin, or 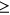 40 mg/kg phenobarbital, total bilirubin
40 mg/kg phenobarbital, total bilirubin 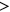 15 mg/dl, treatment with ECMO, or risk of imminent death.
15 mg/dl, treatment with ECMO, or risk of imminent death.
This study had International Review Board approval at all participating NICUs, and parents/guardians provided written informed consent. The trial was prospectively registered with clinicaltrials.gov (NCT00830531).
Measures
cvEEG data were collected for all enrolled neonates, starting soon after or prior to enrollment, if cvEEG was initiated by the clinical team. The cvEEG was continued for at least 48 h postrandomization or longer if seizures persisted. Neonatal seizure burden (in min/h) for each subject was defined as the sum of all minutes of seizure activity from the onset of the first suspected or confirmed seizure to the end of the last EEG-confirmed seizure, divided by the number of hours in the period from first to last seizure. Calculating seizure burden over an arbitrarily chosen standardized duration of recording (e.g., 48 h) could under- or overestimate seizure burden; therefore, seizure burden was measured over the entire period of seizure activity for each subject. Neonatal seizure burden was also calculated as the total minutes of seizure activity recorded by cvEEG. Subjects with clinically suspected seizures but no seizures recorded by subsequent EEG were assigned a seizure burden of zero since seizure diagnosis and seizure burden could not be determined accurately. Incidence of status epilepticus, defined as ≥30 min of EEG seizure activity in a 60-min period, was determined from analysis of EEG data.
Seizure etiology was determined by a review of the clinical data together with cvEEG, neuroradiology reports, and laboratory data. Seizure location and type by cvEEG were compared with the location of any brain injury reported in neuroimaging studies to confirm the etiology of neonates who had multiple diagnoses (e.g., brain malformation and ICH). A neuroradiologist (P. E. G.) reviewed cases of ICH to determine if there was imaging evidence of associated global hypoxic–ischemic brain injury or focal arterial stroke in addition to hemorrhagic brain injury.
Between 17 and 31 months of age, subjects underwent a developmental psychological evaluation. The primary outcome measure was the Bayley Scales of Infant and Toddler Development, 3e (Bayley-III),19 and the secondary outcome measure was the Vineland Adaptive Behavior Scales, 2e (Vineland-II).20 Each child was individually administered the Bayley-III, a comprehensive measure used to identify developmental progress during early childhood. Domains included in our analysis were Cognitive, Language, and Motor. Each family completed the interview version of the Vineland-II, which is designed to measure the adaptive behavior of individuals from birth to the elderly years. Domains included in our analysis were Adaptive Behavior, Communication, and Motor Skills. Bayley-III and Vineland-II composite scores are reported as standard scores (mean = 100, standard deviation = 15 in a healthy, normal population).
Postneonatal seizure recurrence was determined by a review of notes available in the medical record from appointments with neurology, developmental medicine, or comprehensive well-child visits with the primary care physician. Any relevant notes through January 2021 were included in the chart review. Subjects with one or more afebrile seizures were considered to meet the criteria for postneonatal seizure recurrence.
Subjects were divided into groups with normal or abnormal neurologic outcomes based on criteria revised from a study of outcome in neonatal HIE.13 Subjects were defined as having an abnormal neurologic outcome if they had one or more of the following: a diagnosis of epilepsy, Gross Motor Function Classification System (GMFCS) level of three to five,21 sensorineural hearing loss (SNHL) requiring cochlear implant, and/or Bayley-III composite scores less than 85 in all three domains (Cognitive, Language, Motor), or less than 70 in any individual domain. In subjects without Bayley-III composite scores, Vineland-II composite scores were used as a proxy. Subjects not meeting these criteria were defined as having a normal neurologic outcome.
To evaluate the relationship between seizure burden and neurologic diagnosis with neurologic outcome and seizure recurrence, we analyzed neonatal seizure burden, developmental psychological scores, and seizure recurrence data for all enrolled neonates with acute perinatal brain injury and available follow-up data. This analysis included only neonates with HIE, stroke, or ICH; neonates with brain malformation, confirmed genetic disorder, or acute meningoencephalitis were excluded because of the known association of these disorders with long-term epilepsy and adverse developmental outcome independent of neonatal seizure burden.
Statistical analysis
Fisher's exact tests (for categorical variables) and rank-based Kruskal–Wallis tests (for continuous variables) were used to assess differences in demographic and medical characteristics, seizure burden and recurrence, and developmental psychological scores by group (randomized vs. nonrandomized, bumetanide vs. control, and by neurologic diagnosis). Spearman's rank (robust to outlying observations) and linear regression were used to assess the relationship between neonatal seizure burden and developmental psychological scores, including exploratory assessment of interactions with seizure etiology. These findings were confirmed via two robust regression methods: M-estimation with iterated reweighted least-squares (giving lower weight to observations with higher residuals) and generalized estimating equations with sandwich variance estimators (robust to assumptions of normality and homoscedasticity of residuals). Logistic regression and receiver-operating characteristic (ROC) curves were used to assess the relationship between neonatal seizure burden and dichotomous seizure recurrence and abnormal neurologic outcome. R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria) was used for all analyses.
Results
Neonatal characteristics of enrolled trial subjects
Of the 111 subjects enrolled in the study, 10 met the exclusion criteria after enrollment. Forty-three of the remaining 101 enrolled subjects were randomized to treatment (bumetanide, n = 27) or control (standard therapy, n = 16) groups (Table 1 and Figure 1). Fifty-eight subjects were enrolled but did not meet the criteria for randomization (nonrandomized group), either because of a lack of persistent seizures after a loading dose of phenobarbital (n = 54) or missed EEG seizures (n = 4).
| Randomized | Nonrandomized | ||
|---|---|---|---|
| Bumetanide (n = 27) | Control (n = 16) | (n = 58) | |
| Neonatal characteristics | |||
| Male, n (%) | 14 (52) | 7 (44) | 32 (55) |
| Gestational age (weeks), median (IQR) | 39 (39, 40) | 40 (39, 41) | 39 (38, 40) |
| Birth weight (kg), median (IQR) | 3.4 (3.0, 3.7) | 3.3 (3.0, 3.5) | 3.2 (3.0, 3.6) |
| Race, n (%) | |||
| White | 23 (96) | 12 (92) | 33 (77) |
| Black/African American | 0 | 0 | 8 (19) |
| Other | 1 (4) | 1 (8) | 2 (5) |
| Unreported | 3 | 3 | 15 |
| Ethnicity, Hispanic or Latino, n (%) | 2 (7) | 4 (25) | 11 (19) |
| Neurologic diagnosis, n (%) | |||
| HIE | 14 (52) | 8 (50) | 45 (78) |
| Stroke | 7 (26) | 0 | 11 (19) |
| ICH | 3 (11) | 4 (25) | 2 (3) |
| Othera | 3 (11) | 4 (25) | 0 |
| Received therapeutic hypothermia, n (%) | 10 (37) | 5 (31) | 42 (72) |
| Clinical or EEG-confirmed neonatal seizures, n (%) | 27 (100) | 16 (100) | 29 (50) |
| Neonatal seizure burden (min/h), median (IQR) | 3.1 (1.3, 4.9) | 1.2 (0.3, 2.7) | 0.0 (0.0, 0.9) |
| Neonatal status epilepticus, n (%) | 11 (41) | 1 (6) | 5 (9) |
| EEG duration (h), median (IQR) | 88.4 (71.2, 99.9) | 81.9 (69.6, 95.6) | 64.5 (47.9, 83.0) |
| (n = 21) | (n = 12) | (n = 39) | |
|---|---|---|---|
| Neurodevelopmental testing | |||
| Age at testing (months), median (IQR) | 19 (18, 20) | 19 (18, 22) | 19 (18, 22) |
| Bayley-III composite scores, mean ± SD | |||
| Cognitive | 88.2 ± 16.9 | 90.6 ± 16.7 | 95.0 ± 17.3 |
| Language | 86.5 ± 17.6 | 87.9 ± 20.0 | 90.0 ± 17.6 |
| Motor | 84.4 ± 20.3 | 92.3 ± 21.1 | 93.5 ± 16.6 |
| Vineland-II composite scores, mean ± SD | |||
| Adaptive Behavior | 89.7 ± 12.0 | 78.8 ± 23.5 | 92.3 ± 16.0 |
| Communication | 88.4 ± 12.2 | 81.2 ± 22.9 | 90.2 ± 17.5 |
| Motor Skills | 88.8 ± 19.0 | 77.6 ± 31.3 | 96.4 ± 20.1 |
| Postneonatal outcome, n/total (%) | |||
| Postneonatal seizure(s) | 9/26 (35) | 4/13 (31) | 4/51 (8) |
| Confirmed SNHL | 2/26 (8) | 0/13 (0) | 1/54 (2) |
| Suspected or confirmed CVI | 0/24 (0) | 3/13 (23) | 1/46 (2) |
- CVI, cerebral visual impairment; EEG, electroencephalogram; HIE, hypoxic–ischemic encephalopathy; ICH, intracranial hemorrhage; IQR, interquartile range; SD, standard deviation; SNHL, sensorineural hearing loss.
- a Other neurologic diagnoses included brain malformation, confirmed genetic disorder, and acute meningoencephalitis.
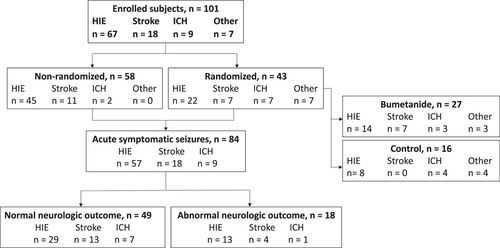
There were no significant differences in neonatal characteristics between the randomized bumetanide and control groups (Table 1), apart from a lower neonatal seizure burden in the control group (p = 0.007), which occurred by chance (pretreatment seizure burden was similarly lower in control subjects).18 Of note, the randomized and nonrandomized groups differed in neurologic diagnosis (randomized: 51% HIE, 16% stroke, 16% ICH, 16% other; nonrandomized: 78% HIE, 19% stroke, 3% ICH, 0% other; p < 0.001), and the nonrandomized group had a higher rate of therapeutic hypothermia compared with the randomized group (randomized: 35%; nonrandomized: 72%; p < 0.001) due to a larger proportion of subjects with HIE. As expected, the presence of neonatal seizures (randomized: 100%; nonrandomized: 50%; p < 0.001), neonatal seizure burden (randomized: median, 2.2 min/h, interquartile range [IQR], 1.0, 3.8; nonrandomized: 0 min/h, IQR, 0, 0.9; p < 0.001), and duration of EEG monitoring (randomized: median, 88.1 h, IQR, 70.9, 99.9; nonrandomized: 64.5 h, IQR, 47.9, 83.0; p < 0.001) were lower in the nonrandomized versus the randomized group.
Postneonatal outcome in enrolled trial subjects
Among the 101 enrolled subjects, eight (7%) died prior to follow-up. Developmental psychological scores (Bayley-III and/or Vineland-II) were available for 72 subjects (21 treatment, 12 control, 39 nonrandomized); 21 subjects did not attend developmental psychological testing (5 treatment, 1 control, 15 nonrandomized). Most subjects (n = 61) had composite scores for both the Bayley-III and Vineland-II; three had only Bayley-III testing and eight had only Vineland-II testing. Seizure recurrence data were available for 90 subjects (26 treatment, 13 control, 51 nonrandomized).
Developmental psychological testing was performed at a median age of 19 months (IQR: 18, 20) (Table 1). Bayley-III and Vineland-II scores were not significantly different between the randomized (n = 33) and nonrandomized (n = 39) groups or between the bumetanide (n = 21) and control (n = 12) groups. Within the control group, mean Bayley-III scores were higher than mean Vineland-II scores due to three subjects who had low Vineland scores but were unable to come to the clinic for Bayley-III testing. For all subjects, developmental outcome and functioning indices were lower than expected for age (one-sample t-test; Bayley-III: Cognitive, 92.3 ± 17.1 (mean ± SD), p < 0.001; Language, 88.7 ± 17.7, p < 0.001; Motor, 90.7 ± 18.6, p < 0.001; Vineland-II: Adaptive Behavior, 89.2 ± 16.8, p < 0.001; Communication, 88.1 ± 17.3, p < 0.001; Motor Skills, 91.1 ± 22.7, p = 0.002).
There was no difference in the rate of postneonatal seizures between the bumetanide (9/26, 35%) and control (4/13, 31%) groups. Compared with the randomized group, the nonrandomized group had a significantly lower rate of postneonatal seizures (randomized: 13/39, 33%; nonrandomized: 4/51, 8%; p = 0.003). Since all randomized neonates had neonatal seizures but only 50% of the nonrandomized neonates had neonatal seizures, we repeated this analysis including only subjects with neonatal seizures. In this latter analysis, the difference in postneonatal seizure recurrence was not statistically significant (randomized: 13/39, 33%; nonrandomized: 4/26, 15%, p = 0.15).
Relationship between neonatal seizure burden and postneonatal outcome in all study subjects
To evaluate the relationship between neonatal seizure burden, neurologic diagnosis, and postneonatal outcome, we analyzed data for the 84 subjects diagnosed with acute perinatal brain injury (HIE, stroke, or ICH; Figure 1) who had developmental psychological testing and/or seizure recurrence data. This included all subjects, including both randomized and nonrandomized groups. The majority had HIE (57, 68%), followed by stroke (18, 21%) and ICH (9, 11%) (Table 2). Only subjects with HIE received therapeutic hypothermia (49/57, 86%). Subjects with ICH had no evidence of global hypoxic–ischemic brain injury or focal arterial stroke by review of neuroimaging and clinical data. Most subjects (70%) had neonatal seizures, and there was a statistically significant difference in neonatal seizure burden across etiology groups; subjects with HIE had the lowest seizure burden (p = 0.03). Seven subjects (8%) had only clinically suspected seizures prior to EEG lead placement, with no seizures captured on subsequent EEG monitoring, and were therefore classified as having a seizure burden of zero. Five of the seven subjects were treated with ASM after their clinically suspected seizure(s); two subjects had a single clinically suspected seizure with no ASM treatment. Time between the first clinically suspected seizure and the start of EEG monitoring was not significantly different between patients with clinically suspected seizures only (median: 7.2 h; IQR: 4.8, 10.6) versus those with EEG-confirmed seizures (5.3 h, IQR: −1.0, 12.2). For all subjects included in the analysis, the median duration of EEG monitoring was 73.4 h (IQR: 50.5, 91.0), with no differences among the HIE, stroke, and ICH groups.
| HIE | Stroke | ICH | |
|---|---|---|---|
| (n = 57) | (n = 18) | (n = 9) | |
| Neonatal characteristics | |||
| Male, n (%) | 30 (53) | 9 (50) | 6 (67) |
| Gestational age (weeks), median (IQR) | 39 (38, 40) | 38 (38, 40) | 39 (39, 40) |
| Birth weight (kg), median (IQR) | 3.2 (3.0, 3.6) | 3.3 (3.1, 3.4) | 3.5 (2.9, 3.6) |
| Race, n (%) | |||
| White | 38 (83) | 12 (86) | 8 (100) |
| Black/African American | 5 (11) | 2 (14) | 0 |
| Other | 3 (7) | 0 | 0 |
| Unreported | 11 | 4 | 1 |
| Ethnicity, Hispanic, or Latino, n (%) | 11 (19) | 3 (17) | 0 |
| Received therapeutic hypothermia, n (%) | 49 (86) | 0 | 0 |
| Clinical or EEG-confirmed neonatal seizures, n (%) | 33 (58) | 18 (100) | 8 (89) |
| Neonatal seizure burden (min/h), median (IQR) | 0.0 (0.0, 3.1) | 1.5 (0.8, 3.6) | 3.4 (0.1, 5.5) |
| Neonatal status epilepticus, n (%) | 9 (16) | 4 (22) | 2 (22) |
| EEG duration (h), median (IQR) | 74.5 (52.1, 92.0) | 57.7 (48.1, 80.5) | 83.4 (71.3, 105.5) |
| (n = 46) | (n = 16) | (n = 7) | |
|---|---|---|---|
| Neurodevelopmental testing | |||
| Age at testing (months), median (IQR) | 20 (18, 22) | 18 (18, 20) | 19 (18, 22) |
| Bayley-III composite scores, mean ± SD | |||
| Cognitive | 93.2 ± 17.9 | 97.7 ± 8.6 | 96.4 ± 9.9 |
| Language | 90.4 ± 18.5 | 92.5 ± 12.3 | 92.1 ± 10.1 |
| Motor | 92.5 ± 18.6 | 93.3 ± 12.3 | 97.4 ± 7.8 |
| Vineland-II composite scores, mean ± SD | |||
| Adaptive Behavior | 90.0 ± 17.6 | 93.7 ± 10.1 | 93.0 ± 4.5 |
| Communication | 88.7 ± 17.7 | 93.3 ± 12.3 | 91.3 ± 6.5 |
| Motor Skills | 92.6 ± 24.1 | 95.3 ± 11.9 | 97.2 ± 7.3 |
| Postneonatal outcome, n/total (%) | |||
| Postneonatal seizure(s) | 8/56 (14) | 4/18 (22) | 1/9 (11) |
| Confirmed SNHL | 3/57 (5) | 0/18 (0) | 0/9 (0) |
| Suspected or confirmed CVI | 3/50 (6) | 0/18 (0) | 0/8 (0) |
| Abnormal neurologic outcome | 13/42 (31) | 4/17 (24) | 1/8 (13) |
- CVI, cerebral visual impairment; HIE, hypoxic–ischemic encephalopathy; ICH, intracranial hemorrhage; IQR, interquartile range; SD, standard deviation; SNHL, sensorineural hearing loss.
Developmental psychological testing was obtained in 69 (82%) of the 84 subjects with acute perinatal brain injury included in the outcome analysis (Table 2). For these subjects, developmental outcome and functioning indices were also lower than expected for age (one-sample t-test; Bayley-III: Cognitive, 94.8 ± 15.2, p = 0.01; Language, 91.1 ± 16.1, p < 0.001; Motor, 93.3 ± 16.0, p = 0.003; Vineland-II: Adaptive Behavior, 91.1 ± 15.5, p < 0.001; Communication, 90.0 ± 15.7, p < 0.001; Motor Skills, 93.6 ± 20.9, p = 0.02). Notably, there was a statistically significant correlation between neonatal seizure burden and scores across Bayley-III Cognitive (Spearman's r = −0.45, p < 0.001), Language (r = −0.33, p = 0.01), and Motor domains (r = −0.31, p = 0.02), as well as Vineland-II Adaptive Behavior (r = −0.37, p = 0.005), Communication (r = −0.37, p = 0.003), and Motor Skills domains (r = −0.34, p = 0.01). In addition to the composite scores used in the above analyses, Bayley-III and Vineland-II subscales were examined for outliers, and no individual subcomponent altered the overall results significantly; these subscales were not individually included in the analysis to help prevent multiple comparison statistical errors.
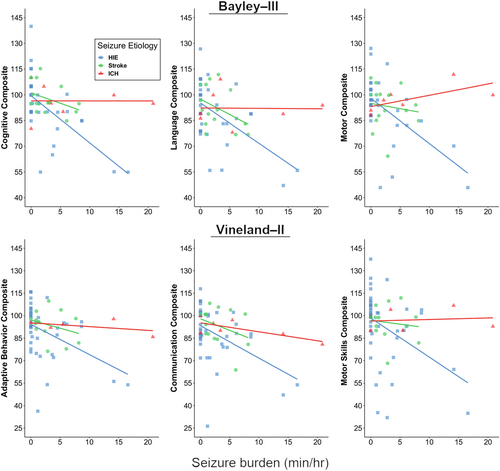
Although higher neonatal seizure burden correlated with worse outcomes, in exploratory analyses the neurologic diagnosis (HIE, stroke, or ICH) modified the relationship between neonatal seizure burden and the Bayley-III Cognitive and Motor composite scores (Cognitive interaction, p = 0.01; Language interaction, p = 0.09; Motor interaction, p = 0.006; Figure 2). These interactions were not statistically significant for the Vineland-II composite scores. We then compared the correlation between neonatal seizure burden and developmental scores in neonates with ischemic injury (HIE, stroke) to those with hemorrhagic injury (ICH). Comparison of these two groups demonstrated a stronger association between seizure burden and developmental psychological scores in the HIE/stroke group than in the ICH group for all Bayley-III domains (Cognitive interaction, p = 0.01; Language interaction, p = 0.04; Motor interaction, p = 0.008). These results are confirmed by comparing Spearman's rank correlations between seizure burden and Bayley-III scores (r = −0.54, p < 0.001 for Cognitive; r = −0.39, p = 0.005 for Language; r = −0.47, p < 0.001 for Motor for the HIE/stroke group vs. r = −0.13, p = 0.79 for Cognitive; r = 0.12, p = 0.80 for Language; r = 0.80, p = 0.03 for Motor for the ICH group). In these exploratory models, the statistically significant interaction effects of seizure burden on developmental psychological scores by neurologic diagnosis were confirmed in robust regression analyses based on M-estimation or generalized estimating equations.
Postneonatal seizure data were available for 83 subjects (Table 2), with postneonatal seizures occurring in 13, all of whom had a history of neonatal seizures. This represented a seizure recurrence rate of 22% (13/58 subjects with neonatal seizures). Neonatal status epilepticus was not significantly associated with postneonatal seizure recurrence, as we found seizure recurrence in 4 of 15 (27%) subjects with neonatal status epilepticus, compared with 9 of 68 (13%) subjects without status epilepticus (p = 0.24). The median age of seizure recurrence was 34 months (IQR: 12, 60). All subjects with seizure recurrence had afebrile focal seizures (n = 13). Some subjects had additional seizure types, including complex febrile seizures (n = 4), afebrile generalized seizures (n = 4), and infantile spasms (n = 2). Twelve of the 13 subjects remained on ASM treatment at the last follow-up, with a median of one ASM (IQR: 1, 2). There were no statistically significant differences in the postneonatal seizure recurrence across the neurologic diagnosis and there was no association between neonatal seizure burden and postneonatal seizures.
Of the 84 subjects with perinatal brain injury included in the outcome analysis, 49 (58%) were classified as having a normal neurologic outcome, 18 (21%) had an abnormal neurologic outcome, and 17 (20%) had insufficient data for classification (Table 2). Neonatal characteristics were similar between abnormal and normal outcome groups. Thirteen of 42 (31%) subjects with HIE, 4 of 17 (24%) subjects with stroke, and one of 8 (13%) subjects with ICH had abnormal neurologic outcomes, with no significant difference across neurologic diagnoses. For subjects with HIE, treatment with therapeutic hypothermia was almost significantly different between the normal (27/30, 90%) and abnormal (9/13, 69%) groups (p = 0.06).
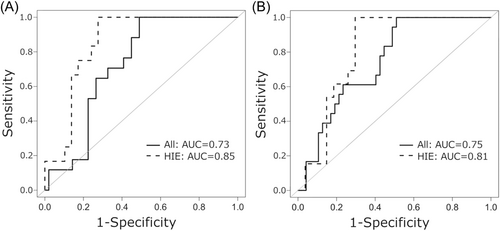
While neonatal seizures were more common in the abnormal outcome group (18/18, 100%) compared with the normal outcome group (32/49, 65%, p = 0.003), neonatal status epilepticus was not significantly associated with abnormal outcomes. The abnormal outcome was found in 6 of 15 (40%) subjects with neonatal status epilepticus versus 12 of 52 (23%) subjects without neonatal status epilepticus (p = 0.21). In contrast, neonatal seizure burden (min/h) was significantly higher in the abnormal outcome group (median: 3.6; IQR: 1.2, 4.5) compared with the normal outcome group (0; IQR: 0, 3.1; p = 0.005). The area under the ROC curve for normal versus abnormal outcome and neonatal seizure burden (min/h) was 0.73 for the entire cohort and 0.85 for HIE only (Figure 3A). Similar results were obtained when seizure burden was analyzed as total seizure minutes recorded, with a higher seizure burden in the abnormal (median: 102.0; IQR: 28.2, 188.3) versus the normal outcome group (3.9; IQR: 0, 73.6; p = 0.002) and comparable areas under the ROC curve (entire cohort = 0.75, HIE only = 0.81; Figure 3B).
Discussion
We present long-term neurologic outcome data from a randomized, controlled, double-blind trial of bumetanide as an add-on therapy to phenobarbital to treat neonatal seizures,18 and find no significant differences in developmental outcome or postneonatal seizure recurrence between treatment and control groups. The trial was an early-phase, dose-finding trial designed to test the safety, pharmacokinetics, and pharmacodynamics of a single dose of bumetanide. As such, the trial was not designed to evaluate the efficacy of bumetanide to reduce total neonatal seizure burden or improve long-term neurologic outcomes. These outcome data demonstrated no long-term benefits or adverse effects of a single dose of bumetanide.
In this study, we evaluated the contribution of neonatal seizures and seizure burden to long-term neurologic outcomes in neonates with acute perinatal brain injury. The effect of bumetanide on long-term outcomes is expected to be secondary to its effects on neonatal seizure burden, and, as noted above, there were no differences between the treatment and control groups in neurologic outcomes after a single dose of bumetanide. Furthermore, all subjects received multiple doses of standard ASMs for neonatal seizures, which would be expected to outweigh by far the effects of a single dose of bumetanide on seizure burden. Although the median half-life (elimination = pharmacokinetics) of bumetanide in neonates was 16 h, the duration of antiseizure action (bumetanide effect = pharmacodynamics) is expected to be considerably shorter than that, particularly compared with the duration of antiseizure action of the other ASMs administered and the period of susceptibility to seizures (days). Therefore, we used neonatal EEG and outcome data from all enrolled subjects, regardless of randomization status. While neonatal seizure burden did not correlate with postneonatal seizure recurrence, there was a significant and consistent correlation between the presence of neonatal seizures and higher neonatal seizure burden with the worse developmental outcome. Interestingly, this relationship between neonatal seizure burden and later neurodevelopmental scores was modified by seizure etiology, with a stronger correlation in subjects with HIE/stroke compared with ICH. This effect was significant despite the small number of subjects with ICH in our study. To our knowledge, this effect of seizure etiology modulating the effect of seizure burden has not been examined previously. Prior studies have focused on neonates with HIE, and our data highlight a need for larger prospective studies of neonatal seizure burden and neurologic outcome in neonates with other seizure etiologies.
The neurologic outcome in all trial subjects is similar to previously published studies in neonates at risk for seizures. Developmental scores from both the Bayley-III and Vineland-II were lower than expected for age, in agreement with previous studies reporting abnormal developmental outcomes in neonates with acute perinatal brain injury, particularly those with neonatal seizures.9-15, 22 The 22% incidence of postneonatal seizures is also consistent with previously reported rates of seizure recurrence, which range from 5 to up to 56% of surviving patients with a history of neonatal seizures.23-31 SNHL was only observed among the subjects with HIE, with three affected subjects (two in the bumetanide group and one in the nonrandomized group). This represented 5% of enrolled neonates with HIE, which is lower than what has been previously reported (10%) in neonates with HIE, likely related to the inclusion of subjects with mild HIE (and no seizures) in the nonrandomized group.32 Cerebral visual impairment was also seen exclusively in the HIE group, at a rate (5%) in line with prior data (3%–4%).33
Our analysis of subjects with acute perinatal brain injury (HIE, stroke, or ICH) demonstrated a strong correlation between continuous measures of neonatal seizure burden and developmental outcome, adding to the growing evidence that higher seizure burden is correlated with worse neurodevelopmental outcomes.12-15 Prior papers examining the relationship between seizure burden and short- and long-term outcome measures have used a variety of measures to quantify seizure burden, including categorization of seizure burden based on seizure number9-11, 14, 31 or amplitude-integrated EEG data,15 quantification of total seizure duration in seconds,12 minutes,13 hours,9 or days,31 and quantification of maximum hourly seizure burden.13 Because of the variability of EEG monitoring duration in our study, we measured seizure burden as minutes of seizure activity divided by the number of hours in the period from first to last seizure (min/h). Additionally, to allow for a direct comparison of our results to a similar study by Kharoshankaya et al.13 conducted in neonates with HIE, we calculated seizure burden as total minutes of seizure activity and used a binary normal/abnormal neurologic outcome scale. Here again, our data show that the group with abnormal outcome were more likely to have had neonatal seizures and a significantly higher neonatal seizure burden, with median seizure burdens and ROC areas under the curve similar to those presented by Kharoshankaya et al.13 In contrast to that study, however, we did not find a clear threshold for seizure burden to predict an abnormal outcome. Indeed, our continuous analysis suggests that with an increasing neonatal seizure burden, there is an increased risk for an abnormal outcome.
Unlike most prior outcome studies, our study included detailed EEG data for neonates with stroke and ICH in addition to HIE, so we were also able to directly compare the effects of seizure burden across different seizure etiologies. It has long been observed that seizure etiology plays an important role in determining the long-term outcome, with worse injury corresponding with worse outcomes (e.g., global HIE with bilateral or diffuse injury having worse outcomes than a unilateral focal stroke).34, 35 Prior work has also shown that certain diagnoses, such as infection, brain malformations, and genetic disorders, are predictors of a worse prognosis, compared with HIE.36 Interestingly, evidence from animal models suggests that injury secondary to hypoxia–ischemia is exacerbated by the presence of seizure activity,16, 17 suggesting that seizure burden may play a more important role in determining outcome in a brain with hypoxic–ischemic injury, versus a brain that did not suffer from lack of oxygen and blood flow. However, the differential effects of neonatal seizure burden with and without hypoxic–ischemic injury have not been carefully studied in the clinical setting. Our results suggest that seizure etiology does indeed affect the relationship between seizure burden and developmental outcome in perinatal brain injury. Specifically, subjects with ICH, as compared with those with HIE or stroke, had a weaker correlation between neonatal seizure burden and developmental psychological scores. Notably, as can be appreciated in Figure 2, there were subjects with ICH who had high developmental scores despite having had a high neonatal seizure burden. Although experimental models show that hemorrhage adjacent to or involving cortical neurons may cause acute seizures and later epilepsy,37 neonatal seizures without ischemic injury are associated with generally favorable neurobehavioral outcomes.38 This preliminary evidence suggests that the injurious effects of seizure activity may be specific to global or local hypoxic–ischemic etiologies of acute seizures, and thus the prognostic importance of seizure burden is dependent on seizure etiology. However, as our study included relatively few subjects with ICH, it will be important to repeat these analyses in a larger cohort.
Another factor to consider is the degree to which seizure burden is a proxy for the severity and/or extent of brain injury. Several studies have demonstrated that, in neonates with HIE, seizure burden correlates with HIE severity22 and/or the extent of brain injury seen on magnetic resonance imaging (MRI).10, 39-41 This has led to the argument that seizure burden is merely a proxy for the severity of brain injury, rather than an additional contributor to the worse neurologic outcome,22 although one study still found a significant correlation between seizures and outcome after controlling for severity of injury seen on brain MRI.10 Additionally, in the studies that examined the relationship between seizure burden and brain MRI,10, 39-41 MRIs were obtained at a mean or median of 5–9 days of age, which is generally after the resolution of neonatal seizures secondary to acute injury. Therefore, it is possible that the injury seen on MRI was in part secondary to seizure activity. In contrast, seizures associated with hemorrhage adjacent to cortical neurons without a large area of associated ischemic injury may not exacerbate brain injury or worsen later cognitive outcomes. Our data support the notion that seizure burden in neonates with hemorrhagic brain injury may be less related to extent of injury or may not exacerbate the brain injury compared with neonates with global or local hypoxia–ischemia such as occurs with HIE and stroke, explaining in part why the correlation between seizure burden and outcome is weaker. Further research with larger populations and detailed neuroimaging analysis will be helpful in clarifying the relationship between injury severity, seizure burden, and long-term outcome.
In terms of postneonatal seizures, we found, not surprisingly, that all subjects with postneonatal seizures had a history of neonatal seizures. However, we did not find a statistically significant correlation between neonatal seizure burden or status epilepticus and postneonatal seizure recurrence. This is in contrast to prior studies, which demonstrated a relationship between status epilepticus and later-life epilepsy in neonates with neonatal encephalopathy,27 as well as the presence of neonatal seizures and risk of later epilepsy in patients with a history of neonatal stroke.29 A recent study in infants with acute brain injury also demonstrated a relationship between days of neonatal seizures and an increased risk of postneonatal epilepsy.31 Several factors may explain why we did not find a significant relationship between seizure burden and postneonatal seizure recurrence. First, as noted above, variable methods for quantifying neonatal seizure burden have been used. Second, the number of subjects with postneonatal seizure recurrence was small overall (n = 13), and a larger number might be required to detect an association with neonatal seizure burden. Indeed, the fact that 27% of subjects with versus 13% without status epilepticus had seizure recurrence suggests that with more subjects we may have been able to detect a statistically significant association between status epilepticus and seizure recurrence. Third, since postneonatal seizure recurrence can be observed years after the neonatal period,27 it is likely that our follow-up period was too short to detect all subjects who will eventually develop postneonatal seizures. Finally, risk factors that we were unable to measure in this study likely play a role in postneonatal seizure recurrence, including the extent and location of brain injury and genetic factors.
Limitations of our study include the lack of a direct measure of brain injury severity. Also, although our study is one of the larger studies of neonates with acute perinatal brain injury to date, the number of subjects with etiologies such as stroke or ICH remains small. It will be important to conduct similar analyses with larger populations of patients to determine if the effects we find are robust. Finally, our follow-up data consisted of developmental testing during the preschool years, which likely does not capture the full spectrum of long-term neurologic impairments that can be seen in patients with a history of acute perinatal brain injury, as more subtle neuropsychological impairments manifest at an older age.42
Strengths of our study include the prolonged neonatal EEG recordings with quantification of neonatal seizure burden. Additionally, the availability of detailed and prospectively obtained developmental psychological testing by a blinded investigator allowed for rigorous and objective assessment of developmental outcomes. Finally, this is one of the few studies to date to include neonates with stroke and ICH in a study of seizure burden and long-term neurologic outcome, and our finding of differences between seizure etiologies is a compelling argument for more studies of this kind. Indeed, heterogeneity in the relationship between seizure burden and outcome across different seizure etiologies could explain in part some of the conflicting findings on the impact of treating neonatal electrographic seizures on long-term outcomes.43
Overall, our data demonstrate the lack of long-term beneficial or adverse effects of a single dose of bumetanide to treat neonatal seizures, when comparing treatment and standard therapy control groups. Our results add to the literature demonstrating a clear link between higher neonatal seizure burden and worse neurodevelopmental outcomes, and we present the preliminary but novel finding that this relationship was stronger for neonates with global or focal arterial ischemic brain injury than intracranial hemorrhage. Thus, there is a need for further research, particularly with the goal of finding more effective treatments for neonatal seizures that might reduce adverse long-term sequelae of neonatal seizures.
Author Contributions
Sara K. Trowbridge: Conceptualization; data curation; formal analysis; methodology; writing – original draft; writing – review and editing. Lois O. Condie: Data curation; formal analysis; methodology; writing – review and editing. Jessica R. Landers: Data curation; formal analysis; methodology; project administration; writing – review and editing. Ann M. Bergin: Data curation; formal analysis; investigation; methodology; supervision; writing – review and editing. Patricia E. Grant: Data curation; formal analysis; writing – review and editing. Kalpathy Krishnamoorthy: Data curation; formal analysis; investigation; writing – review and editing. Valerie Rofeberg: Formal analysis; methodology; writing – review and editing. David Wypij: Formal analysis; methodology; writing – review and editing. Kevin J. Staley: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; writing – review and editing. Janet S. Soul: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; supervision; writing – original draft; writing – review and editing.
Acknowledgments
The authors are grateful for the contributions of many physician, nursing, pharmaceutical, laboratory, and research staff at all four sites, and are especially grateful for the participation of the families. The trial was funded by NIH/NINDS Grant 5R01 NS066929, and grants from the CURE foundation, Harvard Catalyst—Harvard Clinical and Translational Science Center, Tufts Clinical and Translational Science Institute (CTSI), the Charles H. Hood Foundation, the Translational Research Program at Boston Children's Hospital, and the Mooney Family Initiative for Translation and Clinical Studies in Rare Diseases.
Boston Bumetanide Trial Group Members
| Member | Institution |
|---|---|
| Sarah Barnett, MD | Boston Children's Hospital |
| Gerard Berry, MD | Boston Children's Hospital |
| Joseph H. Chou, MD | Massachusetts General Hospital |
| Helen A. Christou, MD | Brigham and Women's Hospital |
| Jonathan M. Davis, MDCM | Floating Hospital for Children/Tufts Medical Center |
| Min Dong, PhD | Cincinnati Children's Hospital Medical Center |
| Carmen Rosa Fortuno, MD | Boston Children's Hospital |
| John N. Gaitanis, MD | Floating Hospital for Children/Tufts Medical Center |
| David A. Griesemer, MD | Floating Hospital for Children/Tufts Medical Center |
| Breda Hayes, MD | Boston Children's Hospital |
| Xiaoping Huang, MD | Boston Children's Hospital |
| Robert M. Insoft, MD, FAAP | Brigham and Women's Hospital |
| Frances E. Jensen, MD | Boston Children's Hospital |
| Fengxin Lu, MD | Boston Children's Hospital |
| Prajakta Mangeshkar, MS | Boston Children's Hospital |
| Deirdre O'Reilly, MD, MPH | Boston Children's Hospital |
| Danielle B. Pier, MD | Boston Children's Hospital |
| Christine Powell, MA | Boston Children's Hospital |
| Arnold J. Sansevere, MD | Boston Children's Hospital |
| Adam Simmons, MPH, CCRC | Boston Children's Hospital |
| Avantika Singh, MD | Boston Children's Hospital |
| Christian Stopp, MS | Boston Children's Hospital |
| Ju Tang, MD, PhD | Floating Hospital for Children/Tufts Medical Center |
| Alexander A. Vinks, PhD, PharmD | Cincinnati Children's Hospital Medical Center |
| Linh N. Vu, MA | Boston Children's Hospital |
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data sets generated during and/or analyzed during the current study are not publicly available due to the protection of patient data but are available from the corresponding author upon reasonable request.




