Deep Phenotyping of the Broader Autism Phenotype in Epilepsy: A Transdiagnostic Marker of Epilepsy and Autism Spectrum Disorder
ABSTRACT
Objective
We conducted deep and minimal phenotyping of the broader autism phenotype (BAP) in people with epilepsy (PWE) and compared its expression with published rates in the general population and relatives of individuals with autism spectrum disorder (ASD-relatives). We then examined the association of clinical epilepsy variables with BAP expression to explore its underpinnings in PWE.
Methods
103 adults with seizures (Mage = 37.37, SD = 12.50; 47% males; 51 temporal lobe epilepsy, 40 genetic generalized epilepsy, 12 other) and 58 community members (Mage = 39.59, SD = 14.56; 35% males) underwent deep phenotyping using the observer-rated Autism Endophenotype Interview and minimal phenotyping with the Broader Autism Phenotype Questionnaire (BAPQ). Published rates of the BAP were ascertained from large randomly selected samples (n > 100) of the general population and ASD-relatives based on BAPQ data.
Results
There was a higher rate of BAP in PWE (15% males, 27% females) compared with the general population (5% males, 7% females) and a similar rate to ASD-relatives (9% males, 20% females). Deep phenotyping identified an additional 22 males and 10 females, with the combined measures indicating elevated rates of the BAP in PWE (44% males, 36% females). Only a shorter duration of epilepsy was weakly correlated with BAP trait expression in males (r = − 0.21, p = 0.05).
Interpretation
PWE have a high rate of BAP, largely unrelated to secondary clinical epilepsy effects. The BAP may provide a trans-diagnostic marker of shared etiological mechanisms of epilepsy and ASD and partly account for psychosocial difficulties faced by PWE with childhood or adult onset of seizures.
1 Introduction
The broader autism phenotype (BAP) refers to mild autism traits in the domains of social and pragmatic language difficulties and restricted and repetitive behaviors and interests (RRBI) [1]. BAP traits are continuously distributed in the general population [2] but are more common in child and adult relatives of individuals with autism spectrum disorder (ASD-relatives) in whom they may reflect genetic and neurobiological mechanisms that increase ASD susceptibility [3, 4]. Thus, studying the BAP can inform our understanding of mechanisms contributing to ASD [5], including its co-occurrence with epilepsy [6].
There is a high rate of co-occurrence of epilepsy and ASD, with population-based studies suggesting a 6% rate of autism in epilepsy [7]. By definition, ASD manifests in childhood. In the past, there was debate about whether ASD in children with epilepsy was primarily due to the secondary effects of seizures. It is now understood that for an important subset of children, this is not the primary cause of autistic traits since epilepsy often presents several years after the onset of autism [8]. This pattern supports shared causal mechanisms of epilepsy and autism and means that one should expect an elevated rate of the BAP not only in pediatric epilepsy but also before seizure onset in later childhood to adulthood. In people with epilepsy (PWE), individual BAP traits spanning the three domains are overrepresented compared with the general population [9-11]. In considering their etiology, epilepsy-related factors including age at seizure onset and seizure type and frequency have been found to have no or only weak associations with BAP traits. This suggests BAP traits, like ASD traits, are not purely secondary to the effects of repeated seizures but may instead reflect shared susceptibility mechanisms for epilepsy and ASD.
The self-report version of the Broad Autism Phenotype Questionnaire (BAPQ) [12] has been widely used to assess the BAP in the general population and in ASD-relatives [4, 12]. However, previous studies have demonstrated that such minimal phenotyping measures under-identify the BAP [13] and have therefore stressed the importance of using a multi-measure, multi-informant phenotyping approach to fully capture the BAP [13-15]. The BAP may also be assessed as a continuous or categorical construct. While the former approach may increase statistical power in quantitative trait analyses [16], classifying individuals with a level of BAP trait expression above a cutoff is considered a more specific marker of ASD susceptibility mechanisms [3-5].
Thus, in the current study, we employed a multi-measure, multi-informant approach to perform the most in-depth characterization of the BAP in PWE to date. We undertook deep phenotyping using a well-validated semi-structured interview previously developed for deep phenotyping of the BAP in ASD-relatives [17]. Participants also self-reported BAP traits using the BAPQ (i.e., minimal phenotyping) [12]. We then examined how BAP classification was influenced by combining data from deep and minimal phenotyping and explored differences in the BAP domains captured by each approach. We hypothesized that more BAP individuals would be identified from the combined data than BAPQ data alone.
We then compared the rate of the BAP in PWE with published rates in the general population and ASD-relatives. We hypothesized the rate would be higher than that of the general population and lower or similar to that of ASD-relatives. We also explored the BAP profile of PWE compared with that of ASD-relatives. Finally, to better understand mechanisms underpinning the BAP in PWE, we examined the association of demographic- and epilepsy-related factors, such as age at seizure onset and seizure type, with the BAP. We hypothesized a limited role of these factors, suggesting the BAP in PWE is underpinned by ASD mechanisms.
The current study is conducted in adults with epilepsy partly because it is expected that an important subset of individuals with co-occurrence of the BAP and epilepsy would experience seizure onset in adulthood. Consequently, one should expect greater accuracy in the identification of adults than children with co-occurrence of the BAP and epilepsy, such that a clearer picture of the co-occurrence, relevant to both children and adults, can be garnered by examining the phenomenon in adults. Moreover, by studying adults, we are more likely to capture persistent BAP traits that may be more strongly related to autism susceptibility mechanisms. Furthermore, greater range in some of the seizure-related variables (e.g., age of seizure onset) may increase sensitivity to identify an association of these variables with the BAP.
2 Methods
2.1 Participant Recruitment
PWE were recruited from the First Seizure Clinic, the Epilepsy Outpatient Clinic, and the Inpatient Services of the Comprehensive Epilepsy Program at Austin Health, Victoria, Australia, as well as via referrals from local epileptologists and a private neuropsychology clinic over a 2-year period. This produced a heterogeneous sample of 103 patients with unprovoked seizures, ranging from individuals who had experienced a single seizure (and may not go on to have another) to patients with drug-resistant epilepsy.
We refer to the overall patient group as PWE; however, we note that 10 first-seizure patients (10%) did not meet criteria for a diagnosis of epilepsy at the time of assessment as they were not considered at high risk for seizure recurrence [18]. Adults who had their first recognized unprovoked seizure in the last 6 months were classified as first-seizure patients (n = 21). Those with longer-standing epilepsy (n = 82) largely comprised patients with temporal lobe epilepsy (TLE; n = 42; 51%) or genetic generalized epilepsy (GGE; n = 37; 45%). Patients were excluded if they had another neurological condition, such as traumatic brain injury or a malignant tumor, or if they had undergone neurosurgery. Thus, participants' increased seizure susceptibility was more likely attributable to inherent than acquired factors.
A comparison group of individuals with no history of epilepsy nor a family history of ASD was recruited from the local community and through snowball sampling (i.e., participants were encouraged to share study recruitment materials with potentially eligible adults they knew; n = 58). To distinguish individuals drawn from the community with and without the BAP, the non-BAP individuals are termed “controls.”
All participants were aged between 18 and 65 years, with IQ > 70 and no psychiatric condition with the exception of depression or anxiety, which are so common in the epilepsy population that exclusion was considered too restrictive [19, 20]. This study was approved by the Research Ethics Committee of Austin Health, and all participants provided written informed consent.
2.2 Measures
The Autism Endophenotype Interview (AEI) is a clinician-administered semi-structured interview that was designed for deep phenotyping of the BAP [17]. It evaluates up to 34 BAP traits (Table 1) that were derived from rigorous phenotyping in large multiplex families with ASD (> 8 individuals with ASD or the BAP per family) spanning four generations. The AEI has been found to segregate individuals with and without the BAP with up to 97% sensitivity and 82% specificity [17].
| AEI subscale | Traits |
|---|---|
| Social traits | Limited capacity to develop rapport |
| Awkward social interactions | |
| Difficult or limited interpersonal relationships | |
| Aloof personality style | |
| Reduced affection | |
| Reduced empathy | |
| Tendency to anger easily | |
| Narcissistic personality style | |
| Pragmatic language traits | Unusual or awkward greeting style (atypical greeting behavior) |
| Reduced eye gaze | |
| Difficulty answering open-ended questions | |
| Making inappropriate comments | |
| Idiosyncratic use of language | |
| Monotonous verbal output | |
| Tendency to monologue (overly talkative) | |
| Tangential pragmatic style (out-of-synchrony communicative behavior) | |
| Overly detailed when producing a narrative (overly detailed) | |
| Overly technical language | |
| Focus on technicalities or minutia | |
| Precise articulation | |
| Unusual speech volume | |
| Reduced quantity of verbal output (terse) | |
| Terse pragmatic style (overly direct) | |
| Opinionated in conversation | |
| Difficulty comprehending or appreciating humor | |
| Restricted and repetitive behaviors and interests | Hobby or interest of unusual intensity |
| Hoarding of items | |
| Preference for structure in activities of daily living | |
| Inflexible response to errors | |
| Fastidious cleaning | |
| Fastidious regarding personal appearance | |
| Perfectionistic personality style | |
| Recurrent thoughts that are not distressing | |
| Recurrent thoughts that are distressing (excess worry) |
- Note: Italicized items were taken from the Pragmatic Rating Scale [21].
Each AEI trait is scored on a 3-point Likert scale capturing the extent to which it interferes with daily functioning based on interview responses and clinical observation of participants. Item scores are summed to generate a total BAP score, as well as scores for the AEI subscales: social traits, pragmatic language, and RRBI. In this study, there was 76% agreement (positive agreement = 18%; negative agreement = 58%) of AEI item scores based on 16 (10%) randomly selected interviews scored by an independent researcher blind to participant status. The absence of a significant difference between raters in marginal frequencies as assessed by McNemar tests (p > 0.05) indicates acceptable agreement.
We used the BAPQ to examine the rate of the BAP in epilepsy compared with the general population and ASD-relatives [4, 12]. The BAPQ has three subscales, aloofness, pragmatic language, and rigidity, and the total score is used to classify the BAP according to established cutoff scores [4]. The BAPQ has demonstrated strong convergent and discriminant validity and internal consistency [4, 12, 22].
Full Scale IQ was estimated for all participants using the Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II) [23], except when they had been recently assessed as part of their clinical care or participated in another research study and consented to our use of these IQ estimates (n = 25). These were obtained using the WASI-I [24] or the Wechsler Test of Adult Reading [25]. Demographic and medical information was ascertained via a purpose-designed questionnaire, as well as a review of patient medical files.
2.3 Data Analysis
2.3.1 Classifying the BAP
We used cluster analysis with AEI and BAPQ scores as indicator variables to classify the BAP (BAP Cluster [BAP-C] approach). Cluster analysis was selected because it does not assume that our community members were representative of the general population. We used transformed total scores, obtained by dividing score deviations from the minimum score of the scale by the total range of scores [26]. Analyses were conducted separately in males (n = 68) and females (n = 93).
A two-step approach was used to identify the most robust clusters. First, four agglomerative methods (Unweighted Group Average Linkage, Single Linkage, Complete Linkage, Ward's Method) [27] indicated the potential number of clusters. Second, the most consistently identified number of clusters from step 1 was entered into an independent K-means cluster analysis to determine final membership of the cluster groups. We used McNemar tests to compare the number of individuals classified with the BAP-C approach versus those identified by the BAPQ alone (BAP Questionnaire [BAP-Q] approach).
We further examined the agreement between the BAP-C and BAP-Q approaches by comparing their classification of individuals with marked expression of each BAP domain. The level of agreement between approaches was assessed using prevalence-adjusted kappa [28]. Domain scores for the BAP-C approach were calculated as the sum of z-scores from the AEI and BAPQ. For the BAP-Q approach, the BAPQ's subscale scores were used. For each BAP domain, individuals with domain scores ≥ 1.96 standard deviations (SD) from the control mean were classified with marked expression of the domain. It was more appropriate to use this criterion for the BAP-Q approach than the established cutoffs [4] because it allowed us to compare the BAP-C and BAP-Q approaches with the same control group for reference.
2.3.2 Profiling the BAP in PWE
2.3.2.1 Comparing the Rate of the BAP in PWE, the General Population, and ASD-Relatives
Published reports of the rate of the BAP using the BAPQ in large randomly selected samples (n > 100) of the general population and ASD-relatives were retrieved through a systematic search of the PubMed, PsycInfo, and Embase databases in April 2022, using the search term “Broad Autism Phenotype Questionnaire.” The search was updated in August 2023. This identified nine relevant studies [4, 13, 29-35] from which we selected two [4, 13] that estimated the rate of the BAP in larger samples with more similar cultural background and context to our sample of PWE [35].
The large Australian sample of mothers of children with ASD from Kulasinghe et al. [31] was not selected as a comparison sample because of their substantially higher rate of the BAP compared with previous estimates [36]. This discrepancy may partly reflect that the diagnostic status of children in the study by Kulasinghe et al. (2021) was based solely on parent report. A second potential contributor that limits the comparability of their sample to ours is the growing momentum of a clinical and cultural shift in diagnosing autism in the years between our data collection (2013–2015) and that of Kulasinghe et al. (2019–2020) associated with the neurodiversity movement [37, 38]. Thus, the rates reported by Sasson et al. [13] were considered more appropriate, despite their study being based in the United States.
We used chi-squared tests to compare the rate of BAP classification in the general population and ASD-relatives from the selected studies with that in PWE. Demographic characteristics of the general population and ASD-relatives in these studies were compared with our sample of PWE using t-tests and chi-squared tests. We report the more conservative two-tailed Fisher exact test p-value for all chi-squared analyses, and where possible, we performed separate analyses for males and females given reported differences in rates of the BAP by sex in some previous studies [13, 14].
2.3.2.2 Comparing the BAP Domain Profile of PWE and ASD-Relatives
To profile the BAP in PWE compared with ASD-relatives, we contrasted expression of the three BAPQ domains in PWE with that previously described in parents of individuals with ASD in Sasson et al. [13]. Specifically, we used chi-squared tests to compare the proportion of PWE with marked expression of each BAP domain to that in ASD-relatives.
2.3.2.3 The Association of Epilepsy-Specific Factors and Demographic Variables With the BAP
We examined the association of epilepsy and demographic variables with BAP domain scores and total BAP score (combining data from the AEI and BAPQ) using Pearson correlations for continuous factors, Spearman correlations for ordinal factors, and t-tests for dichotomous factors. Epilepsy-related variables included age at seizure onset, time since first seizure, seizure frequency, number of antiseizure medications (ASMs), the presence of an epileptogenic lesion, and seizure type (TLE or GGE). Demographic variables included age, sex, and IQ. We further evaluated the influence of sex by conducting chi-squared tests to compare the rate of marked expression of each BAP domain in males and females.
3 Results
3.1 Demographic and Clinical Characteristics
The sample comprised 103 PWE and 58 community members, whose demographic characteristics are presented in Table 2. Groups differed in IQ, with PWE having lower IQ than community members.
| PWE | Local community members | p-value | |
|---|---|---|---|
| N | 103 | 58a | |
| Mean age (SD), range | 37.37 (12.50), 18–65 | 39.59 (14.56), 18–65 | 0.33 |
| Mean IQ (SD),b range | 103.97 (12.41), 78–139 | 112.97 (11.63), 82–135 | < 0.001 |
| Male (%) | 48 (47%) | 20 (35%) | 0.18 |
| Caucasian, N (%) | 99 (96%) | 53 (91%) | 0.29 |
| Family history of ASD, yes:no | 18:80 | — | |
| TLE:GGE | 51:40 | ||
| Lesion, present:absent | 26:73 | ||
| Focus, left:right | 25:17 | ||
| Mean age at seizure onset (SD), range | 22.36 (16.83), 2–64 | ||
| Mean years since first seizure (SD), range | 14.92 (13.58), 0.6–52 | ||
| Median seizures/month,c range | 0.89, 0–3240 | ||
| < 1 seizure/month, N | 51 | ||
| 1–4 seizures/month, N | 26 | ||
| > 4 seizures/month, N | 24 | ||
| Mean number of ASMsd (SD), range | 1.47 (1.10), 0–5 |
- Abbreviations: ASD, autism spectrum disorder; ASMs, antiseizure medications; GGE, genetic generalized epilepsy; PWE, people with epilepsy; TLE, temporal lobe epilepsy.
- a Three participants had a second- to fourth-degree relative with epilepsy. Since none had extreme scores (/Z/ ≥ 3.29) on the AEI or BAPQ or their subscales, they were retained in the sample.
- b An IQ estimate was unavailable for one participant with seizures.
- c Seizure frequency is based on information from the 12 months before assessment. The participant reporting 3240 seizures/month was an extreme outlier with Jeavons syndrome. The median seizures/month when excluding this outlier was 0.82 (range: 0–120). Eighteen (18%) participants had no seizures in the past 12 months. The seizure frequency of two participants was unknown as they had a history of exclusively nighttime seizures.
- d Eighty-five (83%) PWE were taking ASMs.
3.2 Classifying the BAP Using the AEI and BAPQ
Strong positive Pearson correlations between AEI and BAPQ total scores support that these measures assess similar constructs (males: r(67) = 0.61; females: r(92) = 0.66, p < 0.001) and that cluster analysis is appropriate. Figure 1 displays the cluster analysis solutions we selected for BAP classification in males and females. These were the most frequently obtained solutions in the K-means analyses, occurring in 50% and 30% of the 20 runs in males and females, respectively. The next most frequent solutions were obtained in 15% and 25% of the runs in males and females. The second most frequent solution in females differed in the group membership of a single participant and had lower homogeneity than the solution we selected.
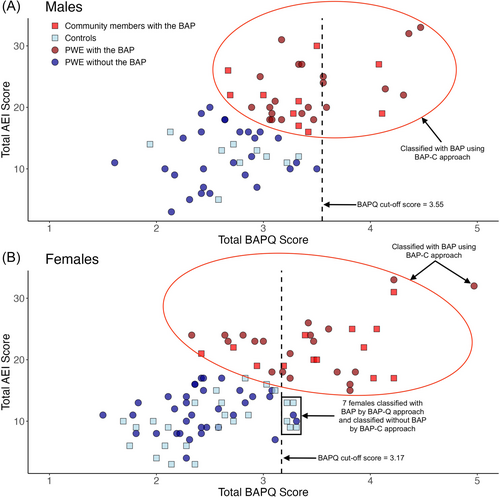
The selected cluster solutions identified an additional 22 males and 10 females with the BAP not classified by the BAPQ alone. Thus, in males with epilepsy the rate of full expression of the BAP identified by the BAP-C approach was 44%. This was significantly higher than the proportion of males with the BAP identified by the BAP-Q approach (15%, p < 0.001). In females with epilepsy, 36% were identified using the BAP-C approach. This was not significantly higher than the proportion of females identified by the BAP-Q approach (27%, p = 0.18). Among participants not identified with the BAP by the BAP-Q approach, significantly more males than females were additionally identified by the BAP-C approach (χ2(1) = 7.22, p = 0.008).
The additional males identified by the BAP-C approach had lower IQ (M = 107.23, SD = 10.92) than males identified with the BAPQ alone (M = 120.44, SD = 9.66; t(29) = −3.16, p = 0.004) but did not differ on any other variables examined (age, proportion of PWE vs. community members, age at seizure onset, time since first seizure, seizure frequency, number of ASMs; p > 0.05). In females, there were no significant differences between those additionally identified by the BAP-C approach compared with the BAP-Q approach (p > 0.05).
All males identified with the BAP using the BAP-Q approach were also identified by the BAP-C approach. In contrast, seven females classified using the BAP-Q approach were not identified by the BAP-C approach. These females' BAPQ scores were just above the established BAPQ cutoff (Figure 1B). They had moderately elevated scores across the three domains; only one (14%) had marked expression of a BAP domain based on her BAPQ score compared with 17/24 (71%) of the females classified by both the BAP-Q and the BAP-C approaches. There were no evident differences in demographic- (age, IQ) or epilepsy-related variables (age at seizure onset, epilepsy duration, seizure frequency, number of ASMs) between these seven females and those classified using the BAP-C approach.
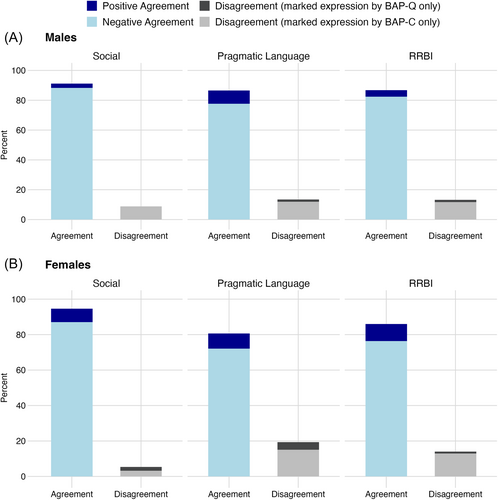
Figure 2 displays the level of agreement in the classification of individuals with marked expression of each BAP domain using the BAP-C and BAP-Q approaches. Percent agreement ranged from 77% to 96%, and prevalence-adjusted kappa supported excellent agreement for the social domain (males: k = 0.82; females: k = 0.89) and good agreement for the pragmatic language (males: k = 0.71; females: k = 0.61) and RRBI domains (males: k = 0.74; females: k = 0.72) [28]. For each domain, Figure 2 shows that disagreement largely or entirely stemmed from the identification of participants with marked expression by the BAP-C but not by the BAP-Q approach.
3.3 Profiling the BAP in PWE
3.3.1 Rate of the BAP in PWE Compared With the General Population
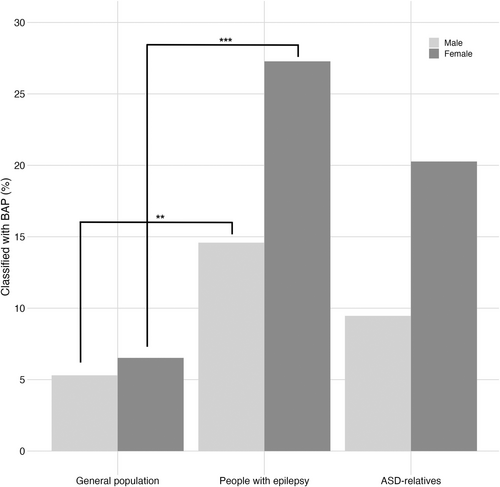
To compare the rate of the BAP in PWE with that of the general population and ASD-relatives, we relied on the BAP-Q approach for consistency in assessment methods across studies. This showed the rate of the BAP in males and females with epilepsy (males: 15%; females: 27%) was significantly higher than in the general population sample of biological parents of children with typical development (Figure 3; fathers: 5%; mothers: 7%), ascertained from Sasson et al. [4] (and personal communication with Sasson). PWE were older (M = 37.37, SD = 12.50) than the general population group (M = 35.95, SD = 5.30; t(1082) = 2.16, p = 0.03) and had fewer years of education, with 40% having primary or secondary school as their highest level of education compared with 13% of the general population group (χ2(1) = 77.51, p < 0.001).
3.3.2 Rate of the BAP in PWE Compared With ASD-Relatives
Males and females with epilepsy had a similar rate of the BAP to biological fathers and mothers of children with ASD ascertained by Sasson et al. [13] (Figure 3; fathers: 9%; mothers: 20%). Males with epilepsy were younger than fathers of children with ASD (M = 38.46, SD = 12.96 vs. M = 41.70, SD = 7.44; t(268) = −2.35, p = 0.02, r = 0.14) and had a higher level of education; they were more likely to have started or completed a college degree than having no secondary education compared with fathers of children with ASD (χ2(1) = 9.35, p = 0.002). Females with epilepsy were younger than mothers of children with ASD (M = 36.42, SD = 12.12 vs. M = 39.52, SD = 6.73; t(275) = −2.55, p = 0.01, r = 0.15) and had lower education, being less likely to have completed a master's or doctoral degree than mothers of children with ASD (χ2(1) = 5.67, p = 0.02).
3.3.3 BAP Trait Expression in PWE
Figure 4 shows the percentage of males and females with epilepsy with marked expression of the social, pragmatic language, and RRBI BAP domains compared with fathers and mothers of children with ASD described in Sasson et al. [13] For consistency across samples, classification of BAP domain expression was based on BAPQ cutoff scores described in Sasson et al. [4]
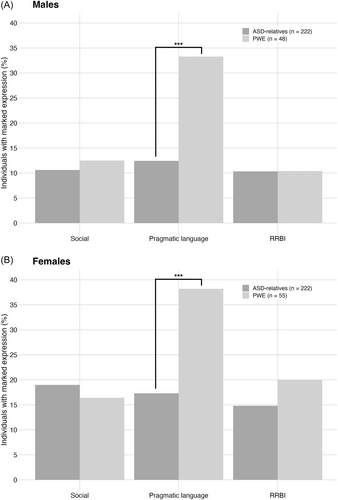
Chi-squared analyses demonstrated that for social and RRBI traits, males and females with epilepsy were equally likely as fathers and mothers of children with ASD, respectively, to report marked expression on the BAPQ (p > 0.05). In contrast, both males and females with epilepsy were significantly more likely to report pragmatic language features of the BAP (males: χ2(1) = 19.24, p < 0.001; females: χ2(1) = 16.78, p < 0.001).
3.3.4 The Relationship of Epilepsy and Demographic Variables With the BAP
In males with epilepsy, a shorter duration of epilepsy was weakly associated with more severe RRBI traits (r(47) = −0.29, p = 0.05). There was no significant association of demographic or epilepsy variables with total BAP scores. This suggests that the longer duration of their seizure disorder was not related to the BAP. In females with epilepsy, lower IQ was moderately associated with more severe pragmatic language difficulties (r(53) = −0.47, p < 0.001) although IQ was not significantly associated with total BAP scores (p > 0.05). There was a weak association between older age and more severe pragmatic language difficulties (r(54) = 0.28, p = 0.04) and total BAP scores (r(54) = 0.30, p = 0.02).
Overall, females with epilepsy had lower social BAP domain scores than males with epilepsy (t(101) = 2.65, p = 0.009, r = 0.25). There was no significant difference in total BAP scores between the sexes (p > 0.05). There was no significant difference between the sexes in the proportion with marked expression of each BAP domain relative to controls (p > 0.05).
4 Discussion
In this study of 103 adults with heterogeneous types of epilepsy, deep phenotyping of the BAP demonstrated imperfect overlap with minimal phenotyping based on self-report. As hypothesized, more individuals were identified with the BAP when combining data from observer-based and self-report measures as compared with using the BAPQ self-report version alone. Our study showed a higher rate of the BAP in PWE compared with the general population, and a similar rate to ASD-relatives. Combined with our finding that expression of the BAP in PWE was not attributable to secondary effects of seizures, this suggests that the BAP is an “essential comorbidity” of epilepsy. In other words, the BAP in PWE is likely a manifestation of causal mechanisms underpinning increased susceptibility to ASD and seizures [10]. This has important implications for the clinical management of PWE as well as for the identification of shared mechanisms of epilepsy and ASD.
Notably, the BAP has been associated with increased loneliness, anxiety, and depression [39]. These psychosocial comorbidities are often stronger predictors of quality of life in PWE than seizure-related variables and consequently, equally important to address [40]. Moreover, if these co-occurring conditions partly stem from the BAP in PWE, management strategies similar to those effective in individuals with ASD may benefit individuals with epilepsy and the BAP (e.g., social skills interventions, adapted cognitive behavior therapy for anxiety and depression) [41, 42]. This may be especially relevant for individuals with pediatric epilepsy, whose developmental outcomes may be negatively affected by the combination of epilepsy-related factors (e.g., language and cognitive deficits, psychosocial challenges) plus social/communication weaknesses associated with the BAP [43, 44]. This underscores the importance of recognizing the BAP in PWE. Our findings support using a multi-measure, multi-informant approach since we found a higher rate of the BAP when combining data from the AEI and BAPQ than when using the BAPQ alone, with the majority of individuals identified by the BAPQ also identified by the BAP-C approach. We found the same pattern of results for the identification of individuals with marked expression of each BAP domain. Moreover, a multi-pronged approach to assessing the BAP is consistent with best-practice recommendations for evaluation of the BAP as well as autism [15, 45, 46].
Our findings indicate that the BAP-C approach had a more pronounced effect on BAP classification in males than females, with significantly more males identified. This is consistent with the finding by Sasson et al. [13] that the self-report version of the BAPQ under-identifies males more than females. This may be due to lower self-awareness of BAP traits in males [13], which is indirectly supported by our finding that males self-reporting the BAP had higher IQ and thus perhaps higher self-awareness than males additionally identified by the BAP-C.
In terms of BAP domain profiles, we found that compared with fathers and mothers of individuals with ASD, males and females with epilepsy had a similar rate of marked expression of social and RRBI BAP traits as assessed by the BAPQ but reported a strikingly higher rate of challenges in the pragmatic language domain. This raises the possibility that in PWE, shared mechanisms of epilepsy and ASD are more likely to influence pragmatic language skills than social or RRBI BAP traits. A particularly elevated rate of pragmatic language difficulties is also notable for being reminiscent of features of the “epileptic personality,” which was described as early as the 1800s [47, 48] and was subsequently operationalized in 1977 by the Bear-Fedio Inventory [49, 50]. In particular, the majority of social/communication traits of the epileptic personality related to pragmatic language (i.e., viscosity, circumstantiality, humorlessness/sobriety), raising the idea that the BAP might account for several features of the epileptic personality (see Table 3).
| Social/communication traits reminiscent of the BAP | |
| Anger | Increased temper, irritability |
| Viscosity | Excessive social cohesion or interpersonal clinging, stickiness; tendency to draw out interpersonal encounters; tendency toward repetition |
| Circumstantiality | Loquacious, pedantic; overly detailed, peripheral |
| Humorlessness; sobriety | Ponderous concern; humor lacking or idiosyncratic |
| Restricted and repetitive behaviours and interests reminiscent of the BAP | |
| Obsessionalism | Ritualism, perseveration, orderliness, compulsive attention to detail |
| Hypermoralism | Attention to rules with inability to distinguish between significant and minor infractions; desire to punish offenders |
| Hypergraphia | Extensive diaries, detailed notes, writing autobiography or novel |
| Religiosity | Holding deep religious beliefs; multiple conversions |
| Philosophic interests | Newly developed interest in metaphysic or moral speculations, theories |
| Non-BAP traits | |
| Emotionality | Deepening of emotions, sustained intense affect |
| Elation, euphoria | Grandiosity, exhilarated mood; diagnosis of bipolar disorder |
| Sadness | Discouragement, tearfulness, self-depreciation; diagnosis of depression, suicide attempts |
| Aggression | Overt hostility, rage attacks, violent crimes, murder |
| Paranoia | Suspicious, overinterpretive of motives and events; diagnosis of paranoid schizophrenia |
| Dependence, passivity | Cosmic helplessness, at hands of “fate” |
| Altered sexual interests | Hyposexualism, hypersexuality, fetishism, transvestism, exhibitionism |
| Guilt | Tendency to self-scrutinize and self-recriminate |
| Sense of personal destiny | Events given highly charged significance |
- Abbreviations: BAP, broader autism phenotype.
The concept of the epileptic personality was abandoned in the research literature partly due to lack of evidence for its specificity to epilepsy compared with other patient groups [51]. If, however, the epileptic personality reflects aspects of the BAP mixed with mood and psychotic symptoms (Table 3), this lack of specificity is unsurprising. Moreover, it is consistent with the notion that, as an endophenotype of autism, the BAP can be used as a “trans-diagnostic” marker of shared genetic, neural, cognitive, or environmental factors that increase susceptibility to both seizures and ASD and can therefore help to uncover shared mechanisms of epilepsy and ASD [10].
The transdiagnostic approach is currently not well recognized as a way to understand the co-occurrence of epilepsy and ASD. However, it has strong promise given that these conditions have similar genetic architecture (i.e., complex interplay of multiple genetic and environmental susceptibility factors [52, 53]) to conditions in which the transdiagnostic approach has proven informative, such as depression, schizophrenia, and bipolar disorder [54, 55]. In contrast, the bulk of our understanding of shared mechanisms of epilepsy and ASD is derived from rare single gene disorders and from drawing parallels between causal pathways implicated in epilepsy independent of ASD, and vice versa [56]. Consequently, it is unclear to what extent the shared mechanisms of epilepsy and ASD identified to date [10] are implicated in the majority of individuals with co-occurring epilepsy and ASD. Using the BAP as a transdiagnostic marker may help to overcome the limitations of current approaches, providing a more accessible and broadly applicable approach that may eventually aid the development of targeted therapies that address symptoms of both conditions [57-59].
Importantly, the core domains of autism have been found to be partly genetically dissociable [60], with studies investigating genetic underpinnings of circumscribed autism features rather than the diagnosis as a whole proving more effective [61]. Consequently, when applying a transdiagnostic approach to interrogate shared mechanisms of epilepsy and ASD, focusing on more circumscribed aspects of the BAP is likely to be more effective. The uneven profile of BAP domain expression in PWE in the current study suggests that targeting pragmatic language differences may be particularly beneficial to uncovering shared mechanisms, given their prominence in our sample. In addition, it is worth noting that sex differences in the level of expression of BAP traits in the current study support the call for separate evaluation of ASD mechanisms in males and females in light of the growing evidence of distinct ASD susceptibility factors across the sexes [62, 63].
A principal strength of this study is the inclusion of a heterogeneous sample of PWE, notably including first-seizure patients in whom the presence of the BAP provides strong support for ASD-susceptibility mechanisms independent of repeated seizures. Moreover, as noted in the introduction, in the context of a cross-sectional study, examining the BAP in adults with epilepsy may provide a clearer picture of the co-occurrence of the BAP and epilepsy, relevant to both children and adults. A key limitation of this study is that our sample of local community members was recruited through convenience sampling and therefore was not representative of the general population. Consequently, we compared the rate of the BAP in PWE with published estimates from large population-based samples. Although differences across the samples (e.g., education, cultural background) may have influenced our findings, the effect of education was likely mitigated by the IQ of our sample falling within the normal range. In other words, the lower educational level in PWE was likely a consequence of the challenges of living with epilepsy rather than of differences in general intellect. A further limitation is the relatively small sample size. Replication studies will thus be particularly important.
5 Conclusion
This study found an elevated rate of the BAP in a heterogeneous sample of PWE, similar to that in a community sample of ASD-relatives, which was unrelated to the secondary effect of repeated seizures. This suggests that the BAP in PWE may represent a transdiagnostic marker of shared etiological mechanisms of epilepsy and ASD, with pragmatic language features providing a focus area for subsequent investigations. The findings also have important implications for the clinical management of psychosocial difficulties in children and adults with epilepsy.
Author Contributions
Annie E. Richard contributed to project conceptualization, formal analysis, funding acquisition, investigation, methodology, visualization, writing–original draft. Ingrid E. Scheffer and Sarah J. Wilson contributed to project conceptualization, funding acquisition, methodology, resources, supervision, and writing–review and editing.
Acknowledgments
This project received financial support from the National Health and Medical Research Council of Australia Project grants (APP1044175, 2013-2016; APP1098255, 2016-2021), Practitioner Fellowship (APP1104831) and Australian Postgraduate Awards.
Ethics Statement
This study was approved by the Research Ethics Committee of Austin Health.
Consent
All participants provided written informed consent.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.




