A Case Series and Literature Review on Zanubrutinib Therapy for the Treatment of Relapsed/Refractory Immune Thrombocytopenia
Funding: This work was supported by the National Natural Science Foundation of China (82070120, 81370615, 81600097).
ABSTRACT
Background
Immune thrombocytopenia (ITP) is an acquired autoimmune disease characterised by low platelet count. Treatment discontinuation or heterogeneity in the pathogenesis of ITP heightens the occurrence of relapsed or refractory ITP. Bruton's tyrosine kinase (BTK) has emerged as a promising target for autoimmune disorders.
Case
In this case series, we have explored the efficacy and safety of Bruton's tyrosine kinase inhibitors (BTKi) in treating relapsed/refractory ITP, by retrospective analysis of the diagnostic history and efficacy of four patients with relapsed/refractory ITP who attended the Affiliated Cancer Hospital of Zhengzhou University and were treated with BTKi. All four patients received > 4 lines of ITP treatment and did not respond to splenectomy or other interventions before and after treatment with BTK inhibitor. After adjusting to the treatment with BTKi, one patient achieved a complete response, and two patients achieved a partial response. All three patients achieved sustained remission with platelet counts of > 50 × 109/L maintained for 1045, 390 and 334 days, respectively. Another patient died of intracranial haemorrhage due to a decline in the platelet count after discontinuation of the drug, and the duration of sustained remission before discontinuation of the drug was 120 days. Four patients had no significant abnormalities in the functions of the liver and kidney monitored during the treatment period.
Conclusion
For patients with relapsed/refractory ITP, BTK inhibitor therapy can be considered as an option, with promising preliminary efficacy and safety. However, more clinical trials are needed to verify the exact data.
1 Introduction
Immune thrombocytopenia (ITP) is an acquired autoimmune disease characterised by low platelet count (< 100 × 109/L), which occurs due to impaired production or immune-mediated destruction of platelets [1]. ITP causes bleeding in the skin and the mucous membranes and even leads to fatal intracranial hemorrhage. The annual incidence of ITP is about ~5/100 000 in children and ~2/100 000 in adults and is more prevalent in geriatric females [2]. Although benign in nature, the Global Burden of Disease Survey on ITP (ITP World Impact Survey, I-WISh) considered ITP as a disorder that could severely impact health-related quality of life (HRQoL) [3]. The therapeutic options for ITP include intravenous immune globulin (IVIG), glucocorticoids (GC), including methylprednisolone, prednisolone (Pred), dexamethasone (DXM), mycophenolate mofetil and anti-RhD immunoglobulin (anti-D) [4], which focuses mainly on reducing bleeding by interfering with platelet destruction to rapidly increase platelet counts [1, 5-7]. Treatment discontinuation or heterogeneity in the pathogenesis of ITP heightens the occurrence of relapsed or refractory ITP.
At present, second- and third-line therapies are widely employed in the treatment of relapsed or refractory ITP. Second-line therapy includes thrombopoietic agents (such as Recombinant human TPO [rhTPO], TPO receptor agonists [TPO-RA], rituximab [RTX] and rhTPO in combination with rituximab), immunosuppressants (such as azathioprine) [8] and splenectomy [9]. Third-line therapy includes low-dose decitabine (DAC), all-trans retinoic acid (ATRA) in combination with danazol and immunosuppressive therapies (e.g., vincristine and cyclosporine A). Apart from autoantibody-mediated platelet destruction, IgG antibodies also target platelets via Fcγ receptors (present on reticular cells and Kupffer cells) and Ashwell-Morell receptors (present on hepatocytes). In this context, novel therapies are being explored to improve the rate of sustained remission [6, 10, 11].
Bruton's tyrosine kinase (BTK) is widely expressed in many cells and plays a key role in B cell maturation, antibody production and Fcy receptor-mediated signalling pathways [12, 13]. Inhibition of BTK, especially in autoimmune disorders, has the potential to decrease Fcy receptor-mediated macrophage function and reduce autoantibody production. The activity of selective BTK inhibitors in collagen-induced arthritis and other rodent models of inflammation has been demonstrated in several studies [14-16].A study by Xu et al. explored the effects of BTKi in rodent models of arthritis and immune hypersensitivity demonstrating significant anti-inflammatory and bone-protective effects [17]. Another recent study by Akasaka et al. demonstrated the therapeutic effects of a different BTKi in experimental models for arthritis and demonstrated effective inhibition of BTK enzyme activity and suppression of various immune-related signaling pathways [18]. These evidences provide a rationale for the use of BTKi in autoimmune diseases.
Zanubrutinib, a second-generation irreversible BTKi, inhibits the kinase activity by binding covalently to the cysteine residue in the BTK active binding site [19]. It has more target occupancy and less off-target binding compared to the first clinically effective covalent BTK inhibitor, Ibrutinib [19, 20]. The enhanced selectivity of Zanubrutinib may be due to its higher reversible binding capability. It showed higher values of IC50 and no inhibition of kinase activities compared to Ibrutinib. The oral absorption and target occupancy of Zanubrutinib is also greater than Ibrutinib. Zanubrutinib has complete BTK occupancy in mononuclear cells. Cardiac side effects and atrial fibrillation were reported to be lower with Zanubrutinib treatment as compared to Ibrutinib [21]. Incidence of other adverse events, such as bleeding and haemorrhage, is lower with Zanubrutinib [22].
Herein, we report four cases treated with BTK inhibitor (BTKi), Zanubrutinib for the treatment of relapsed/refractory ITP. We also conducted a review of the relevant literature to further explore the clinical feasibility of BTKi for treating ITP.
2 Case Reports
2.1 Case 1
Patient 1, a 61-year-old male, diagnosed with thrombocytopenia for > 4 years without any family history of the condition, was hospitalised in August 2022. The patient presented with gingival bleeding with petechiae on both lower limbs in May 2018. In July 2018, the patient's routine blood test revealed white blood cells (WBC) of 4.6 × 109/L, haemoglobin of 119 g/L and platelet count of 3 × 109/L. The bone marrow smear showed active bone marrow hyperplasia, 127 megakaryocytes, 25 classified megakaryocytes, 22 granulocytes, 2 plate giants and 1 naked nucleus. Bone marrow biopsy showed bone marrow hyperplasia as normal (about 45%), with many megakaryocytes and predominantly lobulated nuclei. Reticular fiber staining was seen as MF-0 grade. Upon further examination, the patient was diagnosed with ITP and was treated with a high dose of DXM ([HD-DXM] 40 mg, 4 days). The patient was discharged from the hospital after a repeat platelet test (66 × 109/L).
After discharge, oral pred was gradually tapered off and gingival bleeding reappeared on May 1, 2021, and a gradual decrease in platelet count (3 × 109/L) and was transfused with platelets. Upon hospital discharge, oral methylprednisolone (20 mg, q12h) was prescribed 1 month after the discontinuation of oral pred. Between May 2021 and August 2022, the patient underwent platelet transfusions and received various treatments. The results are detailed in Table 1.
| Case number | Timeline | Clinical events | Laboratory data | Treatment | Outcome |
|---|---|---|---|---|---|
| Case 1 | 25 May 2021 | Patient re-hospitalised | Platelet count: 1 × 109/L |
|
No improvement in condition |
| 3 June 2021 | Admitted to hospital with thrombocytopenia | Platelet count: 2 × 109/L |
|
|
|
| July 2022 | Thrombocytopenia re-diagnosed | Platelet count: 1 × 109/L |
|
No significant increase in platelet count | |
| August 2022 | Admitted to hospital with thrombocytopenia | Platelet count: 6 × 109/L | DAC (6 mg × 3 days + pred) and Herombopag along with HD-DXM, IVIG, and Avatrombopag | No significant improvement in the platelet count | |
| Case 2 | 2 May 2018 and 23 May 2018 | Lymphoma | Platelet count: 25 × 109/L | R-CHOP + Ibrutinib (280 mg, q.d) | Platelet count repeatedly decreased |
| 4 July 2018 | Lymphoma | Platelet count: 30 × 109/L | Peripheral blood haematopoietic stem cells infusion |
|
|
| 18 December 2018 | Thrombocytopenia | Platelet count: 8 × 109/L | Amifostine (0.4 g d1-d5 discontinued for 2 days) was given for 4 cycles | No significant improvement in the platelet count | |
| 18 September 2019 | Thrombocytopenia | Platelet count: 5 × 109/L | RTX (100 mg, q.wk × 4 times) | No sign of lymphoma recurrence | |
| June 2021 | Intracranial haemorrhage due to thrombocytopenia | Platelet count: 1 × 109/L | HD-DXM, pred, IVIG, RTX, and Eltrombopag | Limited effectiveness | |
| Case 3 | July 2017 | Low platelet count | Platelet count: 30 × 109/L | Pred (40 mg) for > 2 months and was gradually tapered off | The platelet count rose to normal levels and then decreased gradually |
| October 2017 | Low platelet count | Platelet count: 9 × 109/L | DXM (12 mg × 3 days) and rhTPO (15 000 IU × 14 days) | Platelet count increased to 30 × 109/L | |
| December 2017 | Thrombocytopenia re-diagnosed | Platelet count: 12 × 109/L | The patient was recommended splenectomy | The patient refused splenectomy | |
| January 2018 | Low platelet count | Platelets decreased to 4 × 109/L |
|
|
|
| February 2018 | Low platelet count | Platelets decreased to 8 × 109/L | Splenectomy | No significant improvement in the platelet count | |
| 12 March 2018 | Thrombocytopenia | Platelet count: 5 × 109/L | Amifostine (0.40 g day 1-day 5 off 2 days, 4 cycles) and recombinant human interleukin-11 | Platelet count increased to 35 × 109/L | |
| 10 November 2018 | Low platelet count | Platelet count: 3 × 109/L | Platelet transfusion | Platelet count increased to 47 × 109/L | |
| 17 March, 2019 | Low platelet count | Platelet count: 3 × 109/L | Platelet transfusion | Platelet count increased to 66 × 109/L | |
| 5 May 2019 | Patient was admitted to the hospital due to bleeding from the oral cavity and posterior pharyngeal wall | Platelet count: 4 × 109/L | Platelet transfusion | Platelet count increased to 34 × 109/L | |
| 16 May 2019 | Left temporal lobe and frontal occipital lobe haemorrhage occurred | Platelet count: 2 × 109/L | HD DXM and IVIG |
|
|
| April 2020 | Patient admitted to the hospital due to oral and posterior pharyngeal haemorrhage | Platelet count: 3 × 109/L | Oral Eltrombopag 75 mg q.d (from April 2020 to June 2021) | No significant improvement in the platelet count | |
| 30 June 2021 | Low platelet count | Platelet count: 3 × 109/L | Avatrombopag 40 mg q.d | Platelet count increased to 48 × 109/L | |
| Case 4 | 8 December 2017 | Thrombocytopenia | Low platelet count | IVIG treatment | Platelet count increased to normal but decreased again after discontinuation of the drug |
| January 2018 | Thrombocytopenia | Low platelet count | HD-DXM, standard Pred, Danazol, Levamisole, and Interleukin-11 | The efficacy could not be maintained | |
| 17 January 2018 | Thrombocytopenia | Platelet count: 1 × 109/L | RTX (100 mg, q.wk × 4 times) | No improvement in platelet counts | |
| February 2018 | Thrombocytopenia | Platelet count: 1 × 109/L | Splenectomy | No improvement in platelet counts | |
| 2018–2022 | Multiple hospital admissions for thrombocytopenia | Platelet count: 0–3 × 109/L | Hormonal therapy and rhTPO | No satisfactory outcomes | |
| 20 April 2022 | Thrombocytopenia | Platelet count: 2 × 109/L | SYK inhibitor (TQB3473 clinical trial) | On June 21, 2022, he withdrew from the group due to poor efficacy | |
| June–July 2022 | Thrombocytopenia | Platelet count: 1–5 × 109/L | Oral Chinese medicine | No significant improvement in platelet counts | |
| 14 July 2022 | Thrombocytopenia | Platelet count: 0 × 109/L | Herombopag (7.5 mg, q.d) | Platelet count: 2 × 109/L | |
| 18 July 2022 | Intracranial haemorrhage | Platelet count: 1 × 109/L | HD-DXM 40 mg × 4 days |
|
|
| 20 July 2022 | Thrombocytopenia and thrombosis | Platelet count: 23 × 109/L | Herombopag (7.5 mg, q.d) |
|
|
| 20 July 2022 | Intracranial haemorrhage and patient went into coma | Platelet count: 23 × 109/L |
|
|
|
| 24 July–6 August 2022 | Thrombocytopenia | Platelet count: 1 × 109/L | IVIG 0.4 g/day × 5 days, HD-DXM 40 mg × 4 days, rhTPO 15000 IU × 14 days, and Herombopag 7.5 mg q.d taken orally | No significant increase in platelets | |
| 7 August 2022 | Thrombocytopenia | Platelet count: 1 × 109/L | Oral Avatrombopag (40 mg, q.d) |
|
- Abbreviations: DAC, decitabine; HD-DXM, high dose dexamethasone; IU, international unit; IVIG, intravenous immune globulin; Pred, prednisolone; q.d, ever day; q.wk, every week; q12h, every 12 h; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine sulfate and prednisone; R-CHOP-M rituximab, cyclophosphamide, doxorubicin, vincristine sulfate, prednisone and methotrexate; rhTPO, recombinant human thrombopoietin; RTX, rituximab; SYK, spleen tyrosine kinase; WBC, white blood cells.
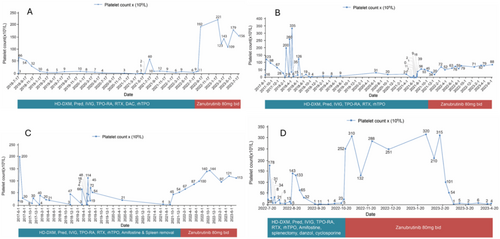
The patient was initiated with Zanubrutinib (80 mg, bid) from August 2022. Patient outcomes from August 2022 to July 2023 after initiation of Zanubrutinib treatment has been described in Table 2. From February to July 2023, the number of days in which platelet values were maintained at 50 × 109/L or higher was 334 days (Figure 1A).
| Case number | Status of patients before treatment with Zanubrutinib | Timeline | Outcome after treatment with Zanubrutinib |
|---|---|---|---|
| Case 1 |
Low platelet count: 11 × 109/L White blood cells: 5.07 × 109/L Haemoglobin: 62 g/L |
15 August 2022 | Gradual increase in platelet counts after initiation of Zanubrutinib (80 mg twice a day) |
| 7 September 2022 | Platelet count increased to 192 × 109/L | ||
| 26 February 2023 |
|
||
|
February–July 2023 20 July 2023 |
|
||
| Case 2 | Low platelet count | 26 June 2021 | Zanubrutinib was initiated which increased platelet count to > 30 × 109/L |
| November 2021–June 2023 |
|
||
| Case 3 | Low platelet count | 10 September 2021 | Platelet count was 8 × 109/L; Patient re-admitted to hospital; Zanubrutinib (80 mg bid.) was initiated |
| January 2022–April 2023 | Platelet count fluctuated between 97 × 109/L and 144 × 109/L after the initiation of Zanubrutinib | ||
| Till July 2023 | Platelet count remained above > 50 × 109/L continuously for 1045 days | ||
| Case 4 | Platelet count decreased to 1 × 109/L, after discontinuation of Avatrombopag | 3 October 2022 | Zanubrutinib was initiated, which improved platelet count to 252 × 109/L and stabilised between 110 × 109/L and 340 × 109/L |
| 5 March 2023 |
|
- Abbreviations: HD-DXM, high dose dexamethasone; IVIG, intravenous immune globulin; Pred, prednisolone; RTX, rituximab.
2.2 Case 2
Patient 2, a 49-year-old male with thrombocytopenia for > 4 years, was admitted to the hospital on 1 July 2021. He presented with skin ecchymosis with a low platelet count of 16 × 109/L in August 2017. Blood tests revealed anti-glycoproteins (GPs) autoantibodies was positive, and the patient was consequently diagnosed with ITP with bone marrow examination. Treatment with HD-DXM and IVIG increased the platelet count to normal levels.
The patient's medical history indicated a diagnosis of diffuse large B-cell lymphoma, identified following a right epididymectomy due to right testicular swelling in January 2018. During follow-up, the platelet count was 23 × 109/L and PET-CT revealed multiple metabolically active soft tissue nodules and masses at various sites, suggesting lymphoma infiltration. No abnormalities were detected in the bone marrow smear and immunophenotyping. In 7 February 2018, the patient underwent four courses of R-CHOP-M (rituximab, cyclophosphamide, doxorubicin, vincristine sulfate, prednisone and methotrexate) chemotherapy regimen. After two courses of chemotherapy, a CR was assessed through PET-CT. From May 2018 to June 2021, the patient received various treatments to treat lymphoma and thrombocytopenia, the details of which are described in Table 1. In June 2021, due to limited effectiveness demonstrated by previous treatments, the treatment was modified to initiate Zanubrutinib (80 mg, bid, oral). Patient outcomes during the treatment with Zanubrutinib from June 2021 to June 2023 have been described in Table 2.
Throughout November 2021 to June 2023 period, the platelet count remained steadily above 50 × 109/L for a continuous period of 390 days (Figure 1B). This case highlights the potential of zanubrutinib as a valuable therapeutic option for managing ITP, particularly in patients with a complex medical history that includes haematologic malignancies.
2.3 Case 3
Patient 3, a 31-year-old, dealing with thrombocytopenia for > 1.5 years received in-patient care in March 2018. In June 2017, the patient's platelet count was 19 × 109/L with scattered bleeding spots. Bone marrow puncture and other examinations were performed, and the patient was diagnosed with ITP. HD-DXM (40 mg × 4 days) and rhTPO (15 000 IU × 7 days) raised platelets to 200 × 109/L but were lowered to 30 × 109/L in July 2017. Between July 2017 to June 2021, the patient received various treatments to manage ITP. The results of each treatment are described in Table 1. The patient's platelet count was 8 × 109/L in September 2021, which led to his re-hospitalisation. On 10 September 2021, Zanubrutinib (80 mg, bid) was initiated. The patient outcomes during the treatment with Zanubrutinib are described in Table 2. Till July 2023, the patient's platelet count remained above > 50 × 109/L continuously for 1045 days (Figure 1C).
2.4 Case 4
Patient 4 was a 51-year-old female who was presented initially in March 2018 with a history of ITP for more than 11 years. The patient was diagnosed with ITP in 2011 and treated with Pred, HD-DXM, Danazol, Caffeic acid tablets, Cyclosporine and so on but showed unsatisfactory outcomes. Bone marrow examinations showed active proliferation with megakaryocytes and normal chromosomes. Platelet count returned to normal after the commencement of IVIG but decreased after its discontinuation. Throughout 2017–2022, the patient received multiple treatment regimens to improve the platelet counts. The details of each treatment are described in Table 1. In October 2022, the patient initiated oral Zanubrutinib (80 mg, bid), as no improvement in platelet count was observed with the previous treatment. The patient outcome during treatment with Zanubrutinib is detailed in Table 2. The patient died in April 2023 due to an intracranial haemorrhage (Figure 1D).
3 Discussion and Literature Review
Over the decades, many scientific works have expanded the knowledge and understanding of autoimmune diseases including ITP. Owing to the ineffectiveness of current therapies against ITP and heterogeneity, the discovery of novel therapies is crucial to curbing this autoimmune disorder. Among various targets, BTK could be a viable target due to various functions, including regulation of B-cell maturation, proliferation, differentiation and apoptosis. The therapeutic strategy of BTK inhibition has become crucial, especially in treating various haematologic malignancies including diffuse large B-cell lymphoma, condylomatous lymphoma, and lymphoblastic leukaemia [23]. Furthermore, the kinase nature of BTK is essential in maintaining the homeostasis of many immune cells, which could effectively treat autoimmune disorders [24-29]. However, few studies have reported the use of BTKis, including ibrutinib, rilzabrutinib, orelabrutinib, zanubrutinib and Zanubrutinib for ITP. In a study, Yu Tianshu et al. studied the effectiveness of BTKi in the inhibition of B-cell proliferation, pro-inflammatory cytokine secretion and plasma cell differentiation (activated via BCR pathway) and could inhibit FcTR-mediated phagocytosis in ITP active mice model [30]. This preclinical study provides a strong basis for the efficacy of BTKi against ITP disease. In a clinical setup, Hampel et al. analysed patients with chronic lymphocytic leukaemia (CLL) and treated them with ibrutinib. Among the 193 patients with CLL, 29 CLL patients had autoimmune cytopenias (AICs) prior to ibrutinib treatment. Patients who received ibrutinib did not show any major variation in EFS or OS based on their prior history of AIC. This indicates that ibrutinib possibly nullified the unfavourable outcome associated with AIC [31].
Rilzabrutinib, an oral, reversible, covalent BTKi used for treating immune-mediated disorders, is currently being studied in a phase 3 trial and was evaluated for its inhibitor effect in 60 patients with relapsed/refractory ITP. The median platelet count was reported to be 15 × 109/L with a duration of 6.3 years and received 4 lines of ITP therapy. On a median of 167.5 days of treatment, 24 patients (40%) treated with rilzabrutinib (400 mg, bid) achieved a platelet response of 50 × 109/L at 11.5 days. This study indicates the safety and clinical feasibility of BTKi for the treatment of ITP [10].
Orelabrutinib is another BTKi used for inhibiting multiple signaling pathways in immune-mediated disorders. In a preclinical evaluation, orelabrutinib exhibited a significant reduction/inhibition of CD69 & CD86 (expressed in isolated polymorphonuclear cells of both patients with ITP and healthy humans). The administration of orelabrutinib significantly increased the platelet counts in the ITP mice model [30]. In another randomised open-label phase II study, orelabrutinib was evaluated for its safety, efficacy and tolerability in persistent/chronic ITP patients. The proportion of patients who achieved at least two consecutive platelet counts ≥ 50 × 109/L without rescue medication in the 4 weeks before the elevated platelet count was considered as the primary endpoint. Overall, patients who achieved the primary endpoint were 36.4% (12/33) of which 40% (6/15) were from the 50 mg arm, and 22.2% (4/18) were from the 30 mg arm. Two patients achieved the endpoint after transferring to the 50 mg arm. This study showed that the patients treated with orelabrutinib exhibited better efficacy especially those who were previously treated with GC/IVIGs [32].
Zanubrutinib is a new-generation BTKi that acts by covalently binding to the cysteine at site 481 of the BTK protein and thereby inhibiting its tyrosine phosphorylation at site 223. It is currently approved for the treatment of adult mantle cell lymphoma (MCL), adult chronic lymphocytic leukaemia (CLL)/small lymphocytic lymphoma (SLL) and Wahl's macroglobulinemia (WM). In our previous case study, we reported a case of a 15-year-old patient with Evans syndrome with platelet elevation after treatment with zanubrutinib, which could be used for the treatment of ITP [33]. In China, clinical studies on zanubrutinib and eltrombopag were considered as second-line treatments for refractory ITP (NCT05369377). Many clinical trials evaluating zanubrutinib as montherapy (NCT05214391)/(NCT05279872)/(NCT05199909) or in combination with high-dose dexamethasone (NCT05369364) or rituximab (ChiCTR2200057058) were ongoing with more focus on its effectiveness towards ITP. It could provide new therapeutic horizons for patients with relapsed/refractory ITP.
In the present case study, all four patients who were treated with different ITP treatment regimens reported significant improvement in platelet counts following treatment with Zanubrutinib. These patients did not respond to splenectomy or other interventions. Overall, one patient achieved CR, and two patients achieved partial response (PR) with sustained remission after Zanubrutinib intervention. The blood analysis revealed that the platelet counts of three patients were maintained above 50 × 109/L for 1045, 390 and 334 days, respectively. One patient died of intracranial haemorrhage due to platelet decline after discontinuation of the drug. However, the deceased patient stayed in a sustained remission for 120 days before treatment discontinuation. During the treatment, no significant abnormalities in liver or kidney functions were reported, suggesting that Zanubrutinib showed a favourable safety profile in these cases. These findings suggest that BTK inhibitors, such as Zanubrutinib, may offer a new therapeutic option for patients who have exhausted traditional treatment options. However, several gaps remain in our understanding of the long-term efficacy and safety of Zanubrutinib for ITP. The study involves only four patients, limiting the generalisability of the findings. A larger cohort would yield more reliable data on the effectiveness and safety of the treatment. The previous treatment regimen variations may further complicate the analysis of Zanubrutinib's effectiveness as a monotherapy. In addition, although no significant abnormalities in liver and kidney function were reported during treatment, long-term monitoring for adverse effects of BTK inhibitors remains crucial to understand potential risks associated with prolonged use.
4 Conclusion
Zanubrutinib demonstrated sustained remission and maintained platelet levels. These findings indicate that Zanubrutinib can be a promising option for treating ITP. However, treatment with BTK inhibitors is still in the exploratory phase and requires further investigation through clinical trials to accurately assess its efficacy and safety in chronic or persistent ITP.
Author Contributions
Hu Zhou designed the study. Bingjie Ding analysed the data and wrote the manuscript. Mengjuan Li, Xuewen Song, Yuanyuan Zhang, Ao Xia and Jingyuan Liu collected and collated the clinical data. Hu Zhou and Liu Liu reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant no. 82070120, 81370615, 81600097).
Ethics Statement
The study was approved by the Medical Ethics Committee of Henan Cancer Hospital.
Consent
Informed consent was obtained from all patients enrolled in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.




