Efficacy of olanzapine for quality of life improvement among patients with malignant tumor: A systematic review
Abstract
Background
Cancer patients always experience an ongoing deterioration in health-related quality of life (HRQoL). There is a strengthening awareness of health care professionals of taking HRQoL, which is a patient-reported outcome measures (PROMs), into consideration when they make an adequate selection in clinical practice. Olanzapine, an antipsychotic agent, has been demonstrated to be a safe and effective agent in improving cancer-related symptoms.
Aim
To review the efficacy and safety of olanzapine in improving HRQoL among adults with malignant tumor.
Methods
Eligible studies were retrieved from an electronic database search of the Cochrane, Medline, CINAHL plus, Pubmed, Embase, PsycINFO, Web of Science, and Scopus. The methodological quality of selected studies was evaluated, and the relevant data were extracted and synthesized.
Results
While studies differed in target population, olanzapine-based treatment regimen, and HRQoL measurement tools, results have shown that olanzapine has a positive impact on cancer patients' general HRQoL status, functional outcomes, and/or symptoms improvement. Besides, no serious toxicities attributable to olanzapine were observed in all studies included.
Conclusion
While further studies are needed especially which adopted the HRQoL as primary outcome through comprehensive measures, olanzapine could still be recommended in the palliative care.
1 INTRODUCTION
For chronic and sometimes incurable diseases, with interventions that could have toxic and side effects and long-term consequences, it is especially important that clinical decisions influencing patient outcomes reflect the patient's own perspective.1 Patient-reported outcome measures (PROMs), which facilitates a systematic and comprehensive approach to patient assessment,2 are increasingly being used in routine cancer care. Reviewing PROMs with cancer patients increases communication between health care professionals and patients, identification problems that are often overlooked, and satisfaction with care.3
PROMs encompass many domains, particularly one of which is health-related quality of life (HRQoL).1 As it was concluded by the National Cancer Institute (NCI)'s Cancer Outcomes Measurement Working Group (COMWG), an HRQoL measure is patient reported, and it involves the patient's subjective assessment or evaluation of important aspects of his or her well-being.1 Although there have not been reached a consensus, the “well-being” always captures physical, psychological (including emotional and cognitive), and social functioning at a minimum.4
Cancer patients always experience an ongoing deterioration in HRQoL. There is growing recognition that improvements in HRQoL may instead serve as an alternative but equally important clinical outcome to survival in advanced cancer patients. Besides, an increasing body of evidence supports that HRQoL are predictive of survival in metastatic and advanced cancer. Some studies even suggest that HRQoL may be better prognostic indicators of survival than conventional clinical parameters for malignancies patients.5 For a patient's baseline HRQoL can predict overall survival (OS) across different cancer types.6 As a result, there is a strengthening awareness among health care professionals of taking the HRQoL into consideration when they make an adequate selection in clinical practice.
Olanzapine, an antipsychotic agent, blocks multiple neurotransmitters: serotonin, at 5H2a, 5H2c, 5H3, and 5HT6 receptors, dopamine at D1, D2, D3, and D4 brain receptor, catecholamines at alpha 1 adrenergic receptors, acetylcholine at muscarinic receptors, and histamine at H1 receptors.7 In previous studies, olanzapine has been demonstrated to be a safe and effective agent in improving cancer-related symptoms, including chemotherapy-induced nausea and vomiting (CINV),7-16 cancer pain,17, 18 cancer-related anorexia,19, 20 and cachexia.21 The efficacy of olanzapine in improving HRQoL of cancer patients was demonstrated in several studies as a secondary endpoint.7, 9, 10, 12, 14, 19, 22 The objective of this review is to evaluate and to summarize the effect and safety of olanzapine in improving HRQoL among adults with malignant tumor.
2 METHODS
No registered protocol exists for this review. The review was conducted according to the PRISMA guidelines.23
2.1 Eligibility criteria
Inclusion and exclusion criteria were used in developing a systematic literature search strategy, and also in judging literatures. Inclusion criteria: (1) original research articles published in peer-reviewed journals; (2) participants with a histological or cytological confirmation of malignant tumor; (3) interventions containing olanzapine applied orally; (4) HRQoL was adopted as an outcome; (5) HRQoL was evaluated using self-assessment questionnaires; and (6) comparative studies or epidemiological studies (prospective observational, cohort, cross-sectional or retrospective). Exclusion criteria: (1) patients with a history of psychiatric disorders such as schizophrenia, bipolar disorder, or dementia; (2) patients with a history of antipsychotic drugs in-take; (3) reference was reviews, case studies, or not a full-text paper (ie, conference literatures, protocol publication, abstracts, letters, or comments); and (4) non-English language articles. Besides, a duplicate publication with same sample and outcomes as the previous study will also be excluded.
2.2 Searching strategy
An electronic database search was conducted on 11 June 2018 using the Cochrane, Medline, CINAHL plus, Pubmed, Embase, PsycINFO, Web of Science, and Scopus. The Medical Subject Heading (MeSH) terms and textwords were combined. (1) MeSH: neoplasms or text words: cancer or malignanc* or malignant neoplas* or malignant tumor; (2) text words: olanzapine or zyprexa or zolafren; (3) MeSH: quality of life or text words: HRQoL or QoL or health-related quality of life or quality of life or life quality; and (4) (1) and (2) and (3).
2.3 Article screening and selection
After exporting the results of literatures searching into EndNote software and removing the duplicates (by author, title, journal, and publication date), two reviewers (Ji and Cui) screened for relevance independently based on the title and abstract. Then, full text reviewed by them followed. The suitability is reviewed according to the inclusion and exclusion criteria listed above. Discrepancies for final selections between Ji and Cui were resolved by consensus.
2.4 Data extraction and synthesis
Data extraction and the assessment of the quality of the studies were conducted independently by two authors (Ji and Xi). The Johanna Briggs Institute (JBI) critical appraisal checklists24-26 were adopted in this review to assess the trustworthiness, relevance, and results of studies (Table 1). Data which was recorded from articles in the final set includes: (1) study characteristics (author, year of publication, country of origin, study design); (2) sample demographics (target population, sample size in each group, age range and mean age, chemotherapy); (3) treatment of olanzapine; (4) instrument used to measure HRQoL; (5) follow-up time; and (6) summary of findings. Meta-analysis of the data was not feasible because of heterogeneity in the designs, interventions, and outcome measures used in studies identified. Therefore, a descriptive synthesis of data was carried out in this review.
| Author | Study Design | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mukhopadhyay S. | RCT | Yes | Unclear | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| James E. | Prospective cohort study | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | NA | Yes | / | / |
| Nikbakhsh N. | Randomized study | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | / | / | / | / |
| Mizukami N. | RCT | Yes | Unclear | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Navari R.M. | Randomized study | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | / | / | / | / |
| Tan L. | Randomized study | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | / | / | / | / |
- Note. Columns 1-13 are answers to the questions in The Johanna Briggs Institute (JBI) critical appraisal checklists (references 24-26).
- Abbreviation: NA, not applicable; RCT, randomized controlled trial.
3 RESULTS
3.1 Study characteristics
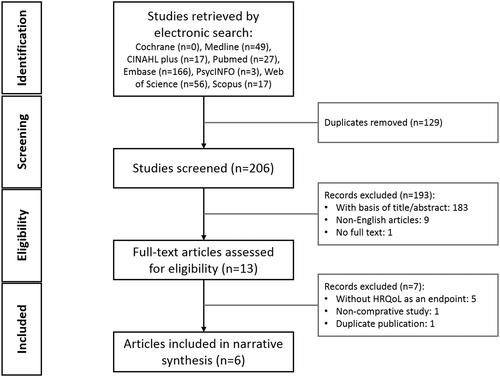
There were 335 studies identified based on our searching strategy (number of relevant studies identified in different databases is shown in Figure 1). After duplicates were removed, we screened the titles and abstracts of the remaining 206 studies according to the inclusion and exclusion criteria. There were 13 full-paper studies being further reviewed, among which seven were excluded owing to noncomparative study design, having not included HRQoL as an endpoint, or being a duplicate publication. In the end, six articles were included in this review, two of which are randomized controlled trials (RCT),12, 14 three had quasi-experimental designs,7, 19, 22 and one is cohort study9 (Table 2).
| Author | Year | Country | Study Design | Target Patients | Sample Size | Age (Mean) | Chemotherapy | Treatment | Follow-Up Time |
|---|---|---|---|---|---|---|---|---|---|
| Mukhopadhyay S. | 2017 | India | RCT | Malignancy | 100 (50/50) | 18-80 (55.04/53.66) | Platinum-based HEC or MECa | Olanzapine 10 mg p.o. Qd (D1-5); Prophylactic antiemetic regimen: (palonosetron, dexamethasone, rescue drug: metoclopramide)b. | 8 days (up to 10 days) |
| James E. | 2017 | India | Prospective cohort study | Malignancy | 42 (21/21) | 45-62 (51.4/54.9) | HEC or MECa | Olanzapine 10 mg p.o. Qd (D1-5); Prophylactic antiemetic regimen: (palonosetron or ondansetron and dexamethasone)b. | 4 cycles of chemotherapy |
| Nikbakhsh N. | 2016 | Iran | Randomized study | Gastric cancer | 30 (15/15) | (61.80/61.33) | Yes | Olanzapine 2.5-10 mg p.o. Qd (D0 to 8 weeks); Routine treatment regimensb. | 8 weeks |
| Mizukami N. | 2014 | Japan | RCT | Malignancy | 44 (22/22) | 22-78 (63) /33-75 (55) | HEC or MECa | Olanzapine 5 mg p.o. Qd (D0-5); Prophylactic antiemetic regimen: (dexamethasone, a 5-HT3 receptor antagonist, a NK-1 receptor antagonist)b; Placebo p.o. (D0-5). | 6 days |
| Navari R.M. | 2010 | USA | Randomized study | Gastrointestinal cancer or lung cancer (stages III and IV) | 76 (39/37) | 39-81 (60/61) | No chemotherapy in the previous 4 weeks | Olanzapine 5 mg p.o. Qd for 8 weeks; Megestrol acetate 800 mg p.o. Qd for 8 weeksb. | 8 weeks |
| Tan L. | 2009 | China | Randomized study | Malignancy | 229a (121/108) | 18-74 (54♂, 48.25♀/54.5♂, 49.58♀) | HEC or MECa | Olanzapine 10 mg p.o. Qd (D1-5); Prophylactic antiemetic regimen: (azasetron, dexamethasone, rescue drug)b. | 6 days |
- Abbreviations: HEC, highly emetogenic chemotherapy; MEC, moderately emetogenic chemotherapy.
- a There were 214 of 299 patients evaluable for HRQoL in Tan L. (2009).
- b Treatments received by both olanzapine group and non-olanzapine group.
3.2 Olanzapine-based treatment regimen
In five studies whose primary outcome was effective in prevention of CINV, text group patients received an olanzapine-based treatment including olanzapine applied orally plus routine antiemetic regimens.7, 9, 12, 14, 22 The add-on olanzapine was started before12, 22 or on the first day7, 9, 14 of chemotherapy at varied dose of 5,12 10,7, 9, 14 or 2.5 to 10 mg/day based on patient tolerance,22 and last days,7, 12, 14 8 weeks,22 or 5 days in every four cycles of chemotherapy.9 In the remaining study whose primary outcome was improvement of cancer-related anorexia, patients in text group were treated with oral olanzapine at a dose 5 mg/day plus oral megestrol acetate (MA) at 800 mg/day lasting for a period of 8 weeks19 (Table 2).
3.3 Assessment of HRQoL
Across studies included in this review, HRQoL was all measured as a secondary endpoint. There were four instruments assessing HRQoL being identified. The European Organisation of Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ) Core 30 (EORTC QLQ-C30) was adopted in three studies (50%).7, 9, 14 The remaining three studies used the Functional Assessment of Cancer Therapy-General (version 3) (FACT-G),19 the World Health Organization Quality of Life (WHOQOL-BREF) questionnaires,22 and the Functional Living Index-Emesis (FLI-E) questionnaire,12 respectively (Table 3).
| Author | Year | Country | Assessment Instrument | Analysis Strategy | Results of HRQoL | Other Variables |
|---|---|---|---|---|---|---|
| Mukhopadhyay S. | 2017 | India | EORTC QLQ-C30 | Comparison within groups before and after treatment | ♦In study group: Be stable in global health status, physical functioning, nausea vomiting, fatigue, anorexia, constipation (P > 0.05); be better in emotional functioning, pain, and sleep (P < 0.05) | (1) CINV |
| ♦In control group: Be worse in global health status, physical functioning, nausea vomiting, fatigue, anorexia, constipation (P < 0.05); be stable in emotional functioning, pain, and sleep (P > 0.05) | ||||||
| James E. | 2017 | India | EORTC QLQ-C30 | Comparison across groups | ♦Global health status (P = 0.01), physical functioning (P < 0.0001), emotional functioning (P = 0.0005), role functioning (P = 0.005), constipation (P = 0.009), nausea and vomiting (P < 0.0001), dyspnea (P = 0.009), insomnia (P = 0.009), and appetite loss (P = 0.001) were improved in the study group compared with the control group | (1) CINV; (2) body weight, fasting blood glucose, lipid profiles |
| Nikbakhsh N. | 2016 | Iran | WHOQOL-BREF | Comparison across groups | ♦Physical health improved at 8th week (P = 0.005) in the study group compared with the control group | (1) CINV; (2) HADS; (3) appetite assessment, BMI, blood glucose (fasting blood glucose and 2-h postprandial glucose), serum lipid profile |
| ♦Total score, mental health, social relationship, environment (P > 0.05) | ||||||
| Mizukami N. | 2014 | Japan | FLI-E | Comparison across groups | ♦Study group experienced a better QoL than the control group (P = 0.0004) | (1) CINV; (2) change in dietary intake; (3) satisfaction, wish to use the drug in the next cycle |
| Navari R.M. | 2010 | USA | FACT-G (version 3) | Narrative analysis | ♦23 of 39 patients in study group, 5 of 37 patients in control group had an improvement in QOL at both 4 and 8 weeks (no P value) | (1) weight, appetite; (2) nausea; (3) MDASI |
| Tan L. | 2009 | China | EORTC QLQ-C30 | Comparison within and across groups | ♦In study group: Global health status, emotional functioning, pain, insomnia improved (P < 0.05) | (1) CINV |
| ♦Across groups: Global health status, emotional functioning, social functioning, fatigue, nausea and vomiting, insomnia, and appetite loss were better in the study group than in the control group (P < 0.01) |
- Abbreviations: BMI, body mass index; CINV, chemotherapy-induced nausea and vomiting; EORTC QLQ-C30, European Organisation of Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ) Core 30; FACT-G, Functional Assessment of Cancer Therapy-General (version 3); FLI-E, Functional Living Index-Emesis questionnaire; HADS, Hospital Anxiety and Depression Scale; MDASI, M.D. Anderson Symptom Inventory; WHOQOL-BREF, the World Health Organization Quality of Life (WHOQOL-BREF) questionnaires.
3.4 Synthesis of studies
3.4.1 General HRQoL status
Five of six studies reported results of general HRQoL change. In two of three studies which used the EORTC QLQ-C30, HRQoL was significantly improved among patients who received olanzapine both in short term (6 days, P < 0.01)7 and in long term (after four cycles of chemotherapy, P = 0.01)9 compared with the non-olanzapine group. The remaining one indicated that the general HRQoL status of patients in the olanzapine group was stable between the 8th and 10th day after chemotherapy, whereas it was worse in the control group.14 There is one study which described that 23 of 39 patients in the olanzapine group got an improvement of general HRQoL at both 4th and 8th week. In the control group, it was only 5 of 37 patients who did (no chi-square or P value).19 However, in one study, the overall quality of life and general health reflected by the total score of the WHOQOL-BREF did not show a significant improvement in patients at the end of treatment (P > 0.05).22
3.4.2 Functional outcomes
- Physical functioning
- Psychological functioning
- Social functioning
- Other components
Besides physical, emotional, cognitive, and social functioning, the EORTC QLQ-C30 also evaluates patients' role functioning. But only one study of those which used the EORTC QLQ-C30 indicated an improved role functioning among patients in the olanzapine group compared with those in the control group (P = 0.005).9 The environment factor of HRQoL assessed by WHOQOL-BREF in one study was not reported to have a significant change (P > 0.05).22
3.4.3 Symptoms improvement
- Symptom-related quality of life
- Symptom scales
The primary objective of five studies included in this review was to examine effectiveness of olanzapine on prevention of CINV.7, 9, 12, 14, 22 Results from four of five studies demonstrated significantly the positive effect of olanzapine plus standard antiemetic regimen on CINV prevention and control compared with standard antiemetic regimen, especially on delayed nausea and vomiting (P < 0.05).7, 9, 12, 14 The remaining one of these five studies has not presented a statistical difference on CINV decline between the olanzapine group and control group (P > 0.05). Nonetheless, patients' anxiety which was rated by the Hospital Anxiety and Depression Scale (HADS) has significantly decreased in the 4th (P = 0.03) and 8th (P = 0.002) week after olanzapine treatment.22
Besides, there were two scales utilized to evaluate patients' symptoms across the studies included in this review, namely the EORTC QLQ-C307, 9, 14 and the M.D. Anderson Symptom Inventory (MDASI).19 Among eight symptoms scales (fatigue, pain, nausea and vomiting, dyspnea, appetite loss, insomnia, constipation, and diarrhea) which can be appraised by the EORTC QLQ-C30, significant improvement of fatigue (P < 0.01),7 nausea and vomiting (P < 0.01),7, 9 dyspnea (P = 0.009),9 insomnia (P < 0.01),7, 9 and appetite loss (P < 0.01)7, 9 were demonstrated in olanzapine groups compared with the control group. Based on data calculated from comparison within the olanzapine group, pain (P < 0.05) and insomnia (P < 0.05) were better after a 5-day treatment.7, 14
The remaining one study included in this review, whose primary purpose was to determine the efficacy of olanzapine for the treatment of cancer-related anorexia (CRA), recorded patients' symptoms by utilizing the MDASI. It was indicated that the combination of olanzapine with megestrol acetate (MA) was more effective in improvement of general activity, mood, work, walking, enjoyment, appetite, and nausea than the use of MA alone (P < 0.01).19 In addition, the maintenance of dietary intake was compared between olanzapine group and control group in one study. The continuous measurement data revealed that the olanzapine group maintained a similar amount of dietary intake throughout chemotherapy. In contrast, the dietary intake in the control group was significantly reduced.12
3.4.4 Safety of olanzapine
All studies included in this review indicated that no serious toxicities attributable to the study drugs were observed. Possible adverse effects related to the olanzapine which were recorded in four studies included somnolence7, 14 or mild sedation,19 and dyspepsia.9 However, one study reported that no patients in the olanzapine group had sedation.9 Besides, several studies particularly explained that olanzapine-treated patients had no significantly increased incidence of hyperglycemia,7, 9, 22 hyperlipidemia,7, 22 deep vein thromboses,19 or extrapyramidal symptoms.12
4 DISCUSSION
PROM instruments are included in clinical trials based on their advantages in knowing patient perspective of a certain treatment's effectiveness.4 Systematic evaluations of patient's perspective may provide valuable information to clinical decision making.1 As a representative PROM, HRQoL is a multidimensional concept. Changes in one domain of HRQoL can further influence perceptions in other domains.27 For example, good physical function is associated with better emotional well-being, treatment tolerance, and survival in cancer patients.28 Therefore, evaluating specific domains of HRQoL, eg, physical functioning, psychological well-being, and social functioning, is necessary in addition to general HRQoL assessment.
Heretofore, a variety of HRQoL measurement instruments have been developed and applied into cancer research. A recent systematic review identified that there were 39 instruments used to measure HRQoL in patients with advanced cancer.29 The four HRQoL measures used in studies included in this review contain three common approaches30: (1) generic measures for any disease group or a general population (the WHOQOL-BREF31); (2) disease-specific measures to be utilized across cancer types (the EORTC QLQ-C3032 and the FACT-G33); and (3) targeted, dimension-specific measures, which focus on depth on specific aspects of HRQOL (the FLI-E questionnaire34). The WHOQOL-BREF, an abbreviated 26-item version of the WHOQOL-100,35 measures four domains: physical health, psychological, social relationships, and environment.31 As a generic instrument, the WHOQOL-BREF capture a broad range of aspects of health status and more general applicability in a wide range of patient groups. However, its risk is therefore of some loss of relevance of questionnaire items when applied in any specific context.36 In this review, half of included studies used the EORTC QLQ-C30, which was considered as the most commonly used HRQoL measurement instruments for patients with advanced cancer.29 The EORTC QLQ-C30, a 30-item questionnaire, reflect the multidimensionality of the HRQoL construct through five functional scales (physical, emotional, cognitive, social, and role), eight symptoms scales (fatigue, pain, nausea and vomiting, dyspnea, appetite loss, sleep disturbance, constipation, and diarrhea), as well as global health and HRQoL status.32 The FACT-G, another major measure of HRQoL in general cancer,1 consists of five domains, including physical, social, emotional, functional well-being, and relationship with doctor.33 As cancer-specific HRQoL measures, the EORTC QLQ-C30 and the FACT-G are intended to have very relevant content when used in cancer patients and clear relevance to the problems presented by the patients. Besides, disease-specific instruments are more likely to detect important changes that occur over time in patients with particular disease. A salient potential disadvantage of a disease-specific instrument is that it may not capture health problems associated with a disease.36 As to the FLI-E questionnaire, a dimension-specific HRQoL instrument, it contains 18 questions (nine for nausea and nine for vomiting) that specifically address the effects of CINV on patients' ability to maintain daily life.37 A unidimensional measure is a much more detailed assessment in the area of concern.36 But just as what the Cancer Outcomes Measurement Working Group (COMWG) suggested, HRQoL measures should be regarded as multidimensional constructs. In particular, HRQoL always incorporates measures of physical and mental/emotional health, although there is less consensus on whether additional domains, such as social functioning, role functioning, spiritual well-being, and symptoms, are essential.30 Therefore, appropriate HRQoL measure instrument needs to be selected based on a certain study's distinct hypotheses and target participants.
Notwithstanding significant heterogeneity of cancer types, olanzapine treatment, and HRQoL measures in included studies, a paucity of clinical trials and observational researches were summarized in this review. The results of this systematic review are consistent with the hypothesis that cancer patients who have received olanzapine treatment are likely to experience a positive change of HRQoL, although the mechanisms of impact on HRQoL caused by olanzapine are still unclear. It is commonly realized that cancer patients experience a variety of distressing symptoms, which usually continue throughout the course of the disease and treatment, leading to a significant deterioration in patients' HRQoL.6, 22 Furthermore, number of cancer-related symptoms is negatively associated with HRQoL.38 So, the positive effect on cancer-related symptoms caused by olanzapine might be an important path leading to HRQoL improvement.
Olanzapine has a high affinity with several important serotonin receptors, including the HT2a, 5HT2c, and 5-HT3 receptors, making it an appealing agent for symptom management in palliative care.39 Hitherto, no consensus has been reached with regard to the optimal set of cancer-related symptoms that should be considered in clinical care.40 Across studies included in this review, the efficacy of olanzapine on CINV and cancer-related anorexia (CRA) was demonstrated as primary indicators. The mechanism behind the benefit in the CINV by olanzapine remained unclear. Multiple neurotransmitters and their receptors are related to the mechanisms of nausea and vomiting.12 Olanzapine blocks multiple neurotransmitters, not only dopamine and serotonin that are known as mediators of CINV but also histamine, acetylcholine, and catecholamine. Some of these neurotransmitters cannot be inhibited by standard antiemetic therapy. Thus, the addition of olanzapine to the standard antiemetic regimen reduced CINV.12 This improvement was more prominent in the prevention of CINV in the delayed phase.14 CINV in the delayed phase, on the other hand, seems to be caused by a different pathogenic mechanism from acute CINV because most antiemetic drugs, such as 5-HT3 receptor antagonists, are less effective in the delayed phase in spite of being effective in the acute phase.12 Moreover, the delayed CINV has a greater negative effect on patient's HRQoL than the acute phase. As a result, the olanzapine's effect on CINV prevention especially in the delayed CINV could lead to improvement of HRQoL of cancer patients.
Cancer-related anorexia (CRA), whose prevalence ranges from 14% to 55%,41 is a loss of appetite associated with the systemic effects of cancer with a resulting loss of weight and diminished global HRQoL.19, 41 Just few studies have evaluated olanzapine for CRA.39 Although the orexigenic action of olanzapine is considered a side effect of the drug in the psychiatric population,12, 39 the olanzapine-associated weight gain may be advantageous, such as in CRA.39
It should be noted that although the olanzapine is recommended as an add-on medication for breakthrough CINV and CRA, the prophylactic use in clinical practice is still limited. It might be owing to the paucity of data on its dosage, efficiency, and adverse effect profile in the palliative care population.42 Side effects reported with olanzapine include postural hypotension, constipation, dizziness, dry mouth, delirium, extrapyramidal side effects, hyperprolactinemia, agranulocytosis, cardiac arrhythmias, weight gain, glucose intolerance, and neuroleptic malignant syndrome in schizophrenia studies.39 None of these were reported in this review. Although somnolence or mild sedation, and dyspepsia were observed among minority patients which were considered olanzapine related.
4.1 Limitations
Our study could be interpreted in the context of the following limitations. Firstly, the sample size calculated based on the primary outcome might not be enough to support the results of secondary parameters, eg, the HRQoL. These highlight the need for high quality RCTs and/or prospective studies with a larger sample size to examine the efficacy of olanzapine in treatment of cancer patients. Secondly, the complexity of the HRQoL concept might not be reflected in current measurement tools. Evaluation of HRQoL based on score of scales might be biased by floor and ceiling effects or restricted in established domains. Health care professionals might benefit from a qualitative approach in understanding, describing, and interpreting patients' real experience of their HRQoL. Besides, whether the improvement in patients' HRQoL was due to the antipsychotic effect of olanzapine is unclear and further relevant studies for clarification are required.
5 CONCLUSIONS
HRQoL improvement remains an important part of caring for patients with malignant tumor. This review summarized the efficiency of olanzapine in improving the overall HRQoL of cancer patients.
While we await more definitive prospective studies and/or RCTs, olanzapine can still be recommended during palliative care. However, it is important to notice that the current evidence supporting the efficacy of olanzapine in improving HRQoL is insubstantial. Further researches on HRQoL concerns and the side effects profile of olanzapine based on comprehensive outcome measures in the palliative care are needed.
ACKNOWLEDGEMENTS
This work was supported by grants from Shanghai Municipal Education Commission-Gaoyuan Nursing (no: hlgy16016kyx), Shanghai, China. The funders had no role in study design, data extraction and synthesis, the decision to publish, or preparation of the manuscript.
CONFLICT OF INTEREST
All authors have declared no competing interests.
AUTHOR CONTRIBUTIONS
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, L.W.; Investigation, Formal analysis, Writing - Original Draft, M.J.; Investigation, Writing - Review & Editing, J.C.; Data curation, Formal analysis, H.X.; Methodology, Y.Y.




