Anion exchange membrane water electrolysis for sustainable large-scale hydrogen production
Abstract
The development of sustainable energy technology has received considerable attention to meet the increasing energy demands and realize carbon neutrality. Hydrogen is a promising alternative energy source to replace fossil fuels and mitigate environmental issues. However, most hydrogen is produced by reforming fossil fuels, called gray hydrogen, and the production of gray hydrogen emits a large amount of carbon dioxide. As a sustainable approach, water electrolysis technology has been developed to produce high-purity hydrogen, called green hydrogen. Among various technologies, water electrolysis equipped with an anion exchange membrane has been regarded as an attractive pathway for large-scale H2 production at a low cost. The status of anion exchange membrane water electrolyzers is approaching toward megawatt-scale H2 production by companies, which has the potential to become competitive technology for existing water electrolyzers (alkaline electrolyzer, proton exchange membrane electrolyser). This review article represents recent advances in the development of major components (membrane, catalyst, membrane electrode assembly) of anion exchange water electrolyzers. By recognizing the current water electrolysis performance and solving the remaining challenges, anion exchange membrane electrolysis can be a leading technology for green hydrogen production.
1 INTRODUCTION
With the increasing energy demands worldwide, consumption of fossil fuels and a huge amount of greenhouse gas generation has been threatening the environment on earth. Therefore, sustainable fuel production has been regarded as one of the most important issues around the world.[1, 2] Hydrogen, as a promising energy carrier, plays a significant role in the carbon-neutrality world because of its various advantages: sustainability, nontoxicity, and high energy contents.[3, 4] The use of hydrogen mitigates the environmental impacts that occur with carbon-based fuels. However, unlike fossil fuels, hydrogen as a form of fuel is not available to use in nature.[5] Over 95% of hydrogen has been produced by steam reforming processes, a mature technology, using fossil fuels that contain mainly CH4.[6-8] However, the reforming of fossil fuels emits a large amount of CO2, and the hydrogen produced by this technology is called gray hydrogen.[9] As a solution to produce green hydrogen where the hydrogen is generated by renewable energy without CO2 emission, water electrolysis (WE) powered by renewable energy sources has been arising as a sustainable and commercial H2 production technology.[10-12]
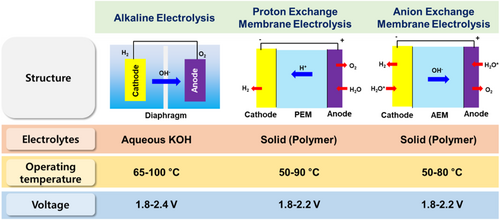
WE as the method where applied electric current is used to split the water molecule into hydrogen and oxygen with high purity.[13] The electrolysis technologies can be categorized by operating temperature[14, 15]; low-temperature electrolysis contains alkaline WE, proton exchange membrane (PEM) WE, and anion exchange membrane (AEM) WE (Figure 1). High-temperature electrolysis is based on solid-oxide fuel cells (SOFC), which will not be addressed in this review. The most mature WE technology is alkaline WE, which is conducted in liquid alkaline electrolytes. The electrode compartment and product gases are separated by a diaphragm, which allows the OH− and water movements. However, due to its challenges, such as limits of operation at high pressure and low current density, new electrolysis systems have been developed using an ion-exchange membrane as a polymer electrolyte.[16] PEMWE, which occurs in a strong acidic environment, reaches the commercial status for H2 production with a compact system by taking advantage of PEM, which reduces the ohmic losses compared to the diaphragm. The limitation of the choice of catalysts, which is stable at harsh acid electrolytes caused the necessity of developing new technology, AEMWE.[17, 18] AEMWE is currently at its early stage of development and possesses the merits of both alkaline electrolysis and PEM electrolysis.[19] With ample consideration of the components (AEM, catalysts, MEA structures) and the system of the AEM electrolysis cell, it is expected to produce H2 at the industrial level using low-cost catalytic materials and AEMs in alkaline environments via an AEM electrolyzer.
We introduce the recent research on membranes and catalysts and their WE performance with MEAs. By identifying the challenges and plausible ways of developing them, AEMWE can be a leading large-scale H2 production technology.
2 CONVENTIONAL WE TECHNOLOGIES
2.1 Alkaline electrolysis
Alkaline electrolysis is a well-matured technology for hydrogen production.[20] Alkaline electrolysis outreach to the larger commercial level up to the megawatt range.[21] Alkaline water electrolyzers are typically operated in aqueous KOH solutions with concentrations of 25–30 wt% and temperatures between 65 and 100°C.[13] The cathode and anode are separated by a porous diaphragm, which allows the conduction of hydroxide ions while separating the product gases.[22] Alkaline electrolysis is advantageous for employing inexpensive nonnoble electrocatalysts with low overpotentials and good stability in alkaline media. However, the porous diaphragm causes large ohmic losses across the liquid electrolytes, resulting in lower operational current densities of less than 400 mA cm−2. The alkaline electrolyzer is not able to operate at high pressure. The aqueous KOH electrolyte is susceptible to atmospheric CO2. The reaction between hydroxides and CO2 forms insoluble K2CO3, which lowers the performance of alkaline electrolysis due to the decrease in the number of hydroxyl ions, ionic conductivity, and blocking of ion transfer. The diaphragm cannot fully separate the produced hydrogen and oxygen, resulting in a nonnegligible gas crossover between the anode and cathode compartment. Related to the safety issue, the product gases crossover can cause the explosion risk; hydrogen gas diffusion to the anode compartment leads to a dangerous level, where the lower explosion limit is 4 mol% H2 in O2. Up to 40% of the lower explosion limit is reached under operation, so the safety limit is usually 50% of the lower explosion limit.[23] Therefore, the operation is only possible within a narrow potential/current window below the safety limit, and the pressure between the anode and cathode should be balanced.[21]
2.2 PEM electrolysis
The kinetics of the WE in PEM is faster than in alkaline electrolysis due to the acidic nature of the electrolyte with high proton conductivity and the structures that lowers the ohmic losses. The advantageous feature of PEM electrolysis is the possibility of using high pressure on the cathode side while the anode can be operated at atmospheric pressure. The purity of the produced gases in the PEM electrolyzer is higher than that of alkaline electrolysis due to the low gas crossover rate of the polymer electrolyte, and the PEM electrolyzer can operate at a current density of 2 A cm−2.[25] Therefore, the PEM electrolysis can achieve high efficiency with a fast response compared to the alkaline electrolysis. However, there is a limitation to choosing suitable catalysts materials for the noble metals because of the highly acidic environment. The cost problems in PEM WE arise due to the use of noble metal catalysts (platinum group metals), Nafion-based membranes, and stack materials (Ti-based materials).[26]
2.3 AEM electrolysis
AEM electrolysis is a promising WE technology because of its several advantages. From the view of cost-effectiveness, transition-metal catalysts are used, replacing the platinum group metal (PGM) catalysts.[29, 30] The membrane used in AEM electrolysis is less expensive than Nafion-based membranes. Furthermore, while the PEM electrolysis uses the Ti as the porous transport layer and bipolar plates, AEM electrolysis can replace it with cost-effective stainless steel.
3 MAIN COMPONENTS OF AEMWE
3.1 Membrane
3.1.1 Cations
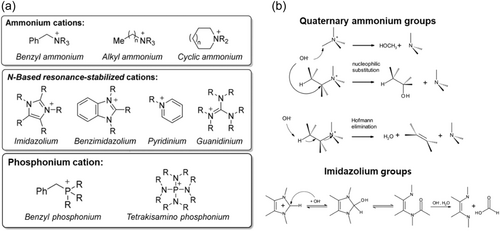
AEM consists of cationic head groups and polymer backbones with functionalities, such as ion exchange capacity, hydroxide conductivity, chemical stability, mechanical stability, and alkaline stability. The commonly incorporated cation or head groups are shown in Figure 2a.[31] First, the cations, or the head groups of the membrane, have been quaternary ammonium (QA) ions because of their ease of preparation and good stability.[33] It has been reported that the thermal/chemical stability of QA was higher than quaternary phosphonium and tertiary sulphonium.[34] However, using AEMs, including QA ions, is faced with poor chemical stability because of the hydroxide ion attack on the ammonium group. The electron-deficient cation moieties are highly susceptible to nucleophile attack (Figure 2b).[32] Imidazolium-based cations are promising in the view of an adaptable structure for adding various functional groups. However, in the view of stability, the imidazolium-based group can be degraded by a ring-opening mechanism.[35] The electron-deficient position of the cation can be protected by introducing large functional groups to hinder OH− attack.[36] Benzimidazolium cation having a benzene ring resonance structure has been shown to improve stability and ion conductivity due to ion cluster formation. Phosphonium and sulphonium cations with large electron-donating groups surrounding the cations have been shown to improve chemical stability. Hugar et al. showed that alkaline stable cations are penta-substituted imidazolium, tetrakis(dialkylamino)phosphonium, piperidinium, and tetraalkylammonium, while benzylammonium cations and simple imidazolium, pyridinium, and guanidinium cations are not proper for alkaline stable AEMs.[31]
3.1.2 Backbones
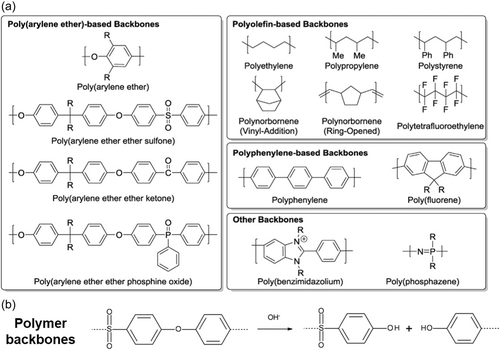
AEM polymer backbone regulates not only chemical stability but also other characteristics, such as mechanical strength, solubility, and so on. It is important to design polymer structures to improve IEC and chemical stability. There is a trade-off between IEC and mechanical stability because of the water uptake (WU) and swelling. Therefore, the AEM polymer has been developed by optimizing cross-linking and ion channel dimensions. As shown in Figure 3a, polymer backbone structures, such as poly(arylene ether), polyolefin, polyphenylene, and polybenzimidazolium have been employed. Polymer backbone stability is an important factor for long-term operating AEMs. However, it is difficult to analyze the backbone degradation since the membrane is broken into pieces after the reaction. AEMs consisting of ether-containing polymers (polysulfone, aryl ether-containing polymers) undergo degradation by aryl-ether bond cleavage.[33] When benzyltrimethylammonium groups are at the ortho position of C–O bonds, nucleophilic aromatic substitution occurs.[37] As shown in Figure 3b, when the chain is broken due to backbone degradation, the membrane is prone to be brittle. The activation energy barrier for cleavage of aryl ether bond can be reduced in the presence of a strong electron-withdrawing group (sulfone linkage, benzyltrimetyhlammonium group, etc.). The sulfone linkage influences on electron-drawing effect greater than benzyltrimethylammonium group, which directly attached aromatic ring. When the QA groups are absent at the benzylic position, the polysulfone backbone is known to be stable in alkaline conditions.[37] Therefore, it has been suggested that heteroatom-free all-carbon backbones (polyolefins, polyphenylenes, etc.) and polymer backbones not containing aryl ether bonds can improve the alkaline stability for AEM electrolysis.
3.1.3 Evaluation of membrane
Ion exchange capacity (IEC)
Water uptake
Membrane water content
Note that the WU is multiplied by 10 to account for the WU being reported in percent and the IEC being reported in mmol/g.
Swelling ratio (SR)
Hydroxide conductivity
Alkaline stability
The alkaline stability, which is one of the most important properties of AEM, can be tested by soaking AEM in a high pH solution at a given temperature for time and checking the IEC of AEMs according to the time. It has been shown that the hydration level of the OH− affects the alkaline stability. In the circumstance of higher hydration levels, the nucleophilicity of OH− is reduced by the surrounding water molecules that fill the solvation sphere, which leads to enhanced alkaline stability.
3.1.4 Commercial AEMs
As the public interest in AEM is rising, various AEMs have been developed and used for applications, such as AEM fuel cells and AEM CO2 electrolysis.[32, 41] For the CO2 reduction reaction, for example, the desired properties for AEMs are summarized by the Berlinguette group,[42] including the mechanical and chemical stability of AEMs. Similar to the required properties of AEM for CO2 electrolyzers, AEMs for WE have been developed, which have suitable properties, such as OH− conductivity, WU, IEC, and so on. Recent research has been reported on the use of AEM for WE. The widely used products for AEM water electrolyzers are summarized in Table 1. Zakaria et al. summarized the characteristics of various AEMs, which have been applied to AEMWEs.[47]
| Company | Membrane | IEC (mmol g−1) | Ion conductivity (mS cm−1) | Tensile strength (Mpa) | Thickness (μm) | ASR (Ω cm2) |
|---|---|---|---|---|---|---|
| Dioxide Materials[43] | Sustainion 37-50 | 80 (1 M KOH) | - | 50 | 0.045 (1 M KOH) | |
| Fumatech | FAA-3-30 | 1.67–2.04 (Cl)a | >5a | - | 26–34a | <2.0 (Cl)a |
| FAA-3-50 | 1.6–2.1 (Cl)a | 3–8 (Cl)a | 25–40a | 45–55a | 0.6–1.5 (Cl)a | |
| FAA-3-PK-75 | 1.2–1.4 (Cl)a | 4.5–6.5 (Cl)a | 30–60a | 70–80a | 1.2–2.0 (Cl)a | |
| Ionomr | AF1-HNN5-25 | 1.4–1.7 (OH)a | 56 ± 1(OH)[44] | 60[32] | 25a | 0.21–0.33[32] |
| AF1-HNN5-50 | 1.4–1.7 (OH)a | 40 ± 2(OH)[44] | 60[32] | 50a | 0.42–0.67[32] | |
| AF1-HNN8-25 | 2.1–2.5 (OH)a | 131 ± 1(OH)[44] | 60[32] | 25a | 0.063[32] | |
| AF1-HNN8-50 | 2.1–2.5 (OH)a | 102 ± 3(OH)[44] | 60[32] | 50a | 0.13[32] | |
| Tokuyama[45] | A201 | 1.7 (OH) | 42 (OH) | - | 28 | - |
| A901 | 1.7 (OH) | 38 (OH) | - | 10 | - | |
| Versogen | PiperION-A-20 | ~2.35a | ~150 (OH)a | >30a | 20a | |
| PiperION-A-80 | ~2.35a | ~150 (OH)a | >50a | 80a | - | |
| Orion[46] | TM1 | 2.19 (OH) | >60 (OH) | - | 30 | - |
- Abbreviations: AEM, anion exchange membrane; IEC, ion exchange capacity.
- a Technical sheets.
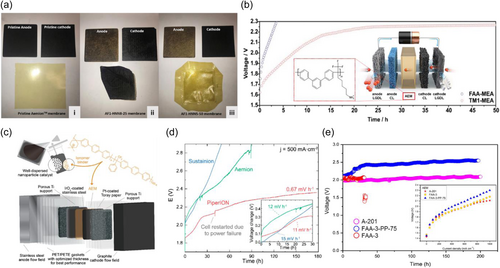
Dioxide Materials' Sustainion membranes are imidazolium functionalized styrene- and vinylbenzyl chloride (VBC)-based polymers. Functional groups such as 1-methyl imidazole by Masel group,[48] and tetramethylimidazolium groups by Hugar et al. have been used to synthesize membranes with alkaline stable polystyrene backbone.[35] Hugar et al. reported that substituting the position of imidazolium rings is very important for achieving the alkaline stability of imidazolium cations. Dioxide Materials provide the Sustainion 37–50 membranes for AEMWE. The Sustainion 37–50 membrane has a thickness of 50 μm, and due to the characteristic polystyrene, the membrane is brittle and cracks easily in the dry state. For the Sustainion X37-50 Grade 60, the membrane with reduced plasticizer becomes more brittle. Sustainion X37-50 Grade T is a polytetrafluoroethylene (PTFE) supported type, and it is easy to handle and shows increased durability. The PTFE support decreased the transport of water in the membrane. The performance of Sustainion 37–50 membranes was demonstrated by the Masel group in comparison with commercial membranes. With NiFe2O4 anode catalysts and NiFeCo cathode catalysts at loadings of 2 mg cm−2, the performance of the membranes in the water electrolyzer was obtained with 1 M KOH fed and at the operating temperature of 60°C.[43] As shown in Figure 4a, the current density as a function of voltage curves of the membranes was obtained. Under the same cell voltage of 1.9 V, the Sustainion 37–50 membrane showed the highest current density of 1 A cm−2 and FAS-50 (Fumatech) of 0.5 A cm−2, FAPQ of 0.16 A cm−2, and PBI of 0.05 A cm−2 were obtained. In the case of the Sustainion 37–50 membrane, which recorded the highest current density among membranes, the effects of temperature and the supporting electrolytes on the conductivity of the membrane were investigated. As the temperature increased, the conductivity increased regardless of the supporting electrolytes, and with high alkaline 1 M KOH fed, the Sustainion X-37 membrane recorded the highest conductivity. The stability of the AEM electrolyzer using different membranes was compared in 1 M KOH at an operating temperature of 60°C.[49] The cell voltage indicates the voltage needed to maintain a current density of 1 A cm−2 in an AEM electrolyzer. As shown in Figure 4b, the Sustainion Grade-T operated 12,000 h at a constant current of 1 A cm-2 with voltage change, implying the possibility of satisfying industrial performance needs. The comparison of the MEAs with different AEMs is represented in Figure 4c, and Sustainion membranes recorded the highest stability among reported studies.
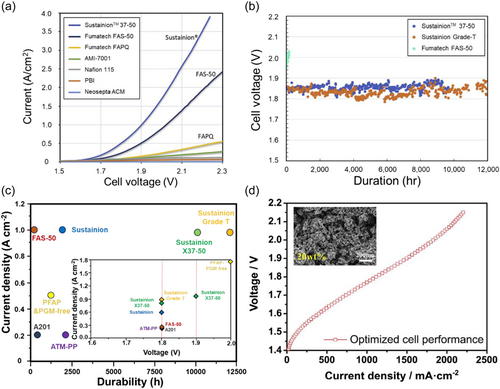
Fumatech's Fumion FAA-3 membrane is based on a brominated polysulfone backbone with QA cation side chain groups. The widely used membranes are FAA-3-30, FAA-3-50, and FAA-3-PK-75. The FAA-3-30 and FAA-3-50 membranes are nonreinforced membranes with a thickness (dry) of 26–34 and 45–55 μm, respectively. FAA-3 represents not only the unreinforced membrane but also the anion exchange ionomer. The FAA-3 ionomer solution (10 wt% in NMP, b.p. 203°C) is delivered in Br− form. Sung group demonstrated the AEM electrolyzer with FAA-3-50 and FAA-3-Br as the AEM and anion exchange ionomer, respectively.[51] With optimizing variables, such as the amount of catalyst loadings, the thickness of GDLs, and ionomer contents, AEMWE showed high performance of 1.15 and 1.5 A cm−2 at 1.8 and 1.9 V, respectively, at 70°C (Figure 4d). In other studies, the moderate durability of 1,000 h was driven using FAA-3-50 membrane by conducting cycling tests at 50°C.[52]
The FAA-3-PK-75 membrane is a polyketone (PK) reinforced AEM with a thickness of 70–80 μm. Lim et al. used FAA-3-PK-75 as AEM and achieved the 1 A cm−2 at 1.8 V with an optimized manufacturing process at the operating temperature of 90°C. Furthermore, the FAA-3-PP-75 membrane, which is reinforced with PP and has a thickness of 70–80 μm, has been utilized for AEMWE. The FAA-3-PP-75 had a lower WU of 74% and a swelling rate of 13%–15% compared to FAA-3 but exhibited lower ionic conductivity of 38 mS cm−1.[6]
The performance of AEMWE was investigated by varying MEA pressing time, torque of cell assembly, operating temperature, and electrolyte prefeed methods.[53] The MEA was fabricated by catalyst-coated substrate method where Ti and C paper was used as the conducting substrates and IrO2 and Pt/C as OER and HER catalysts, respectively. The FAA-3-PK-75 membrane was located between fabricated anode and cathodes to form MEA. With the optimized fabrication methods, the AEM electrolyzer with FAA-3-PK-75 recorded 1 A cm−2 at 1.8 V at the operating temperature of 90°C.
Ionomr's Aemion AF1-HNN8 membrane was developed based on methylated polybenzimidazoles. In the case of alkylated polybenzimidazoles, which contain cationic moieties along the backbone, the stability of the benzimidazolium group directly affects the polymer backbone degradation.[54] Methylated poly(benzimidazolium) starts to degrade by the attack of hydroxides on the C2 positions of the imidazolium moiety.[55] The benzimidazolium C2 positions can be crowded sterically by introducing the mesityl groups, preventing the access of OH−.[56] Holdcroft group reported the scale-up synthetic approach of methylated hexamethyl-p-terphenyl poly(benzimidazolium) (HMT-PBMI) as a universal hydroxide conducting polymer.[54] The fabricated HMT-PBMI with fully hydrated Cl− form showed outstanding mechanical properties (tensile strength of 33 MPa, Young's Modulus of 225 MPa) compared to the commercial Nafion 212 membrane. The WE performance was achieved using 1 M KOH at 60°C. While the FAA-3-based water electrolyzer showed a rapid inoperable voltage-time curve at 20 mA cm−2, the HMT-PMBI-based AEM electrolyzer maintained over 7 days at 25 mA cm−2. In 2020, they investigated the effects of membrane thickness and ion-exchange capacity of HMT-PMBI membrane on AEMWE performance.[44] Commercial Pt/C and Ir black were used as cathode and anode catalysts, respectively, and AP1-HNN8 ionomers were used. The AF1-HNN8-25 membrane with high ion-exchange capacity and lower thickness is promising for achieving good AEMWE performance at a low KOH concentration. As shown in Figure 5a, the catalysts were transferred to the AF1-HNN8-25 membranes, maintaining the intact contact between membrane and catalysts. In contrast, the AEM electrolyzer with AF1-HNN5-50 membrane lost the contact between catalyst and membrane due to the decrease of catalyst loading into the liquid electrolytes, leading to severe performance degradation.
In 2022, the transport properties of the Aemion anion exchange membranes (AF1-HNN5-25, AF1-HNN5-50, AF1-HNN8-25, AF1-HNN8-50) have been reported.[58] As reported in the previous research, the AF1-HNN8 has higher ion exchange capacity and membrane conductivity than AF1-HNN5, and the AF1-HNNX-25 and AF1-HNNX-50 are membranes of which the thicknesses are in μm scale. The term permselectivity refers to the membrane's ability to distinguish between mobile cations and anions preferentially. The permselectivity of the Aemion membrane showed trends of AF1-HNN5-50 > AF1-HNN5-25 > AF1-HNN8-50 > AF1-HNN8-25. The fixed charge group concentration, which is correlated with the water content, turned out to be the most relevant characteristic to draw permselectivity.
Orion Polymer's m-TPN1 membranes were developed using meta-terphenyl as an aromatic monomer. The Orion TM1 membrane has a QA functional group and polyphenylene backbone and showed high conductivity and enhanced stability.[59] The polymer without an aryl-ether backbone structure is known to be stable by preventing degradation by OH− attack. In 2019, NREL conducted the membrane characterization, and a membrane with a polyphenylene backbone (Orion TM1) showed the high conductivity and durability among various membranes.[59] Bae group developed the Orion TM1 membrane on AEMWE and investigated the influence of changing the backbone structure of the membrane on the performance of the AEM electrolyzer.[46] Two different terphenyl aromatic monomers were used to form para-terphenyl (p-TPN1) and meta-terphenyl (m-TPN1). The m-TPN1 showed a higher ion conductivity due to the better formation of ion clusters through the self-assembly of hydrophobic backbone and side chains. Henkensmeier group reported the reinforced mTPN using polybenzimidazole (PBI) nanofiber mats as porous supports.[40] After forming PBI nanofiber mats via electrospinning, bromomethylated precursors of mTPN filled the pore of PBI nanofiber mats. Reinforced PBI/mTPN was obtained after quaternization and showed the 37% reduced length swelling, 56% increased Young's Modulus, and 17% increased tensile strength, maintaining the hydroxide conductivity of 62 mS cm−1.
Kang et al. reported the use of the Orion TM1 membrane in the AEM electrolyzer in comparison with the FAA-3 membrane, which includes the ether bond in the backbone.[41] Orion TM1 membrane with aryl-ether-free polymer structure and hydrophobic backbone structure with the aid of hydrophilic QA groups can prevent the access of OH− and reserve the polymer structure with high ionic conductivity. Therefore, it showed higher performance and durability than the FAA-3 membrane (Figure 5b).
Versogen's PiperION membrane was developed from the functionalized poly(aryl piperidinium) polymer.[60] Anion conductivity and water absorption performance of poly(aryl piperidinium) based on terphenyl (PAP-TP-85) membrane were explored by Luo et al. The conductivity of the membrane was determined depending on the hydration level,[61] which affects the transport characteristics. The water adsorption and conductivity of the membranes were turned out to be affected by the kinds of counter-anion, especially on the anion size. Boettcher group reported the performance and durability of AEM electrolyzers using PiperION in comparison with the Sustainion and Aemion membrane.[57] With pure water and Pt black and IrO2 catalysts, the PiperION-based system operated over 175 h with a degradation rate of 0.67 mV h−1, while systems with Sustainion and Aemion membranes failed much earlier (Figure 5d).
Tokuyama's membrane (A201 and A901) was developed by Tokuyama, and the membrane consists of a hydrocarbon backbone and QA functional groups.[45] The structure of the A-201 membrane forms a dense network of the ionic pathway, and therefore it is possible to achieve improved ionic conductivity. Wang group reported the WU, ionic conductivity, and swelling characteristics of a 28-μm-thick A201 membrane at various temperatures and water activities.[62] The percolation threshold value for the A201 membrane is 0.34 of hydrophilic fraction for ionic conductivity, revealing that the membrane's functional groups aggregate as cubic hydrophilic domains are in random dispersion. Vincent et al. reported the MEA with the A-201 membrane and compared the AEM electrolysis performance with FAA-3 and FAA-3-PP-75 membrane.[6] The Acta 3030 (CuCoOx) and Acta 4030 (Ni/(CeO2-La2O3)/C) were adjusted as anode and cathode catalysts, respectively. The performance was carried out at 60°C in 1% K2CO3 electrolytes. At a cell voltage of 1.95 V, the AEMWE with A201 showed a current density of 500 mA cm−2 was achievable, and the 200 h operation at a current density of 500 mA cm−2 was recorded. However, AEMWE with FAA-3 drastically degraded after 31 h measurement (Figure 5e). The results showed that the FAA-3 membrane became swollen and, thus, mechanically weak. Considering the similar polarization cures of FAA-3 and A-201 membranes, engineering the mechanical properties of the FAA-3 membrane can be a proper alternative to A-201.
Polybenzimidazole (PBI) is an ion-solvating polymer, and PBI-based anionic membranes with supporting electrolytes have high chemical, mechanical, and thermal stability.[63] The advantage of PBI-based AEMs is their hindrance to cationic degradation occurred by OH− attack. The PBI requires a KOH feed solution, and it cannot be used in pure water. Kraglund et al. demonstrated the key features of ion-solvating membranes as a hydroxide conducting separator.[64] The Celazole's PBI membrane has a chemical structure of poly-2,2′-(m-phenylene)-5,5′-bibenzimidazole. Related to the AEMWE using PBI membranes, Vincent et al. reported that the fabricated AEMWE recorded the best performance of 1000 mA cm−2 at 1.9 Vat at a temperature of 60°C, showing the efficiency of AEM electrolysis of 74%. Also, 100 h of operation was achieved with a current density of 1000 mA cm−2, indicating the efficient OER/HER catalysts and PBI membranes with high stability. Blending polymers with ionomers is one of the promising strategies to enhance the performance of AEM.[65] In the case of PBI, the scissoring of the dense packing of the PBI chain is promising to improve water/KOH sorption ability. Henkensmeier group demonstrated the effects of blending Dapazol PBI (mPBI) AEM and FAA3 anion exchange ionomers on AEMWE performance.[66] By controlling the blend ratio of PBI to FAA3, the tensile strength, conductivity, and alkaline stability were improved than pristine PBI.
3.2 Catalysts
3.2.1 Mechanism of hydrogen evolution reaction (HER)
Volmer step electrochemically reduces a water molecule into an adsorbed hydrogen intermediate and hydroxide anion. Heyrovsky step is the electrochemical hydrogen desorption, while the Tafel step involves the chemical desorption of hydrogen gas. These three steps consecutively result in the generation of hydrogen gas molecules from the metal active site at the catalyst surface. HER mechanism considering transition metal electrocatalysts was typically found to follow the Volmer-Heyrovsky mechanism in which either Volmer or Heyrovsky is a rate-determining step. Unlike the HER process in acid conditions, HER in an alkaline medium faces much slower kinetics due to the lack of protons derived from the hydronium ions usually present in acids to form the adsorbed hydrogen in the Volmer step and required additional energy to split the H–OH bond to obtain the adsorbed hydrogen and releasing OH−. Based on Sabatier's principle, the Gibbs free energy of hydrogen adsorption, namely ΔGH, of an ideal catalyst should be optimum, with a value close to zero for the optimized H adsorption and desorption in the same place.[67] When ΔGH is too positive, the weak binding between the active site with H-intermediate will hinder reactions from taking place. On the other hand, more negative ΔGH calls for a very strong interaction between active sites and reactants, intermediate, and product species, which would result in blocking the active site and poisoning the overall reaction. Pt/C catalysts exhibit a near-optimum ΔGH close to zero, and that explains the excellent HER catalytic activity of Pt/C.
3.2.2 Cathode electrocatalysts in AEMWE
Up to date, the widely used cathodes for ion exchange membrane electrolysis are Pt black or Pt/C, where Pt possesses the excellent HER catalytic activity. However, Pt is a highly scarce metal with a strikingly expensive cost, which would hinder the commercialization effort of AEMWE. Although there have been some efforts to reduce the loading of Pt on HER catalysts, clearly eliminating the use of expensive noble metal electrocatalysts and replacing them with cheap transition metal-based materials is cost-effective considering the large-scale production of hydrogen for industrial AEMWE. Recent studies have aimed to obtain HER catalysts with comparable or even lower overpotentials than the benchmark Pt/C catalyst.
This review introduces the reports of various types of cathode electrocatalysts for AEMWE. The metal alloy is one of the most widely used materials for HER catalysts due to its high conductivity and abundance of catalytic active sites. Wang et al. used the atmospheric plasma spraying method to apply NiAlMo and NiAl as cathode and anode catalysts on stainless steel gradient porous metal framework electrodes, respectively.[68] An AEMWE cell was then fabricated with an MEA structure composed of the catalyst-coated electrodes and HMT-PBI AEM, which achieved a potential of 2.086 V at a current density of 2 A cm−2 and kept stable operation for 154 h at a high current density of 1 A cm−2. Unfortunately, the plasma sprayed catalyst layer was too thick and led to an increased charge transfer resistance with limited catalytic activity. To solve this problem, Chen et al. synthesized an amino group-containing NiMo catalysts (NiMo-NH3/NH2) with a rather thin catalyst layer via annealing in a mixed NH3/H2 atmosphere and reported an exceptional HER performance of 500 mA cm−2 at an overpotential of 107 mV.[69] The elemental distribution of Fe-NiMo-NH3/H2 catalyst after anodic activation was revealed via elemental mapping (Figure 6a). The Tafel slope of NiMo-NH3/NH2 catalyst was 35 mV/dec, which was comparably low as a state-of-the-art Pt/C catalyst. On the other hand, Fe ion-doped NiMo-NH3/H2 catalysts (Fe-NiMo-NH3/H2) yielded 500 mA cm−2 at an overpotential of 244 mV under OER conditions. These catalysts were combined into an MEA in AEM electrolyzer with X37-50 Grade T AEM. The performance of MEAs employing a series of cathode/anode pairs in 1 M KOH at 20°C was demonstrated by the relationship between current density and cell voltage (Figure 6b). Also, the MEA fabricated with a catalyst combination of Fe-NiMo-NH3/NH2 | | NiMo-NH3/NH2 successfully delivered 1.0 A cm−2 at 1.57 V at 80°C in 1 M KOH (Figure 6c). The required voltage to reach a current density of 1.0 A cm−2 for Fe-NiMo-NH3/NH2 | | NiMo-NH3/NH2 MEA was even slightly lower than 1.59 V of IrO2 || Pt/C MEA. The energy conversion efficiency at this current density was determined as 75%. The superior performance might be stemmed from the highly active HER and OER catalysts. Besides, the MEA voltage remained almost unchanged during a short-term 25 h electrolysis test at 500 mA cm−2, owing to the enhanced durability of both cathode and anode catalysts. Durovic et al. fabricated nanocrystalline Fe60Co20Si10B10 alloy via ball milling and applied it on the top of Ni support as cathode catalyst in an MEA with NiCo2O4 as an anode.[73] The surface area of the Fe60Co20Si10B10 catalyst was increased through cyclic polarization and successive leaching in 1 M KOH at 25°C. The overpotential to reach a current density of −10 mA cm−2 was around −439 mV, and NiCo2O4 || Fe60Co20Si10B10 AEMWE single-cell yielded 360 mA cm−2 at a cell voltage of 2 V in 10% KOH at 50°C. It turned out that Fe-based Fe60Co20Si10B10 cathode catalysts failed to outperform the state-of-the-art Ni-based cathode catalysts.
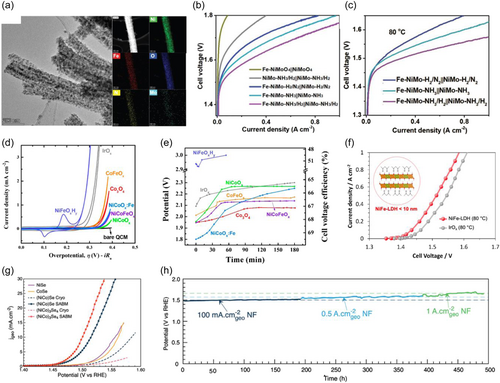
In addition to alloy HER catalysts, metal oxides also have been extensively studied as HER catalysts. Compared to metal alloy, chemical bondings between metal and oxygen atoms in metal oxide catalysts hinder the metal dissolution during reactions, hence ensuring the stability of catalysts. Besides, an introduction of oxygen induces a transformation of electronic orbitals of the metal elements, which might give a chance for a synergistic electronic structure modulation and an enhanced catalytic activity. Faid et al. reported NiCu mixed metal oxide catalysts with high activity for HER due to the rapid kinetics on the Volmer step of HER reaction.[74] The presence of NiO sites facilitated water dissociation and optimized OH adsorbate binding energy, while Ni metallic sites affected H adsorbate binding energy. CuO sites played a role in stabilizing NiO sites under HER conditions. AEM electrolyzer cell was built with NiCu mixed metal oxide as cathode catalysts and Pt/C or Ir/C as anode catalysts to test the activity of NiCu mixed metal oxide in an actual AEMWE environment and achieved AEMWE performance of 1.85 A cm−2 at 2 V in 1 M KOH at 50°C. Chanda et al. reported NiFe2O4 spinel oxide catalyst with promising HER catalytic activity and durability.[75] Cooperative participation of Fe ions in electrode reaction facilitated an overall HER mechanism. In a single-cell test, the MEA with an anion-selective quaternized polyphenylene oxide (qPPO) ionomer showed 125 mA cm−2 at a cell voltage of 1.85 V and lasted with a continuous operation for 143 h.
Recently, Park et al. prepared Co3S4 nanosheets on Ni Foam for HER by calcination of electrodeposited Co(OH)2 and successive sulfurization.[76] The morphology and composition of Co3S4 nanosheets were highly affected by the duration of the sulfurization. It was confirmed that a sulfurization time longer than 2 h was effective on S-conversion on the surface of curled Co3O4 nanosheets to fabricate Co3S4 nanosheets with vertical or oblique 3D-structure with a large surface area. However, the sulfurization time longer than 4 h caused desulfurization, and Co3S4 nanosheets were converted to Co9S8 with less HER activity. With an optimized sulfurization time of 3 h, Co3S4 nanosheets on Ni Foam showed the lowest HER overpotential of 93 mV at −10 mA cm−2 in 1 M KOH and a Tafel slope of −55.1 mV/dec. A single AEMWE cell was fabricated with Co3S4 nanosheets on Ni foam as cathode and Cu0.81Co2.19O4 on Ni foam as an anode, which showed a current density of 431 mA cm−2 at a cell voltage of 2.0 V.
Very recently, catalysts with atomically dispersed metal active sites have emerged and outperformed almost all other HER catalysts, including the Pt/C catalyst. For example, Chen et al. synthesized atomic Ru loaded on nickel hydroxide as layered sheets on nickel nanofoams.[77] Abundant edge hydroxide groups on an ultrathin nanoribbons structure promoted a facile trap of atomic Ru, which hindered Ru atoms from agglomerating into Ru nanoparticles. A synergistic combination of nanoribbon morphology of nickel hydroxide and atomically dispersed Ru active sites resulted in an ultralow overpotential of 16 mV for HER to achieve the current density of −10 mA cm−2 and a Tafel slope of 40 mV dec−1 in 1 M KOH. Compared to the commercial Pt/C catalyst, which exhibited an overpotential of 17 mV at −10 mA cm−2 and a Tafel slope of 43 mV dec−1 in 1 M KOH, a dispersed Ru single-atoms on nickel hydroxide nanoribbons is one of the promising state-of-the-art HER catalysts.
3.2.3 Mechanism of oxygen evolution reaction (OER)
To assess the theoretical catalytic performance with first-principle calculation, Norskov et al. set up the ground model for a theoretical OER overpotential calculation on a surface of transition metal oxides.[78] By calculating the adsorption energies of OH, O, and OOH intermediates from step, the theoretical overpotential can be derived from the Gibbs free energy for intermediate adsorption from each step in the overall OER reaction pathway. The OER limits the energy efficiency of electrocatalytic systems due to poor reaction kinetics and corresponded higher overpotential than HER counterparts, stemming from the complex four-step reaction pathway. In specific, the formation of the M–O bond from the M–OH intermediate state or the formation of the M–OOH bond from the M–O intermediate state is the rate-determining step, so the energy barrier of the rate-determining steps should be decreased with the help of the appropriate catalysts to lubricate the overall OER process. Also, the stability of the catalysts should be achieved to ensure a consistent catalytic performance without degradation.
3.2.4 Anode electrocatalysts in AEMWE
One of the most powerful advantages of AEMWE is that platinum group metal (PGM) metal-free catalysts can be used and contributes to low-cost and high-performance AEMWE. It is well-known that transition metal-based electrocatalysts have high catalytic activity, and they are comparable to noble-metal-based catalysts. Anode electrocatalysts for AEMWE using PGM-free catalysts have been studied. High-surface-area nickel-based catalysts have drawn a key interest among PGM-free catalysts, similar to traditional OER catalysts for alkaline WE. One of the promising nickel alloy catalysts for OER is NixFey, and Li et al. reported improved performance of an AEM electrolyzer to state-of-the-art PEM electrolyzers by selecting NiFe nanofoam catalyst as anode catalyst instead of commercial IrO2 catalyst.[79] By varying cathode catalysts and electrolytes from NiMo to PtRu and pure water to 1 M NaOH, respectively, MEAs built with NiFe nanofoam anode catalyst and HTMA-DAPP AEM outperformed a state-of-the-art IrO2/Pt PEM electrolyzer in a kinetic region at cell voltages of less than 1.58 V. For example, the current density of MEA with NiFe as anode catalyst and PtRu as cathode catalyst recorded a current density of 300 mA cm−2 at 1.5 V, which was almost doubled than that of PEM MEA catalyzed by IrO2 and Pt. However, in a region where a cell current density was greater than 1 A cm−2, mass-transport became the critical limiting factor and the PEM-based MEA outperformed AEM-based MEA in pure water electrolyte conditions.
The anode catalysts are exposed to a high electric potential during OER. Hence the surface of alloy and metal catalysts will be oxidized and transformed into (oxy)hydroxide or metal oxide forms. A modulated oxidation state of metal (oxy)hydroxide and metal oxide might enhance the catalytic activity toward OER. For example, Xu et al. prepared various NiFeOxHy oxyhydroxide catalysts and demonstrated the correlation between OER catalytic activity and AEM WE.[70] The initial polarization curves of AEM electrolyzers with different OER catalysts operated at 50°C with nanopure water fed to the anode showed that the AEM electrolyzer assembled from NiFeOxHy, which was the best OER catalysts in alkaline media (Figure 6d), yielded the worst AEMWE performance (Figure 6e). Further investigation revealed that Ni-based (oxy)hydroxides without oxidization showed very poor electrical conductivity. Since the AEM electrolyzer operated under pure water, oxidation of the catalyst and water oxidation could only occur at the phase boundary with the ionomer. Therefore, unlike in alkaline electrolytes, the NiFeOxHy bulk catalyst remained unoxidized throughout the entire working condition and became an electrical insulator with a conductivity of about 10−8 S cm−1, which was insufficient to drive the OER efficiently.
Maximizing the electronic conductivity of catalysts is important to the catalytic performance in AEMWE working conditions. Among many candidate catalysts for OER, NiFe-layered double hydroxide (LDH)-based electrocatalysts have shown the highest activity in an alkaline condition. To resolve the poor electrical conductivity issue in pure water AEMWE, hybridization with conductive carbonaceous materials was often applied. However, it turned out that those carbonaceous materials degraded and caused detrimental stability issues at industrially relevant current density operation in AEMWE. Jeon et al. enhanced the electrical conductivity of NiFe-LDH-based electrocatalysts by applying facile monolayer structuring without using hybridization of carbonaceous materials.[71] The monolayer NiFe-LDH deposited on Ni foam exhibited much higher electrical conductivity and hydrophilicity compared to the bulk NiFe-LDH. By overcoming the electrical insulating issue and enhancing the mass transport property, a high energy conversion efficiency of 72.6% and long-term stability over 50 h at a current density of 1 A cm−2 were achieved on a fabricated AEM electrolyzer cell. Koshikawa et al. offered another strategy to resolve the intrinsic drawback of bulk NiFe-LDH catalysts by fabricating NiFe-LDH nanoparticles with a lateral size of less than 10 nm via liquid phase reaction.[80] Current-voltage curves were obtained for MEAs using NiFe-LDH and commercial IrOx as an anode catalyst in 1 M KOH at 80°C to investigate the applicability of NiFe-LDH as an anode catalyst in AEMWE (Figure 6f). The onset voltage to achieve a current density of 10 mA cm−2 for NiFe-LDH was 1.37 V, which was lower than 1.40 V for IrOx. Also, the MEA with NiFe-LDH required a lower voltage than MEA with IrOx to achieve the same current up to 1.0 A cm−2 region. The fabricated and optimized AEMWE single-cell achieved an energy conversion efficiency of 74.7% for flowing 1.0 A cm−2 in 1 M KOH at 80°C.
Similar to the aforementioned metal (oxy)hydroxide catalysts, metal oxide catalysts are promising candidates for anode catalysts with enhanced stability. Based on the Pourbaix diagrams, an alloy of Cu and Co is predicted to be stable in harsh OER conditions. To optimize the ratio of Cu and Co, various CuxCo3−xO4 oxide thin films were deposited by magnetron sputtering at oblique angles on carbon paper and showed exceptional OER catalytic performance.[81] Thanks to the porous structure, an increased ECSA led to a huge leap in geometrical current density. With an optimized Co/Cu atomic ratio of around 1.8, the optimized CuxCo3−xO4 showed long-term stability under OER conditions. An AEM electrolyzer cell was fabricated with the optimized anode catalysts and the Ni film cathode yielded a current density of 100 mA cm−2 at a cell voltage of 2.0 V in 1.0 M KOH electrolyte. The researchers further optimized the CuxCo3−xO4 anode catalysts with the modification of the microstructure and chemical properties.[82] The nanostructure of CuxCo3−xO4 catalysts might contribute to increasing the catalytic OER activity due to the optimization of the ratio of a partial exchange between Co2+ and Co3+ active sites and showed an optimal compromise between a catalytic load and particle agglomeration.
Chanda et al. prepared a series of Ni1+xCo2−xO4 anode catalysts to investigate their catalytic activity toward OER. It turned out that NiCo2O4 oxide from the hydroxide preparation method and calcined at 325°C yielded the highest OER activity.[83] However, the catalyst had some stability issues stemming from decreased catalyst particle size and partial loss of active sites over time. Besides, gradual degradation of catalytic activity from irreversibly oxidized Ni3+ species from Ni2+ hindered a reversible transformation of the Ni3+/Ni2+ couple that facilitated the overall OER process. Furthermore, Wu et al. reported CoFe0.25Al1.75O4 anode catalysts for AEMWE with a competitive potential in alkaline electrolysis applications. The mass activity of CoFe0.25Al1.75O4 catalysts outperformed commercial IrO2 catalysts and the benchmark transition metal oxides.[84] Due to the usage of low-priced elements, the cost of CoFe0.25Al1.75O4 catalyst for a given performance is lower than that of noble metal oxides and other reported transition metal perovskites by orders of magnitude. The CoFe0.25Al1.75O4 exhibited a notably higher mass efficiency as well as higher geometrical area activity than IrO2.
In perovskite oxides, partial dissolution and amorphization of the surface were observed during alkaline OER. Using the flame spray method (FS), Fabbri et al. demonstrated a dynamic local surface reconstruction of prepared Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF-FS) and La0.2Sr0.8CoO3−δ (LSC-FS) perovskite anode catalysts.[85] Increased edge-sharing of octahedral units from their initial corner-sharing structure was observed in both cases after activation in 0.1 M KOH, accompanied by increased OER activity pseudo-capacitance. However, there was no change in activity and structure observed for LaCoO3. As a result, perovskites with extensive edge-sharing on a porous nanostructure with a large electrocatalytic surface area allowed the material to be penetrated by water. The lattice OER (LOER) process led to Ba2+ and Sr2+ cation dissolving and leaching out from the perovskite structure. Also, Co and Fe cation was dissolved during the LOER process, but the relative insolubility in electrolyte triggered Co and Fe cation redeposition on the catalyst surface. Besides, OH− from the KOH electrolyte replenished the consumed lattice oxygen by the LOER process. This stable dynamic cycle allowed the coexistence of a self-assembled active surface layer with the original BSCF-FS perovskite structure. The performance of AEM electrolysis cell with MEA having BSCF-FS and commercial IrO2 as anode electrode was verified with a steady-state current of 200 mA cm−2 at 50°C and 0.1 M KOH electrolyte, respectively.
The materials beyond oxygen-containing catalysts, such as metal nitrides, sulfides, phosphides, and selenides, are also one of the possible candidates for the best-performing anode catalysts for AEMWE. These materials are known to be thermodynamically unstable under oxidizing conditions of OER, hence the surface of the catalysts might be transformed into oxide or (oxy)hydroxide and form a core-shell structure. Therefore, a careful structural investigation is required to accurately trace the origin of modified catalytic activity. Recently, Abed et al. synthesized nanocrystalline Ni-Co-Se using ball milling at low temperature with a liquid nitrogen environment to ensure the homogeneous dispersed atomic mixture.[72] Activated (NiCo)3Se4 electrocatalyst on a nickel foam electrode showed an enhanced OER performance than NiSe or CoSe catalysts (Figure 6g). Besides, a continuous yielding of 500 mA cm−2 at 279 mV and 1 A cm−2 at 329 mV of overpotential in 1 M Fe-free KOH electrolyte for more than 500 h was achieved (Figure 6h). By integrating with Cu catalysts loaded carbon paper as cathode and Sustainion X37-50 as AEM, a single cell of AEM electrolyzer was fabricated and successfully delivered 1 A cm−2 at 1.75 V of cell voltage for 95 h without decay in performance. Meena et al. facilitated a crystalline-amorphous interface of nickel phosphide from an active mesoporous Ni2P precatalyst on FePOxHy.[86] Thanks to the abundant interface of crystal nickel phosphide and amorphous nickel phosphide, the number of active sites and accessible surface area with fast charge transfer and mass diffusion were increased. These OER catalysts required an overpotential of 360 mV to yield the current density of 1000 mA cm−2 in 1 M KOH. Besides, the AEMWE cell was built up by coupling anode catalyst Ni2P on FePOxHy with cathode catalyst MoNi4/MoO2 and resulted in an excellent AEMWE performance requiring 1.65 and 1.715 V for the commercially required current density of 500 and 1000 mA cm−2, respectively. Recently, the heterostructure of nickel nitride and monoclinic molybdenum disulfide (Ni3N@2M-MoS2) showed a boosted OER catalytic activity,[87] which was stemmed from regulated active electronic states of Ni and N atoms through the charge transfer between Ni3N and Mo–Mo bonds of 2M–MoS2. Since Ni3N@2M-MoS2 also showed a high HER catalytic activity, this bifunctional catalyst was applied on both anode and cathode to build an AEMWE cell and achieved an ultralow cell voltage of 1.644 V at a current density of 1000 mA cm−2. The AEMWE cell exhibited incredible durability over 300 h without cell voltage increase to maintain the current density of 1000 mA cm−2, which far exceeded both catalytic performance and durability of the commercial electrolyzer with Pt/C as a cathode catalyst and RuO2 as an anode catalyst.
4 MEA
WE performance depends on the MEA used in an electrolyzer. In general, the MEA consists of a polymeric membrane and an anode and cathode catalyst layer on each side of the membrane. The catalyst can be coated on either the membrane, by the catalyst-coated membrane (CCM) method, or on the porous transport layer interfaced with AEM by the catalyst-coated substrate (CCS) method (Figure 7a). In the CCS method, the electrocatalyst ink is directly deposited on the porous transport layer and calcined to form the catalyst-contacted electrode with or without binding ionomers. The porous transport layer transfers the electric current between bipolar plates and the catalyst layers while mechanically supporting the membrane. A metal fiber, metal foam, or woven metal network can be applied as the porous transport layer. Stability upon alkaline conditions with high anodic potential is required, hence Ni foam and stainless steel felts are widely used as the porous transport layer in AEMWE cells. Hot pressing is often applied to reduce the contact resistance and increase the MEA performance. On the other hand, the CCM method can be achieved by directly coating a catalyst ink to form a catalytic layer on the surface of the membrane before the assembly process. CCM method has some advantages over the CCS method like catalyst loading reduction, ionic conductivity increase, membrane electrode contact enhancement, and decrease in mass transport resistance. Although the electrical contact between the current collectors is worse than the CCS method, decreased electrical conductivity can be compensated with the increased ionic conductivity through the electrolytes, and the overall MEA performance can be achieved in a reasonable trade-off. However, a suitable polymeric binder to properly connect the catalyst layer and membrane should be developed to fully unlock the potential of MEA fabricated via the CCM method. The anion-exchange ionomer or polymer can play a role as a binder that enables a mechanically stable catalyst layer from the catalyst powder. Besides, the binder promotes efficient electronic contacts from the catalyst layer to the current collector for the electrochemical reactions, minimizing the electrical interfacial resistance.
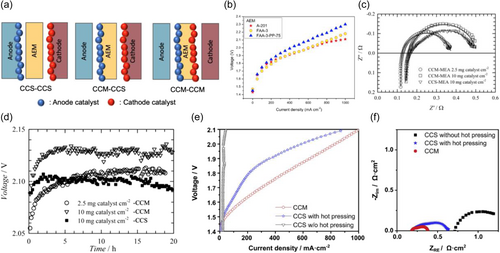
Recently, Pavel et al. applied the CCS method to fabricate MEA with a commercial polymeric AEM A201 (Tokuyama, Japan). Acta 3030 (CuCoOx) was applied as the OER catalyst in the anode, and Acta 4030 (Ni/(CeO2-La2O3)/C) was employed on the cathode to facilitate HER. For the cathode part, Ni-based nanostructure was deposited on the mixed phase of CeO2 and La2O3 supported by carbon. The same MEA cell was also prepared by Vincent et al. via the CCS method and coated Acta 3030 and Acta 4030 catalysts on the anode and cathode, respectively.[6] Since CuxCo3−xO3 spinel anode catalysts were shown to have optimized performance with Acta 4030, the catalyst combination was maintained, and for the membrane part, not only the A201-Tokuyama membrane but also the FAA-3 membrane containing MEA was fabricated and tested (Figure 7b). The catalyst loading of the anode was about 30 and 7.4 mg cm−2 for the cathode. AEM WE with pure DI water exhibited a current density of 500 mA cm−2 at the cell voltage of around 2.0 V. However, the degradation rate was significant with an average voltage increase of 2.37 mV h−1. Hnat et al. fabricated MEAs via CCM or CCS method with varied catalyst loading of NiCo2O4 and NiFe2O4 as anode and cathode catalysts on the Ni foam, respectively.[88] Nyquist plot corresponds to the MEA fabricated with the CCM method, and catalyst load of 2.5 mg cm−2 showed the lowest ohmic resistance (Figure 7c). When the catalyst loading increased to 10 mg cm−2, the thickness of the catalyst layer increased and resulted in higher ohmic resistance with degraded the performance of MEA, even worse than MEA fabricated from the CCS method. The stability of MEAs fabricated via CCS or CCM with different catalyst loading was represented by a dependence of cell voltage on the operation time of AEMWE at a constant current density of 250 mA cm−2 in 10% KOH electrolyte and 45°C (Figure 7d). The MEA cell voltage increased during the initial operation of 5 h until a stable voltage value was achieved.
Park et al. fabricated CCM and CCS with and without hot-pressing.[51] Polarization curves revealed the poor performance of CCS made without hot-pressing. It turned out that hot-pressing increased the adhesion between catalyst layer and substrate, eventually decreasing the contact resistance and showing relatively better performance. However, excessively high temperatures during hot-pressing could invoke the chemical rupture and damage the polymeric membrane, so the optimal hot-pressing temperature was limited to up to 70°C. Among all, MEA fabricated with the CCM method showed the highest AEM WE performance. Nyquist plot acquired from EIS performed at 1.9 V (Figure 7f) revealed that the MEA fabricated via the CCM method exhibited the lowest ohmic, charge transfer, and mass transport resistances. The MEA from the CCS method without hot pressing had the highest ohmic resistance of the other two MEAs, while MEA from the CCS method with hot-pressing and CCM method showed almost the same ohmic resistance, which could be indicated by the intercept in the high-frequency region. The AEMWE performance difference between those two MEAs stemmed from mass transport resistance, indicated by the arc in the low-frequency region. Therefore, the CCM method was the optimal MEA fabrication method for AEMWE.
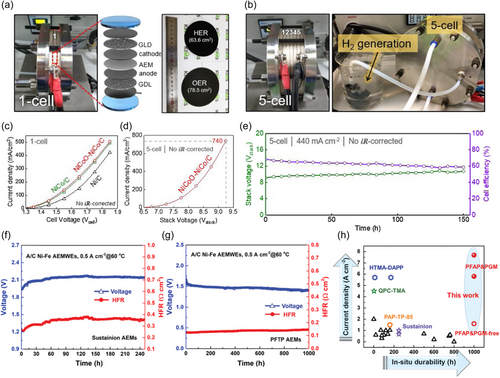
AEMWE is a technology that strengthens and makes up for the weakness of existing alkaline WE and PEMWE. Over the past years, the research of AEMWE has made a remarkable growth in WE technology, showing the possibility of realizing the practical AEMWE. As selected progressive research, the Choi group reported an AEMWE stack with a 5-cell structure using PGM-free electrocatalysts.[89] They prepared a single-cell and stack cell (5-cell) as shown in Figure 8a,b. The CuCoO was used as OER catalysts and NiCoO-NiCo/C as the HER electrocatalysts. They prepared the electrodes by CCS methods. The single-cell showed the current density of 504 mA cm−2 at the potential of 1.85 V (Figure 8c). By staking and operating 5 cells with the same configuration, a current density of 740.23 mA cm−2 at 9.25 Vstack (1.85 Vcell) was achieved (Figure 8d). The increase in the current density of the stack cell is attributed to the beneficial fluid dynamical behavior of electrolyte feed between the combination of laminar and turbulent flow generated by the stack structure. The stack cell operated for 150 h under the applied current density of 440 mA cm−2 (Figure 8e). Although the performance of the stack cell should be improved for the actual system, it opened the door for future research toward large-scale stack levels. Chen et al. developed a rationally designed AEMWE with poly(fluorenyl-co-aryl piperidinium) (PFAP)-based anhydrous cathode, which exceeds the current density of reported state-of-the-art PEMWEs.[50] The elaborate approach toward highly efficient and durable AEMs resulted in the development of PFAP-13 AEM having high ion conductivity and water diffusivity, which enhanced the water and OH− transport in anhydrous cathode AEMWEs. PFAP-based AEMWEs using PGM catalysts showed an outstanding current density of 7.68 A cm−2 at a cell voltage of 2 V, surpassing the performance of PEMWEs. Furthermore, PFAP-based AEMWEs with PGM-free catalysts reach the current density of 1.62 A cm−2 at 2.0 V. Furthermore, while AEMWEs using PFTP-based AEMs operated for 1000 h stably, PTFE-reinforced Sustainion AEM-based AEMWE showed high initial cell voltage and fast decay after 250 h under the same operation condition (Figure 8f,g). The reported performances of AEMWEs are summarized in Table 2.
| Anode catalyst | Cathode catalyst | Membrane | Electrolyte | Operation temperature (°C) | Current density (A cm−2 at V) | Ref |
|---|---|---|---|---|---|---|
| Fe-NiMo-NH3/H2 | NiMo-NH3/H2 | X37-50 Grade T | 1 M KOH | 80 | 1.0 (1.57) | [69] |
| IrO2 | Pt/C | PFTP-13 | 1 M KOH | 80 | 7.68 (2) | [50] |
| IrO2 | Pt/C | Orion TM1 | 1 M KOH | 30 | 2.75 (1.9) | [41] |
| CuCoO | NiCoO-NiCo/C | X37-50 Grade T | 1 M KOH | 50 | 0.504 (1.85) | [89] |
| IrO2 | Pt black | PiperION | 1 M KOH | 50 | 1.0 (1.9) | [57] |
| NiFeV LDH | Pt/C | X37-50 Grade T | 1 M KOH | 50 | 2.1 (1.8) | [19] |
| Ir black | Pt/C | AF1-HNN8-25 | 1 M KOH | 60 | 2.0 (1.82) | [44] |
| NiMn2O4 | Pt/C | FAA3-50 | 1 M KOH | 80 | 0.53 (2) | [52] |
| NiFe | NiFeCo | X37-50 Grade T | 1 M KOH | 60 | 1.0 (1.9) | [43] |
| Acta 3030 | Acta 4030 | FAA-3 | 1 M KOH | 60 | 0.4 (1.91) | [6] |
| IrO2 | Pt/C | A201 | DI water | 50 | 0.399 (1.8) | [57] |
| IrO2 | Pt/C | FAA-3-PK-75 | 0.5 M KOH | 90 | 1 (1.8) | [53] |
| NiAl | NiAlMo | HMT-PMBI | 1 M KOH | 60 | 2.0 (2.086) | [68] |
| NiCu mixed oxide | Ir black | FAA-3-50 | 1 M KOH | 50 | 1.85 (2) | [74] |
| Ni foam | NiFe2O4 | Dowex Marathon A | 10 wt% KOH | 50 | 0.125 (1.85) | [75] |
| NiCo2O4 | Fe60Co20Si10B10 | PSEBS-CM-TMA | 10 wt% KOH | 50 | 0.36 (2) | [73] |
| Cu0.81Co2.19O4 | Co3S4 | X37-50 | 1 M KOH | 40 | 0.431 (2) | [76] |
| NiCoFeOx | Pt black | FAA-3-130 | Nanopure water | 50 | 1 (2.492) | [70] |
| NiFe-LDH | Pt/C | X37-50 Grade T | 1 M KOH | 50 | 1 (1.69) | [71] |
| NiFe-LDH | Pt/C | X37-50 Grade T | 1 M KOH | 80 | 1 (1.59) | [80] |
| CuxCo3-xO4 | Ni | FAA-3-50 | 1 M KOH | 70 | 0.11 (2.2) | [81] |
| NiFe | PtRu/C | HTMA-DAPP | 1 M NaOH | 60 | 5.32 (1.8) | [79] |
| BSCF | Pt | A201 | 0.1 M KOH | 50 | 0.2 (1.78) | [85] |
| (NiCo)3S4 | Pt/C | X37-50 | 1 M KOH | 60 | 1 (1.75) | [72] |
- Abbreviation: AEMWE, anion exchange membrane water electrolysis.
5 STATUS OF COMMERCIALIZATION OF AEMWES
Carbon-neutral green hydrogen production system has been increasing the portion of the energy production industry. In the case of alkaline electrolyzer, the Japanese company Asahi Kasei developed the 10 MW large-scale single-stack alkaline WE system. For PEM electrolyzer, the U.S Department of Energy (DOE) set the goals of durability and stack costs of 80,000 h and ≤100 $/kW, respectively. Hydrogenics provides the PEM electrolyzers, HyLYZER with the different hydrogen production on the Nm3/h scale. Cummins (Hydrogenics) is preparing a 20 MW PEM electrolyzer plant based on the HyLYZER-1000. Among existing WE technologies, AEMWE has been considered a promising pathway toward large-scale hydrogen production at a low cost. The representative companies for developing commercial AEMWEs are Enapter and Ionomr. Enapter introduced the EL. 4.0 electrolyzer with 1 module, which can produce the H2 with the production rate of 1.0785 kg/hr. As a megawatt-class electrolyzer system, they developed the AEM Multicore with 420 stacks, with a production rate of 210 Nm3/h. The expected stack life is at least 35,000 h. Considering the target of PEMWEs, the AEMWE should achieve the durability of a similar stack lifetime and reach over 100,000 h in the future (2050).[90]
It is required to increase the system capacity for the cost-effectiveness of the electrolyzer system.[91] Electrolyzer costs are related to the system capacity, the electricity costs, and so forth. The system capital costs comprised of stack costs and costs of a balance of plants (BoP), which refers to the parts consisting of the hydrogen production system except for the stacks where WE occur. The DOE's 2020 target of electrolyzer system capital costs were reported to be 300$/kW. Ionomer innovations compared the investment costs of 1 MW and 5 MW electrolysis systems and showed that increasing the system capacity of an electrolysis system can significantly lower the costs. Among the WE system, AEMWE without PGM showed the costs of $444/kW, which indicates the cost competitiveness.[92] Although the investment cost is higher than the DOE target ($300/kW), it is expected to be achieved by increasing the system capacity and optimizing the BoP. According to the IRENA analysis, the target (2050') of AEM electrolyzer's capital costs (min. 1 MW) is <$100/kW and a 20 MW system for <$200/kW. Therefore, the AEMWE should be developed with the target of MW-scale system capacity, large area, system costs (<300 $/kW), and durability over 100,000 h for commercial green H2 production.
6 CHALLENGES AND PERSPECTIVES
- 1.
The development of AEM materials should be conducted in the direction of securing various properties (stability, IECs, ion conductivity, water uptake, swelling, etc.). Fundamental research should be conducted to identify how the degradation of the AEM proceeds in a certain operating environment and engineer the materials to supplement it. Exploiting in-situ analysis and calculation approaches are helpful in designing optimal membranes and comprehending the working mechanisms.
- 2.
Efficient HER and OER catalysts have been investigated in both computational and experimental approaches. However, depending on the operating environment of AEMWE, the required catalyst materials change. The AEMWE performance is evaluated under KOH, bicarbonate/carbonate, and water, and a lot of research have been reported in a KOH-fed environment, where the PGM-free catalysts show excellent catalytic properties. However, AEMWE should be operated in a pure water-fed condition ultimately. The challenges arise that the performance of the most widely used Ni catalysts is significantly degraded under pH 9. The development of stable catalysts in the pure-water fed operation is inevitable.
- 3.
The optimization of AEMWE is necessary. Even though the catalysts and membranes have excellent properties, AEMWE performance would be lower than the expected level during fabricating MEAs or assembling the components due to the mass transfer losses, electrochemical resistances, and so on. Also, depending on the cell operation condition, the performance of AEMWE varies, implying that the proper operating conditions should be tested to achieve high current density and long-term stability. When implementing a stack cell beyond a single cell, the behavior of the electrolytes should be considered because the laminar or turbulent electrolyte flow affects the stack cell performance.
- 4.
It is difficult to quantitatively compare the performance of membrane or AEMWEs since the operating condition or the measurement methods largely affect the characteristics. In the case of the existing water electrolytic half-cell, the properties of catalysts or the electrodes can be compared with Tafel slopes, overpotentials, and so forth. It is well-standardized and possible to derive the performance in a similar operating environment. However, it is difficult to compare the properties of AEMs in real operating conditions, although there are standardized measurement techniques. The discrepancies exist between the properties of reported commercial MEAs and the value measured in the laboratory. The performances of the AEMWEs are largely affected by the operating temperatures, feed solution, and so forth. Therefore, it is imperative to complement the measurement techniques, which reflect the real operating circumstance and develop specific standards to evaluate the efficiency of AEMWEs.
In summary, the development of highly durable membranes and catalysts should be conducted on a preferential basis. With the synthesis of membranes with proper cations and backbones, which is the bottleneck for high-performance AEMWEs, and PGM-free catalysts with stability in specific electrolytes,[94, 95] deliberate approaches toward MEA fabrication should be applied to minimize the performance losses. Lastly, all components should be joint optimally to reduce systematic losses, as shown in Figure 9. With the aid of the fundamental study of the membranes, catalysts, and MEAs, the AEMWE can become a mature technology for hydrogen production. Furthermore, shifting the water source of electrolysis from ultrapure water to seawater electrolysis might even massively increase the scale of hydrogen production compared to the traditional AEMWE with pure water. To realize the seawater electrolysis, an optimized catalyst must be developed since the local changes in pH near the electrode are accelerated in seawater, which might lead to severe catalyst degradation.[96] Also, chloride ions in seawater might participate in chloride oxidation reaction under high anodic potential, which calls for appropriate catalysts with high selectivity toward OER than chlorine evolution reaction to further mature the AEM technology for large-scale hydrogen production.[97]
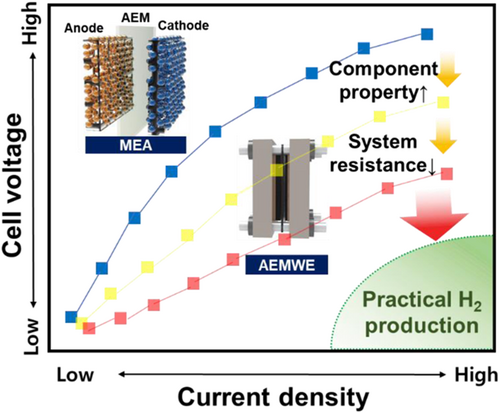
We believe that balanced research in developing AEMWE is one of the promising approaches to unleash the potential for large-scale H2 production and reach the profundity and analysis of AEMWE at the same time. We hope this review article provides a milestone toward developing high-performance AEMWE, from developing the components of the cells to standardizing the evaluation of the performance.
AUTHOR CONTRIBUTIONS
Sol A Lee: Conceptualization, Investigation, Writing (original draft, review & editing). Jaehyun Kim: Conceptualization, Investigation, Writing (original draft, review & editing). Ki Chang Kwon: Conceptualization, Investigation. Sun Hwa Park: Conceptualization, Supervision. Ho Won Jang: Conceptualization, Writing (review & editing), Supervision.
ACKNOWLEDGMENTS
Sol A Lee and Jaehyun Kim contributed equally to this study. This study was supported by the KRISS (Korea Research Institute of Standards and Science) MPI Lab. Program and the National Research Foundation of Korea (NRF) grant funded by the Korea Government MSIT (2021R1C1C2006142). The Inter-university Semiconductor Research Center and Institute of Engineering Research at Seoul National University provided research facilities for this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Biographies

Sol A Lee received her PhD from the Department of Materials Science and Engineering of Seoul National University in 2021. She is currently a postdoctoral researcher under the supervision of Prof. Ho Won Jang. Her research interests include the synthesis and design of transition metal-based electrocatalysts for energy conversion devices.

Sun Hwa Park is a senior research scientist at Korea Research Institute of Standards and Science (KRISS). She received her PhD from the Nanomaterials Science and Engineering at University of Science and Technology in 2013. She worked at University of California, Riverside in 2018 as a visiting scholar. Her current research interests focus on the synthesis of nanoengineered materials based on electrochemistry and the application of these materials in a variety of fields including sensors, energy storage systems, thermoelectrics, and electrocatalysis.

Ho Won Jang is a full professor at the Department of Materials Science and Engineering in the Seoul National University. He received his PhD from the Department of Materials Science and Engineering at Pohang University of Science and Technology in 2004. He worked as a research associate at the University of Madison-Wisconsin from 2006 to 2009. Before he joined Seoul National University in 2012, he worked at the Korea Institute of Science and Technology (KIST) as a senior research scientist. His research interests include materials synthesis and device fabrication for (photo)electrocatalysis, chemical sensors, memristors, micro-LEDs, and thin film transistors.




