Exorhodopsin and melanopsin systems in the pineal complex and brain at early developmental stages of Atlantic halibut (Hippoglossus hippoglossus)
ABSTRACT
The complexity of the nonvisual photoreception systems in teleosts has just started to be appreciated, with colocalization of multiple photoreceptor types with unresolved functions. Here we describe an intricate expression pattern of melanopsins in early life stages of the marine flat fish Atlantic halibut (Hippoglossus hippoglossus), a period when the unpigmented brain is directly exposed to environmental photons. We show a refined and extensive expression of melanopsins in the halibut brain already at the time of hatching, long before the eyes are functional. We detect melanopsin in the habenula, suprachiasmatic nucleus, dorsal thalamus, and lateral tubular nucleus of first feeding larvae, suggesting conserved functions of the melanopsins in marine teleosts. The complex expression of melanopsins already at larval stages indicates the importance of nonvisual photoreception early in development. Most strikingly, we detect expression of both exorhodopsin and melanopsin in the pineal complex of halibut larvae. Double-fluorescence labeling showed that two clusters of melanopsin-positive cells are located lateral to the central rosette of exorhodopsin-positive cells. The localization of different photopigments in the pineal complex suggests that two parallel photoreceptor systems may be active. Furthermore, the dispersed melanopsin-positive cells in the spinal cord of halibut larvae at the time of hatching may be primary sensory cells or interneurons representing the first example of dispersed high-order photoreceptor cells. The appearance of nonvisual opsins early in the development of halibut provides an alternative model for studying the evolution and functional significance of nonvisual opsins. J. Comp. Neurol. 522:4003–4022, 2014. © 2014 Wiley Periodicals, Inc.
Light has an important impact on life. Almost all animals are dependent on light to modulate their behavior and physiology, and as the intensity of light alters with the solar cycle animals have to adapt to the photic changes. The vertebrate eye is responsible for image-forming vision, and the retina can detect spatial and spectral differences of light. In addition, nonvisual photoreception supplies animals with measurements of irradiance and nondirectional photoreception. Although mammals are dependent on ocular photoreception to adapt to changes in the ambient light, photoreceptors in nonmammalian vertebrates have been detected in a wide range of tissues, including dermal melanophores, pineal organ, and deep brain cells (for review see Peirson et al., 2009; Davies et al., 2010). In contrast to mammals that are dependent on photic information from the retinal ganglion cells (Berson et al., 2002), nonmammalian vertebrates have photoreceptors in the pineal organ, which regulate the melatonin synthesis important for photoentrainment of the circadian rhythm directly (for review see Korf, 1994). The existence of additional photoreceptive capacity in the teleost brain was early indicated in studies of blinded and pinealectomized European minnows, in which skin pigmentation was altered in response to light stimulation of the head (Frisch, 1911). These deep brain photoreceptors have received little attention in the literature, although a few studies in teleosts have indicated an involvement in behavioral responses to light (van Veen et al., 1976; Tabata et al., 1989; Fernandes et al., 2012) and in birds, in which hypothalamic photoreceptor cells are thought to be responsible for photoperiodic responses (Halford et al., 2009; Davies et al., 2012). Numerous photoreceptive cell types and their photosensitive pigments have been identified over the last decades, but how these cells contribute to the behavioral and physiological responses to light is just emerging. In the Atlantic halibut, the hatching mechanism is known to be regulated by light (Helvik and Walther, 1992). This physiological process takes place long before the retina is differentiated (Kvenseth et al., 1996). Hence, this study takes advantage of Atlantic halibut in order to study nonvisual photoreception at early stages of teleost development.
The nonvisual opsin melanopsin was originally identified in dermal melanophores of Xenopus laevis (Provencio et al., 1998) and since has been shown to have a significant role in photoentrainment of the circadian rhythm in mammals (Provencio et al., 2000; Gooley et al., 2001; Panda et al., 2002; Hattar et al., 2003). In nonmammalian vertebrates, two different melanopsin classes have been identified, the mammalian-like (opn4m) and the Xenopus-like (opn4x) (Bellingham et al., 2006). In teleosts, melanopsins are expressed both in the eye and in the brain. In zebrafish, expression of melanopsin has been detected in all retinal layers of adult eyes (Davies et al., 2011), and melanopsin is detected in discrete regions of the zebrafish brain already prior to retinogenesis (Matos-Cruz et al., 2011). In the brain of juvenile Atlantic cod, expression of melanopsin is detected in the habenula and suprachiasmatic nucleus (Drivenes et al., 2003). Current studies with Atlantic salmon demonstrate expression of different melanopsin genes in multiple regions of the brain and cell populations that are located in regions associated with different neuroendocrine systems (Sandbakken et al., 2012). Studies in teleosts have located different melanopsins in the same retinal layers (Davies et al., 2011), and melanopsin is also suggested to be colocalized with vertebrate ancient opsin in the horizontal-cell layer (Jenkins et al., 2003; Cheng et al., 2009) and in cells resembling amacrine cells of the inner nuclear layer (Sandbakken et al., 2012). In the Atlantic salmon brain, colocalization of melanopsin and vertebrate ancient opsin has been observed in the left habenula and in the dorsal thalamus (Sandbakken et al., 2012). The nonvisual opsin exorhodopsin has been shown be a pineal-specific opsin expressed in the pineal organ from early in development of teleosts and has been indicated to have a role in the circadian rhythm (Pierce et al., 2008). By using fluorescence double-labeling techniques, the present study reveals how melanopsin is expressed in close connection with the pineal-specific exorhodopsin in the early developmental stages of Atlantic halibut.
Halibut eggs hatch at an early developmental stage, and the hatched larvae have a large yolk sac and a primitive larval body (Lønning et al., 1982; Haug, 1990). In halibut, the pineal has been indicated to be important in perceiving and mediating photic information in the dark-dependent hatching mechanism. It has been suggested that the pineal may influence the time of hatching, because the pineal contain molecules involved in the phototransduction cascade already before hatching (Forsell et al., 1997). Recent studies in zebrafish have, however, indicated other regions of the brain as being important for light-dependent physiological processes. In unhatched preretinal zebrafish, photoreceptors in the hindbrain were shown to be responsible for a “photomotor response” after exposure to intense light (Kokel et al., 2013). In addition, zebrafish larvae lacking eyes and pineal organs demonstrate a light-seeking behavior triggered by loss of illumination, and melanopsin-expressing cells in the preoptic area were found to regulate this behavior (Fernandes et al., 2012).
To investigate further the early embryonic and preretinal light responses in fish, we have characterized melanopsin and exorhodopsin nonvisual systems in Atlantic halibut. Halibut have a more than 1-month-long early life history prior to a functional retina, and during this period a complex expression pattern of the melanopsins is detected, coincident with the light-regulated hatching mechanism. We find expression of melanopsin in various brain regions and an extensive expression of exorhodopsin in the pineal organ. By fluorescence double-labeling techniques, the relative distribution of a mammalian-like melanopsin and exorhodopsin is shown in the pineal region. In addition, this study evaluates the ontogeny of the melanopsin-expressing photoreceptor cells at early stages (preretinal) in relation to the melanopsin expression in the brain and retina of halibut larvae with functional eyes.
MATERIALS AND METHODS
Animals
Eggs, larvae, and juvenile Atlantic halibut (Hippoglossus hippoglossus) were obtained from the Institute of Marine Research, Austevoll Aquaculture Station, Norway. All experiments described follow the local animal care guidelines and were given ethical approval by the Norwegian Veterinary Authorities.
Molecular cloning
Total RNA was isolated from the retina and brain of juvenile Atlantic halibut (Hippoglossus hippoglossus) by Trizol reagent (Life Technologies, Bethesda, MD). Purification of poly-A+ mRNA was performed with Oligotex resin (Qiagen, Germany), and preparation of double-stranded cDNA and adaptor-ligated cDNA was performed with a Marathon cDNA Amplification Kit (Clontech, Palo Alto, CA).
Isolation of the halibut mammalian-like melanopsins (opn4m1 and opn4m3) was performed with a nested approach with degenerative primers described by Sandbakken et al. (2012). For the first round of PCR the annealing temperature was 52°C, and 30 cycles were used. PCR product from the first round of PCR served as a template for the second round of PCR with annealing temperature 50°C and 30 cycles. The halibut Xenopus-like melanopsin (opn4x2) was identified by using opsin specific degenerative primers described by Helvik et al. (2001) and then melanopsin-specific degenerative primers with a nested approach. Annealing temperature for the first round of PCR was 46°C with 30 cycles, and the nested PCR had an annealing temperature of 46°C with 35 cycles. Generation of full-length sequence for opn4m1, opn4m3, and opn4x2 were obtained by 5′- and 3′-rapid amplification of cDNA ends (RACE) nested PCR. The reactions were performed according to the recommendations of Clontech by touchdown PCR (primers are listed in Table 1). To verify the assembly of the RACE products, a PCR with primer binding sites located in the predicted the 5′- and 3′-untranslated regions (UTRs) was performed. All PCR products were extracted from agarose gel with QIAEX II Gel Extraction Kit (Qiagen, Germany) or MinEluteGel Extraction Kit (Qiagen, Germany) before cloning into StrataClone PCR cloning vector pSC-A-amp/kan (Agilent Technologies, LA Jolla, CA) or pGEMT-Easy Vector (Promega, Madison, WI) and sequencing at the University of Bergen Sequencing Facility.
| Primer name | Sequence (5′–3′) | Use |
|---|---|---|
| Opn4mFw2 | GGGCATCACMGGCATGSTGGGAAACYT | Degenerative primer for opn4m1 and opn4m3 |
| Opn4mFw2-N | ATCTGCTCSATGATCACRCTSAYRTKAT | Nested degenerative primer for opn4m1 and opn4m3 |
| Opn4mRv2 | GATGWGTKATGGCRTADATGATGGGGTTGT | Degenerative primer for opn4m1 and opn4m3 |
| Opn4mRv2-N | CCARGAGATGACAWAMADCAGTAGSACWAT | Nested degenerative primer for opn4m1 and opn4m3 |
| OpsinFw | AAGAAGYTCMGTCMACCTCTYAAYT | Degenerative primer for opn4x2 |
| OpsinRv | GTTCATGAAGACRTAGATDAYAGGGTTRTA | Degenerative primer for opn4x2 |
| MopsF | GCTKTSTTCGGMATMACGTCMATG | Nested degenerative primer for opn4x2 |
| MopsR | GMAGCAGCASAGCAKCAWSGTGTA | Nested degenerative primer for opn4x2 |
| HhMelF1 | GGGTCTGCTGACTTCCTGTTCCT | 3′-RACE opn4m1 |
| HhMelF2 | GGGGAAGTTTAACGGCAGCACTC | Nested 3′-RACE opn4m1 |
| HhMelR1 | CGCACCGACGGCGTAAAGGTCAT | 5′-RACE opn4m1 |
| HhMelR2 | GTCCCAGGAACAGGAAGTCAGCA | Nested 5′-RACE opn4m1 |
| HhMelF3 | CGCTCCTACACGATGCTGCTCTT | 3′-RACE opn4m3 |
| HhMelF7 | TGTGGCCCTCACTGCATTCG | Nested 3′-RACE opn4m3 |
| HhMelR3 | GTAGACCCAGGCAACAGCCAAGA | 5′-RACE opn4m3 |
| HhMelR4 | GCTAAGGGCTTTCCTGCGAGACA | Nested 5′-RACE opn4m3 |
| HMops1F3 | GAGGGGCTGATGACGTCTTGT | 3′-RACE opn4x2 |
| HMops1F2 | ACGTCTTGTACGTGGGATTACGTC | Nested 3′-RACE opn4x2 |
| HMops1R3 | GATATAAGAGCTCCAGCCGACGA | 5′-RACE opn4x2 |
| HMops1R2 | TGATAACCACGTAGCGGTCGAT | Nested 5′-RACE opn4x2 |
Recently, the halibut genome was sequenced on an Illumina HiSeq200 (Illumina, San Diego, CA; pair end, 100-bp reads) to 100× coverage, and a contig assembly was made with the CLC software (CLC Bio, Copenhagen, Denmark). Opn4m1, opn4m3, and opn4x2, identified by degenerative PCR and RACE PCR, were verified by searching the genome with BLASTN (NCBI, Bethesda, MD, RRID:nlx_153932) and TBLASTN (NCBI, RRID:OMICS_00999). In addition, the genome was searched by TBLASTN using available teleost protein opsin sequences as a query in order to obtain more halibut opsin genes, and a second Xenopus-like melanopsin (opn4x1), a remnant of a melanopsin gene and exorhodopsin (exorh) was found. Putative opsin genes were predicted based on the BLAST alignments and GENSCAN (Burge and Karlin, 1997; RRID:nif-0000–30609), and the annotation was based on BLASTX (NCBI, MD, RRID:nlx_153933) against GenBank (NCBI, RRID:nif-0000-02873) and phylogenetic analysis. Verification of the predicted opsins were done by PCR using primers with binding sites in the 5′- and 3′-UTRs, and cloning and sequencing were performed as described for the other melanopsins.
Sequence and phylogenetic analyses
Analysis and assembly of the sequences were carried out with the Vector NTI9 software (Invitrogen, Carlsbad, CA), and primer design was performed in ApE-A plasmid Editor v2.0.36. ClustalX 2.1 (Larkin et al., 2007) was used to align the amino acid sequences of melanopsins. Phylogenetic analysis was carried out by constructing a maximum-likelihood tree in MEGA version 5 (Tamura et al., 2011; RRID:nlx_156838), and 1,000 bootstrap replicates were applied to ensure the statistical robustness of each node. The four melanopsins identified in Atlantic halibut were named according to the nomenclature of Bellingham et al. (2006) and Davies et al. (2011).
Riboprobes
Digoxigenin (DIG)-labeled and/or fluorescein-labeled riboprobes for the four halibut melanopsins and exorhodopsin were made following the manufacturer's instructions (Roche Diagnostics, Mannheim, Germany). In the synthesis of the riboprobes, PCR product was used as template for the reaction as described by Thisse and Thisse (2008), and the synthesized probes were precipitated by LiCl and EtOH together with tRNA (Roche Diagnostics). Sequence alignment shows that the similarity between the sequence targets of the melanopsin probes does not exceed 75%.
In situ hybridization on whole embryos and larvae
Embryos and larvae of different developmental stages of Atlantic halibut were fixed in 4% paraformaldehyde-buffered PBS (pH 7.4) for 48 hours at 4°C. After a brief wash in 1× PBS, the whole embryos and larvae were dehydrated in methanol and stored at −20°C in 100% methanol until use.
Whole mount in situ hybridization was started by rehydrating the embryos and larvae in methanol (75−25%) and then rinsing them for 2 × 5 minutes in 1× PBS, pH 7.4. Embryos were dechorinated, and the yolk was removed. Larvae with pigmentation were bleached in 3% H2O2/0.5% KOH as described by Thisse and Thisse (2008), and the bleaching was stopped by first washing for 5 minutes in 1× PBS and then for 4 × 5 minutes in 1× PBST (0.1% Tween20 (Sigma, St. Louis, MO), in 1× PBS). The tissue was permeabilized with proteinase K (Promega) treatment (10 μl/ml in 0.1 M Tris-HCl, pH 8.0, and 50 mM EDTA), and the time of treatment was optimalized for the size of the embryos and larvae. After a rinsing step with 1× PBST, the embryos and larvae were fixed in 4% paraformaldehyde-buffered PBS (pH 7.4) and then washed for 4 × 5 minutes in 1× PBST. Prehybridization was carried out at 65°C for 2 hours in hybridization solution without probe before incubation with hybridization solution with probe overnight at 65°C. The hybridization solution was composed of 10 mM Tris-HCl, pH 7.5, 300 mM NaCl, 1 mM EDTA, 0.2% Tween20, 1% blocking reagent (Roche Diagnostics), 10% dextransulfate (Sigma), and 50% formamide (Sigma-Aldrich). After hybridization washing series of 2 × 15 minutes in 50% formamide (VWR, West Chester, PA) in 2× SSCT (300 mM NaCl, 30 mM C6H5Na3O7 × 2H2O, 0.2% Tween [Sigma]), 1 × 30 minutes in 2× SSCT, 2 × 15 minutes in 2× SSCT, and 2 × 15 minutes in 0.2× SSCT were performed at 65°C. To remove unhybridized probe, the embryos and larvae were treated with RNase A (0.02 mg/ml; Sigma) for 20 minutes at 37°C before washing with RNase buffer (10 mM Tris-HCl, pH 7.5, 0.5 M NaCl, 1 mM EDTA) for 20 minutes at 65°C. The embryos and larvae were incubated in 2× SSC, 0.05% Triton X-100 (Sigma) and 2% blocking reagent for 2−3 hours before overnight incubation with antidigoxigenin-alkaline phosphatase Fab fragments (1:2,000; catalog No. 11093274910, Roche Diagnostics, RRID:AB_514497) in 2× SSC, 1% blocking reagent, and 0.3% Triton X-100. To remove redundant antibody, the embryos and larvae were washed for 4 × 20 minutes in 1× PBST and for 2 × 10 minutes in visualization buffer (100 mM Tris-HCl, pH 9.5, 100 mM NaCl, 50 mM MgCl2). Visualization was done in darkness with freshly made chromogen substrate 45 μl 4-nitroblue tetrazolium chloride (Roche Diagnostics) and 35 μl 5-bromo-4-chloro-3-indolyl-phosphate (Roche Diagnostics) in 10 ml visualization buffer. Sense probes were used as a control for nonspecific DIG probe labeling. Visualization was stopped by washing in stop solution (10 mM Tris-HCl, pH 7.5, 1 mM EDTA, and 150 mM NaCl) before mounting in 70% glycerol (Sigma) in stop buffer.
In situ hybridization on sectioned embryos and larvae
Embryos (14 days postfertilization [dpf]) and larvae (47 days posthatching [dph]) of Atlantic halibut were fixed in 4% paraformaldehyde-buffered PBS (pH 7.4) for 48 hours at 4°C. After a brief wash in 1× PBS, the embryos and larvae were incubated in a solution of 25% sucrose, 25% Tissue Tek (Sakura Finteks, Netherlands) and 50% 1× PBS overnight at 4°C. They were mounted in a mold of Tissue Tek and rapidly frozen on an iron block precooled in liquid nitrogen. Parallel sectioning (10 μm) was performed with a Leica CM 3050S cryostat, and before storage at −20°C the tissue was air dried for 1 hour at room temperature and for 10 minutes at 65°C. One parallel of the 47 dph sectioned larvae was Nissl-stained with 0.5% cresyl fast violet (Chroma-Gesellschaft, Germany), and the other parallel was stained by in situ hybridization. In situ hybridization was carried out as described by Sandbakken et al. (2012).
In situ hybridization on whole embryos using fluorescence double-labeling techniques
Fluorescence double-labeling in situ hybridization was used to identify expression of two genes in the same embryo. Fluorescein-labeled riboprobe for exorhodopsin (exorh) and digoxigenin-labeled riboprobe for the mammalian-like melanopsin (opn4m1) were used. Preparation and in situ hybridization were performed as described above, with the following modifications. At the hybridization step, both probes were applied. The fluorescein-labeled probe was first visualized by using the antibody antifluorescein-horseradish peroxidase (POD) Fab fragments (1:400; catalog No. 11426346910, Roche Diagnostics, RRID:AB_840257) and the TSA Plus Fluorescein System (catalog No. NEL741001KT; PerkinElmer, Waltham, MA) according to the producer's protocol. Before applying antidigoxigenin-alkaline phosphatase Fab fragments (1:2,000; catalog No. 11093274910, Roche Diagnostics, RRID:AB_514497) the embryos were blocked for 4 hours in 2% blocking reagent (Roche Diagnostics) and 2× SCC, and the digoxigenin probe was visualized by use of Fast Red tablets as recommended by the manufacturer (catalog No. 11496549001, Roche Diagnostics). The stained embryos were mounted in DABCO anti-fading medium (triethylenediamine, Sigma) and stored in darkness.
Immunohistochemistry
Immunohistochemistry with antibody against serotonin (5-HT) to mark the serotonergic system was performed. Whole embryos at 14 dpf were washed in 2% PBST (2% Triton X-100; Sigma) in 1× PBS) for 2 days at 4°C before washing for 2 × 30 minutes with 1.5% PBST (1.5% Triton X-100 in 1× PBS). Incubation with primary antibody for 3 days at 4°C was done with 1.5% PBST/1% bovine serum albumin (BSA; Sigma). The primary antibody was polyclonal anti-serotonin antibody produced in rabbit (catalog No. 20080; DiaSorin Italy, RRID:AB_572263) with a concentration of 1:1,000. Embryos were washed for 2 × 30 minutes in 1× PBS prior to incubation overnight at 4°C with the second antibody, anti-rabbit IgG (H + L), CF 555 antibody produced in goat 2 mg/ml (catalog No. SAB4600068, Sigma-Aldrich, RRID:AB_2336059). A concentration of 1:100 was used for the secondary antibody in 1× PBS/1% BSA (Sigma). The incubation was ended by washing for 2 × 30 minutes in 1× PBS before mounting in DABCO antifading medium (Sigma) and storage in darkness.
Fluorescent in situ hybridization and immunohistochemistry
Fluorescent in situ hybridization together with immunohistochemistry was used to evaluate the expression pattern in the region of the pineal. In situ hybridization on embryos was performed with the TSA Plus Fluorescein System together with fluorescein-labeled exorhodopsin riboprobe as described above. Before immunohistochemistry on the fluorescent-stained embryos, they were incubated for 4 hours in 2% blocking reagent (Roche Diagnostics). Monoclonal antiacetylated tubulin antibody produced in mouse (clone 6–11 B-1; 1:1,000; catalog No. T7451, Sigma-Aldrich, RRID:AB_609894) was incubated overnight in room temperature with 1% blocking reagent in 2× SSC. Prior to incubation with secondary antibody, the embryos were washed with 1× PBS for 3 × 10 minutes. The secondary antibody used was anti-mouse IgG (H + L), CF 555 antibody produced in goat 2 mg/ml (1:100; catalog No. SAB4600066, Sigma-Aldrich, RRID:AB_2336060) in 1× PBS/1% BSA. The incubation took place overnight at room temperature before washing for 2 × 30 minutes in 1× PBS and mounting in DABCO antifading medium (Sigma) and storage in darkness.
Antibody characterization
Rabbit antiserotonin (catalog No. 20080, DiaSorin, RRID:AB_572263) is a polyclonal antibody against serotonin and has been shown to have similar staining in classical serotonergic neuronal populations in vertebrates (Ekström and Ebbesson, 1988; Ebbesson et al., 1992; Sandbakken et al., 2012). Mouse antiacetylated tubulin (catalog No. T7451, Sigma-Aldrich, RRID:AB_609894) is a monoclonal antibody that recognizes an epitope located on the α3 isoform of Chlamydomonas axonemal α-tubulin. The antibody has been shown to recognize a single 55-kDa band (the predicted molecular weight of acetylated tubulin) on Western blots of teleost brain extracts (Liu and Lessman, 2007). In addition, the antibody has also been shown to label specifically axons in the developing CNS of zebrafish (Chitnis and Kuwada, 1990). The antibody has been used to label axons in many organisms, including teleosts (Ledizet and Piperno, 1991; Hunter et al., 2011; Verpy et al., 2011). See Table 2 for details.
| Antibody | Immunogen | Manufacturer, host species, mono- vs. polyclonal, catalog number, RRIDs | Dilution used |
|---|---|---|---|
| Antiserotonin | Serotonin (5-HT) | DiaSorin (Italy), rabbit polyclonal, 20080, RRID:AB_572263 | 1:1,000 |
| Antiacetylated tubulin | Chlamydomonas axonemal α-tubulin within four residudes of acetylated Lys-40 | Sigma-Aldrich (St. Louis, MO), mouse monoclonal, IgG2b, T7451, RRID:AB_609894 | 1:1,000 |
| Antidigoxigenin-alkaline phosphatase Fab fragments | Digoxigenin (DIG) | Roche Diagnostics (Germany), sheep polyclonal, 11093274910, RRID:AB_514497 | 1:2,000 |
| Antifluorescein-horseradish peroxidase Fab fragments | Fluorescein | Roche Diagnostics, sheep polyclonal, 11426346910, RRID:AB_840257 | 1:400 |
| Anti-rabbit IgG (H + L), CF 555 | Rabbit IgG (H + L) | Sigma-Aldrich, goat polyclonal, SAB4600068, RRID:AB_2336059 | 1:100 |
| Anti-mouse IgG (H + L), CF 555 | Mouse IgG (H + L) | Sigma-Aldrich, goat polyclonal, SAB4600066, RRID:AB_2336060 | 1:100 |
Microscopy and photographic manipulation
All brightfield photographs were taken witjh a digital camera (Leica DFC 320) attached to a Leica DM 6000B microscope. For fluorescent microscopy, an ebx75mc-L90 lamp (Leistrungselektronik GmbH, Jena, Germany) was used together with the same microscope, a digital camera (Leica DFC 350), and the filter cubes GFP and Y3 (Leica). Adobe Photoshop CS5 was used for adjustments of brightness, contrast, and color levels and to sharpen the pictures.
RESULTS
Identification and characterization of opsins in Atlantic halibut
Three full-length cDNAs of melanopsins in Atlantic halibut were identified by PCR and later verified by searching the halibut genome. In addition, one melanopsin, a remnant of a melanopsin gene, and exorhodopsin were found by searching the halibut genome with a BLAST algorithm. Two of the melanopsins, opn4m1 and opn4m3, are comparable to the mammalian-like melanopsins (opn4m) and two, opn4x1 and opn4x2, are similar to the Xenopus-like type (opn4x). The PCR run with primer binding sites located in the predicted the 5′- and 3′-UTRs revealed that the mammalian-like melanopsin (opn4m3) has two splice variants (long and short) differing in the cytoplasmatic tail. The long isoform has a stretch of 22 amino acids not seen in the short isoform, and by searching the halibut genome we identified an exon coding for 22 amino acids between the last two exons of the short isoform. In zebrafish, an additional intronless mammalian-like melanopsin has been identified, thought to have arisen from retrotransposition (Bellingham et al., 2006). In the degenerative PCR screen for halibut melanopsins, no orthologue for the intronless zebrafish gene was detected. However, by searching the halibut genome with a BLAST algorithm we identified a remnant of a potential intronless gene. The remnant is a pseudogene that does not span the seven-transmembrane region, and it has deletions and frameshifts (data not shown). The other four melanopsin genes detected have an exon−intron structure. GenBank accession No., cDNA length, open reading frame (ORF), and amino acid sequence are listed in Table 3 for the four functional melanopsins and exorhodopsin.
| Name | GenBank No. | cDNA length (bp) | Predicted ORF (bp) | Predicted aa length | Binding site for the in situ probe (5′–3′) (bp) | Probe length (bp) |
|---|---|---|---|---|---|---|
| opn4m1 | KF941289 | 2,138 | 1,527 | 508 | 159–1,392 | 1,172 |
| opn4m3short | KF941290 | 1,780 | 1,638 | 545 | 1–1,089 | 1,089 |
| opn4m3long | KF941291 | 1,846 | 1,704 | 567 | 1–1,089 | 1,089 |
| opn4x1 (partial) | KF941292 | 1,321 | 1,302 | 434 | 1–569 | 569 |
| opn4x2 | KF941293 | 2,328 | 1,671 | 556 | 657–1,859 | 1,203 |
| exorh | KF941294 | 1,274 | 1,059 | 352 | 1–1,274 | 1,274 |
An alignment (Fig. 1) of the four functional melanopsins in Atlantic halibut together with published melanopsin amino acid sequences of zebrafish (Opn4m) shows that they all contain the seven-transmembrane α-helical domains (TM1−7) characteristic of the opsins, and they have the lysine (K) in the TM7 that serves as a site for Schiff base linkage of the chromophore 11-cis retinal (for review see Terakita, 2005). In the third transmembrane domain, the glutamic acid (E), a counterion typical for visual opsins, is replaced with an aromatic tyrosine (Y) residue (Provencio et al., 1998) common with the glutamic acid (E) in the extracellular loop between the TM4 and TM5 being the putative displaced counterion of the Schiff base (Terakita et al., 2000). The tripeptide DRY in the interface between TM3 and the second intracellular loop and an asparagine (N) in TM2 thought to be critical for G-protein activation are conserved in the halibut melanopsins (Bockaert and Pin, 1999). To stabilize the tertiary structure, a disulfide bridge between cysteins (C) of the first and second extracellular loops is formed (Karnik and Khorana, 1990); these cysteins are also present in the halibut melanopsins.
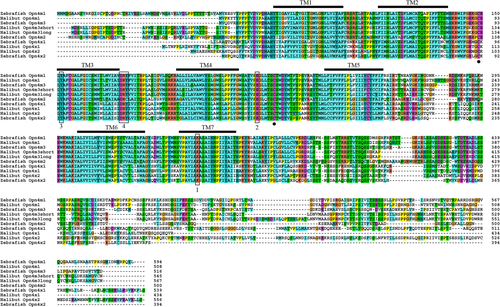
Alignment. Alignment of the deduced amino acid sequence of Atlantic halibut melanopsins against published melanopsin sequences of zebrafish. The seven transmembrane (TM) domains are indicated by TM1-7 (in accordance with Matos-Cruz et al., 2010), and the alignment shows that all the halibut melanopsins span the domains. The retinal attachment site (K) in TM7 (1), the potential Schiff base counterions E and Y (2,3), and the DRY tripeptide (4) are outlined. Cysteins involved in the disulfide bridge formation are indicated with solid circles.
Phylogenetic analysis
The maximum likelihood tree (Fig. 2) based on amino acid sequences shows the putative evolutionary relationship between the halibut melanopsins and various melanopsins of different species. In addition, the visual opsins and exorhodopsin of halibut and zebrafish are included. As shown by Bellingham et al. (2006) the melanopsins divide into two branches, the mammalian-like (opn4m-like) and the Xenopus-like (opn4x-like) melanopsins. The halibut melanopsins are positioned in these two branches, and the teleost duplication of melanopsins is also present in halibut. Exorhodopsin, the pineal-specific opsin (Mano et al., 1999; Philp et al., 2000), branches together with rod opsin, because they arose from an ancient duplication in teleosts (Bellingham et al., 2003).
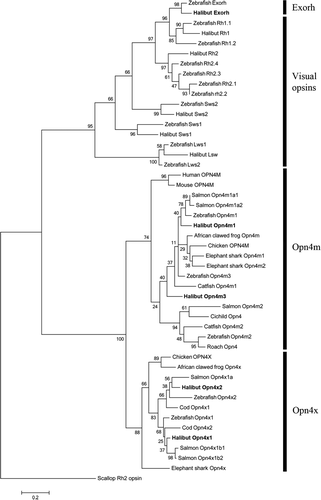
Phylogenetic tree. The maximum likelihood tree shows the phylogenetic relationship between the halibut melanopsins and melanopsins of other species. In addition, the visual opsins and exorhodopsin of halibut and zebrafish are included. Scallop (Mizuhopecten yessoensis) BAA22218 (Rh2 opsin) has been used as an out-group. A bootstrap value of 1,000 has been applied. The opsin sequences used for generating the tree are 1) exorhodopsin: zebrafish (Danio rerio) NP571287 (Exorh); halibut (Hippoglossus hippoglossus) KF941294 (Exorh); 2) visual opsins: zebrafish (Danio rerio) NP571159 (Rh1.1); halibut (Hippoglossus hippoglossus) AAM17918.1 (Rh1), zebrafish (Danio rerio) ADK38855; halibut (Hippoglossus hippoglossus) AAM17916.1 (Rh2); zebrafish (Danio rerio) NP571328 (Rh2.1), NP878311 (Rh2.2), NP878312 (Rh2.3), NP571329 (Rh2.4); zebrafish (Danio rerio) NP571267 (Sws2); halibut (Hippoglossus hippoglossus) AAM17920.1 (Sws2); zebrafish (Danio rerio) NP571394 (Sws1); halibut (Hippoglossus hippoglossus) AAM17917.1 (Sws1); zebrafish (Danio rerio) NP571250 (Lws1); halibut (Hippoglossus hippoglossus) AAM1792.1 (Lws1); zebrafish (Danio rerio) NP001002443 (Lws2); 3) Mammalian-like melanopsin: human (Homo sapiens) ENSP00000361141 (OPN4M); mouse (Mus musculus) ENSMUSP00000022331 (OPN4M); salmon (Salmo salar) AFI61534.1 (Opn4m1a1), AFI61536.1 (Opn4m1a2); zebrafish (Danio rerio) ENSDARP00000109133 (Opn4m1); halibut (Hippoglossus hippoglossus) KF941289 (Opn4m1); African clawed frog (Xenouus laevis) ABD37674.1 (Opn4m); chicken (Gallus gallus) ABX10832.1 (OPN4M); elephant shark (Callorhinchus milii) AFU50495.1 (Opn4m1), AFU50496.1(Opn4m2); zebrafish (Danio rerio) ENSDARP00000070530 (Opn4m3); catfish (Ictalurus punctatus) ACP43590.1 (Opn4m1); halibut (Hippoglossus hippoglossus) KF941291 (Opn4m3); salmon (Salmo salar) JN210550.1 (Opn4m2); Cichild (Astatotilapia burtoni) ACB29678.1 (Opn4); catfish (Ictalurus punctatus) ACP43591.1 (Opn4m2); zebrafish (Danio rerio) ENSDARP00000070530 (Opn4m2); roach (Rutilus rutilus) AAO38857.1 (Opn4); and 4) Xenopus-like melanopsin: chicken (Gallus gallus) ABX10830.1 (OPN4X); African clawed frog (Xenouus laevis) NP_001079143.1 (Opn4x); salmon (Salmo salar) AFI61533.1 (Opn4x1a); halibut (Hippoglossus hippoglossus) KF941293 (Opn4x2); zebrafish (Danio rerio) ENSDARP00000123655 (Opn4x2); cod (Gadus morhua) AAO20043.1 (Opn4x1); zebrafish (Danio rerio) ENSDARP00000100318 (Opn4x1); cod (Gadus morhua) AAM95160.1 (Opn4x2); halibut (Hippoglossus hippoglossus) KF941292 (Opn4x1); salmon (Salmo salar) AFI61531.1 (Opn4x1b1), AFI61532.1 (Opn4x1b2); elephant shark (Callorhinchus milii) AFU50497.1 (Opn4x).
Expression of melanopsin at the stage of hatching
In situ hybridization with whole embryos (Fig. 3) shows that the two mammalian-like melanopsins (opn4m1 and opn4m3) and one of the Xenopus-like melanopsins (opn4x2) are expressed in the brain and spinal cord at the time of hatching. No expression of opn4x1 was detected at the stage of hatching. Sense probes were included as controls for nonspecific DIG probe labeling for all the melanopsins, showing no staining (data not shown).
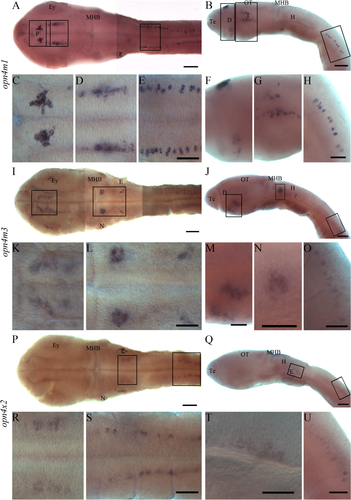
In situ hybridization on whole halibut larvae at the stage of hatching. Expression in the brain and spinal cord is shown from a dorsal and lateral view with different magnifications. The higher magnifications are indicated in the overview pictures by squares. The overview pictures (A,B,I,J,P,Q) were made by placing several pictures with different focal planes together. A-H: Expression of opn4m1 close to the pineal (P), ventrally in diencephalon (D), in tegmentum (T), in optic tectum (OT), ventral to the neuromasts (N), and in spinal cord. I-O: Expression of opn4m3 in ventral diencephalon, in the hindbrain (H), medial to the ear (E), and in the spinal cord. P-U: Expression of opn4x2 medial to the ear and in the spinal cord. For abbreviations see list. Scale bars = 100 μm in A, B, I, J, P, Q, 50 μm C-H, K-Q, R-U.
Expression of opn4m1 (Fig. 3A−H) was detected in several regions of the brain and in the spinal cord. Dorsally in diencephalon, opn4m1 is expressed close to the pineal, and a dorsal view of the expression pattern (Fig. 3A,C) shows that the expression is located a few cells lateral to the midline on both sides. Ventrally in diencephalon, two clusters of cells of opn4m1 were detected in the presumptive future preoptic area (Fig. 3B,F). In the midbrain, an opn4m1-positive cell was seen in the optic tectum, and a cluster of cells is situated just ventral to the optic tectum (Fig. 3B,G). In the tegmentum, a broad cluster of opn4m1 expression was detected (Fig. 3B,G), and seen from a dorsal view the expression is just medial to the developing eye (Fig. 3A,D). In the hindbrain, a small cluster of opn4m1 cells was detected ventral to the neuromast cell on both sides (Fig. 3B). In the spinal cord, distinct cells expressing opn4m1 were seen just dorsal to the developing notochord (Fig. 3A,B,E,H).
Expression of opn4m3 was also detected in several regions of the brain and in the spinal cord (Fig. 3I−O). In the brain, two regions of the ventral diencephalon have opn4m3 expression (Fig. 3I−K,M). The most rostroventral cluster is located in the presumptive future preoptic area (Fig. 3K), whereas the other cluster is situated more dorsally (Fig. 3J,M). In the hindbrain, a bilateral ball of opn4m3-positive cells was seen. The central cells in the cluster lack opn4m3 expression. The clusters are just caudal to the midbrain−hindbrain boundary and at the level of the lateral neuromasts (Fig. 3I,J,L,N). Expression of opn4m3 was also detected medial to the ear (Fig. 3I,J,L). In the spinal cord, distinct cells expressing opn4m3 were seen just dorsal to the developing notochord, but the opn4m3-positive cells start more caudally than the opn4m1 cells (Fig. 3I,J,O). Expression of opn4x2 (Fig. 3P−U) is located medial to the ear (Fig. 3P−R,T) and in the spinal cord in a pattern similar to that of opn4m3 (Fig. 3P,Q,S,U). Staining seen in the ear (Fig. 3P) is not consistent and may be artificial, resulting from trapping of probe in the ear.
Expression of exorhodopsin and melanopsin in the region of the pineal organ
In situ hybridization revealed expression of exorhodopsin in the halibut pineal at the stage of hatching (Fig. 4A), and the mammalian-like melanopsin (opn4m1) is also expressed in the same region (Fig. 4B). Cryosections on embryos (14 dpf) show exorh-positive cells in the pineal (Fig. 4C) and opn4m1-positive cells surrounding the pineal (Fig. 4E). The cryosection also showed that the melanopsin expression is not in the epithelial cells covering the pineal (Fig. 4E). Fluorescence double labeling of whole embryos further compared the expression of the exorh (Fig. 4D) and opn4m1 (Fig. 4F) in the region of the pineal and revealed that the mammalian-like melanopsin is expressed in cells just adjacent to the exorhodopsin-expressing cells of the pineal (Fig. 4G). The two opsins are not expressed in the same cells (Fig. 4G). A combination of in situ hybridization (exorh) and immunohistochemistry (antiacetylated tubulin) demonstrated an axonal connection between the exorhodopsin-expressing pineal and deep brain cells (Fig. 4H−M) at the stage of hatching. A lateral view (Fig. 4H,J,K) shows an axon from the exorhodopsin-expressing pineal descending deep in the diencephalon anterior to the posterior commissure. This corresponds to previous studies in halibut suggesting the presence of neural signaling pathways between the pineal and the brain at the time of hatching (Forsell et al., 2001). From a dorsal view, it is evident that pineal neuronal cell bodies are located medially, where no exorhodopsin expression is seen, and the axons from these cell bodies descend laterally in the developing brain (Fig. 4I,M). Detection of antibody against 5-HT showed 5-HT-positive cells in the pineal (Fig. 4N, O) and in the ventral part of the diencephalon and midbrain (Fig. 4N,P) at the stage of hatching (see Supp. Info. Fig. 1 for a magenta/green copy),
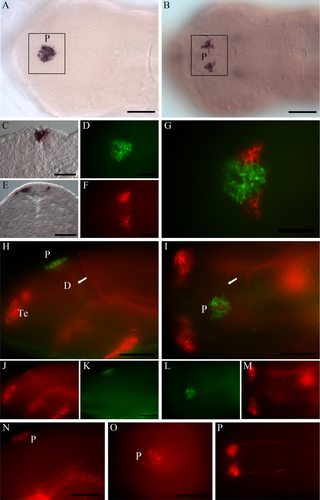
At the stage of hatching, exorhodopsin- and melanopsin-positive cells are apparent in the pineal region. A,B: Dorsal view showing the expression of exorh (A) and opn4m1 (B) in the pineal region (P). C,E: Transverse sections through the pineal region demonstrate the localization of exorh - (C) and opn4m1 positive cells (E). D,F,G: Expression of exorh (D; green) and opn4m1 (F; red) in the same embryo show that the two genes are expressed in adjacent cells (G). H-M: The neuronal projections (antiacetylated tubulin; red) from the exorhodopsin (green)-expressing pineal are shown in lateral (H,J,K) and dorsal (I,L,M) views, illustrating that the projections from the pineal descend deep in the brain (white arrows). N-P: 5-HT-positive cells and projections (red) at the stage of hatching shown from lateral (N) and dorsal (O,P) views. Cells are detected in the pineal and the ventral part of diencephalon and midbrain. For abbreviations see list. See Supplorting Information Figure 1 for magenta/green copy. Scale bars = 100 μm in A,B,H-P; 50 μm in C-G.
The mammalian-like melanopsin (opn4m1) is extensively expressed in the brain of halibut larvae with functional eyes
The spatial expression pattern of opn4m1 in the brain of halibut larvae with functional eyes (47 dph) was compared with adjacent serial Nissl-stained sections (Fig. 5). Positive cells of opn4m1 are apparent in the pineal region (Fig. 5A3), and expression of opn4m1 was detected in the left habenula (Fig. 5B3). Expression in the habenula was also detected at an earlier stage (27 dpf), and a rostral view of a larva with opn4m1 expression shows the distinct cells around the pineal and in the left habenula (Fig. 5B4−5). Expression was also detected in the anterior preoptic area (Fig. 5C3), in the suprachiasmatic nucleus, in the ventral thalamus and presumably in the nucleus pretectalis superficialis magnocellularis (Fig. 5D3). In addition, expression was seen in the optic tectum and dorsally in tegmentum (Fig. 5D5). In ventral diencephalon, expression was observed in cells that are most likely the nucleus lateralis tuberis (Fig. 5E3). In tegmentum, opn4m1-positive cells were detected ventrally, and they are most likely located in the raphe nuclei (Fig. 5F3). Expression was also detected in the hindbrain, presumably in the nucleus medialis octavolateralis and in the descending octaval nucleus (Fig. 5G3).
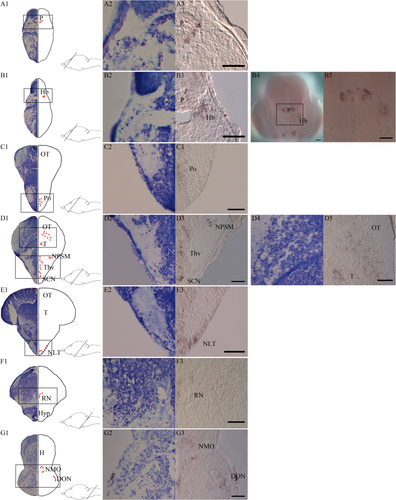
Melanopsin (opn4m1) expression in the brain of Atlantic halibut larvae. A1-G1: Nissl-stained transverse sections at the equivalent level of melanopsin-expressing cells illustrated by red dots in a 47 dph larva. A2-G2,D4: The Nissl-stained cell populations of interest with a higher magnification. A3-G3,D5: Melanopsin expression at the same level and with the same high magnification. B4,B5: A whole larva 27 dpf seen from a rostral view, whole head (B4), and the region of pineal and habenula (B5). A1-A3: Expression around the pineal (P). B1-B5: Melanopsin-positive cells in the left habenula (Hb). C1-C3: Expression in the preoptic area (Po). D1-D3: opn4m1 in the suprachiasmatic nucleus (SCN), in the ventral thalamus (Thv), and presumably in cells of the nucleus pretectalis superficialis magnocellularis (NPSM). D1,D4-D5: Expression in the dorsal tegmentum (T) and in optic tectum (OT). E1-E3: Expression of opn4m1 in the nucleus lateralis tuberis (NLT) of the hypothalamus (Hyp). F1-F3: Melanopsin expression most likely in raphe nuclei (RN). G1-G3: Melanopsin positive cells in the hindbrain in cells resembling nucleus medialis octavolateralis (NMO) and descending octaval nucleus (DON). Scale bars = 50 μm.
Melanopsin and exorhodopsin in the brain of halibut larvae with functional eyes
Expression of the other halibut melanopsins (opn4m3, opn4x1, and opn4x2) and exorhodopsin (exorh) was also investigated at 47 dph. Three of them (opn4m3, opn4x2, and exorh) had expression in the brain, and the spatial expression pattern was compared with adjacent serial Nissl-stained sections (Fig. 6). The pineal-specific exorhodopsin is extensively expressed in the pineal at 47 dph (Fig. 6A3). The mammalian-like melanopsin (opn4m3) is expressed in the suprachiasmatic nucleus (Fig. 6B3), in ventral and dorsal thalamus (Fig. 6C3), in ventral tegmentum (most likely in raphe nuclei; Fig. 6D3), and dorsally in the tegmentum and optic tectum (Fig. 6E3). The only Xenopus-like melanopsin (opn4x2)-positive cells detected in the brain were seen medially, rostrally in the hindbrain (Fig. 6F3).
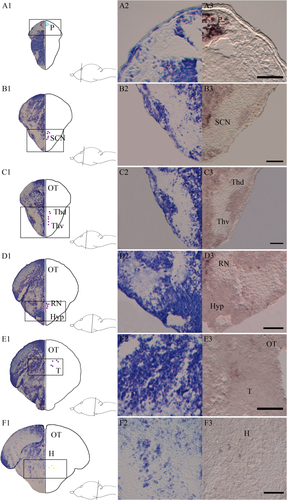
Melanopsin (opn4m3 and opn4x2) and exorhodopsin expression in the brain of Atlantic halibut larvae at 47 dph. A1-F1: Nissl-stained transversal sections at the equivalent level of exorh-expressing cells illustrated by light blue dots (A1), opn4m3-expressing cells illustrated by purple dots (B1-E1), and opn4x2-expressing cells illustrated by yellow dots (F1). A2-F2: The Nissl-stained cell populations of interest at a higher magnification. A3-F3: Exorhodopsin and melanopsin expressions at the same level and at the same magnification. A1-A3: Expression of exorh in the pineal (P). B1-B3: Expression of opn4m3 in the suprachiasmatic nucleus (SCN). C1-C3: Expression of opn4m3 in dorsal and ventral thalamus (Thv and Thd). D1-D3: Expression of opn4m3 presumably in rape nuclei (RN) E1-E3: Expression of opn4m3-positive cells dorsal in tegmentum (T) and in optic tectum (OT). F1-F3: Expression of opn4x2 medial in rostral hindbrain (H). For abbreviations see list. Scale bars = 50 μm.
Melanopsin expression in a functional retina
At the stage of hatching, no melanopsin-positive cells were found in the neuroblast cells of retina. In contrast, at first feeding, when the larvae have functional eyes, expression of three of the melanopsins (opn4m1, opn4x1, and opn4x2) was detected (Fig. 7). No transcripts of opn4m3 were found. The mammalian-like melanopsin (opn4m1) is expressed in the ganglion cell layer and in the inner nuclear layer. In the inner nuclear layer, opn4m1 was found in cells resembling amacrine cells, and distinct cells probably similar to horizontal cells were seen close to the outer plexiform layer (Fig. 7A). The Xenopus-like melanopsin (opn4x1) was located in the ganglion cell layer and in cells resembling amacrine cells of the inner nuclear layer (Fig. 7B). Expression of opn4x2 was seen only in the inner nuclear layer. Close to the outer plexiform layer, distinct cells were observed that are most likely horizontal cells. In addition, a more diffuse expression was seen in cells similar to bipolar cells (Fig. 7C). A schematic drawing of the retina illustrates the distribution of the melanopsin-positive cells (Fig. 7D).
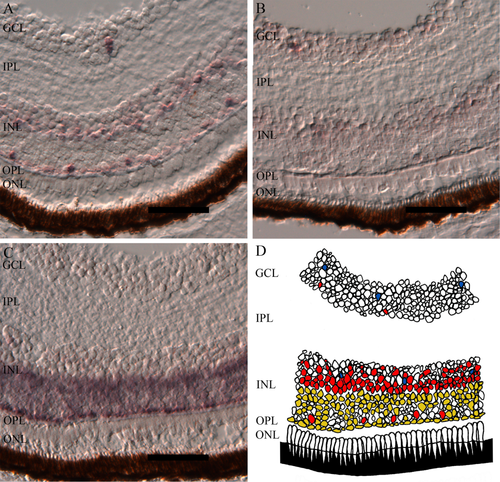
Distribution of melanopsin positive cells in a functional retina at 47 dph. A: The mammalian-like melanopsin opn4m1 is expressed in the in the ganglion cell layer (GCL) and in the inner nuclear layer (INL), probably in cells resembling amacrine cells and in cells that are most likely horizontal cells. B: Distribution of opn4x1 in GCL and in INL possibly in cells resembling amacrine cells. C: Expression of opn4x2 in the INL, most likely in horizontal cells close to the outer plexiform layer (OPL) and in a diffuse manner in bipolar cells. D: Schematic drawing of the relative melanopsin expression, opn4m1 (red), opn4x1 (blue), opn4x2 (yellow). For abbreviations see list. Scale bars = 50 μm.
DISCUSSION
The appearance of nonvisual opsins early in development of halibut provides an alternative model for studying the evolution and functional significance of nonvisual opsins. The present article describes nonvisual photoreception during early life stages of teleosts, when the brain is uncovered and transparent and directly exposed to environmental photons. We show a refined and extensive expression of melanopsin in Atlantic halibut larvae illustrating the importance of nonvisual photoreception from early stages of development. For the pineal complex (Ekström and Meissl, 1997), we detect expression of both melanopsin and exorhodopsin in adjacent cells, suggesting that two different photoreceptor systems may be active in the pineal complex.
The transparent halibut brain has extensive melanopsin expression
The literature has defined two distinct groups of melanopsins in nonmammalian vertebrates, the mammalian-like melanopsins (Opn4m) that encode melanopsin proteins more similar to human and mouse and the Xenopus-like melanopsins (Opn4x) resembling Xenopus laevis melanopsin (Bellingham et al., 2006). In agreement with findings for other teleosts (Davies et al., 2011; Sandbakken et al., 2012) and as a result of a whole-genome duplication event early in the evolution of ray-finned fish (Christoffels et al., 2004; Jaillon et al., 2004; Naruse et al., 2004), this article identifies two halibut melanopsins in each group and a pseudogene of a potential intronless melanopsin. The halibut melanopsins are expressed already at an early developmental stage, when light-regulated hatching takes place. Illustrated in Figure 8, the transparent unpigmented halibut embryo (Fig. 8A) at the stage of hatching has differential expression of the melanopsins in several distinct regions of the brain and in the spinal cord (Fig. 8C). After retinogenesis, when the eyes are functional, the melanopsins are expressed in several retinal layers (Fig. 8D). However, the extensive melanopsin expression in the brain at a preretinal stage persists in the transparent brain of halibut larvae after retinogenesis (Fig. 8B,D), indicating that photoreception directly through the brain is still vital even though the eyes are functional. An apparent exception is the opn4m3 expression in the hindbrain. At hatching, ball-shaped aggregated clusters of cells are seen medial to the neuromasts, but this expression pattern is not detected when the eyes become functional, indicating that the clusters may be involved in an early light-receptive function, e.g., hatching.
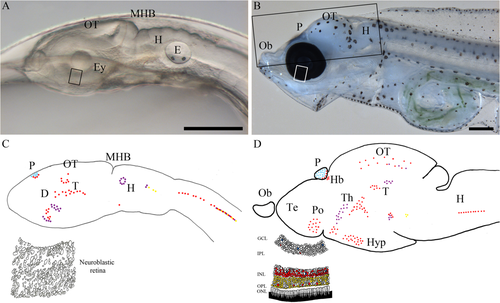
Melanopsin and exorhodopsin expression in the transparent halibut brain at the stage of hatching and at first feeding. A: Lateral view of an unpigmented halibut embryo inside the eggshell at the stage of hatching. B: Lateral view of a halibut larva with functional eyes at the time of first feeding, showing the pigmented eye and the transparent brain with scattered pigmentation. C: Lateral schematic drawing of the halibut larvae (approximately with the same view as in A) at the stage of hatching illustrating the pineal-specific exorhodopsin expression and the extensive melanopsin expression in the brain and spinal cord. A drawing of a section through the presumptive retina illustrates the neuroblastic cells. D: Schematic drawing of a halibut brain (lateral) in a larva with functional eyes showing the distribution of exorhodopsin and melanopsin. A drawing of a section through the retina showing the differentiated retinal layers and the relative distribution of melanopsins in light blue, opn4m1 in red, opn4m3 in purple, opn4x1 in blue, and opn4x2 in yellow. For abbreviations see list. Scale bar = 500 μm.
Interaction of exorhodopsin and melanopsin in the pineal complex
As illustrated in this article, melanopsin (opn4m1) flanks the pineal-specific exorhodopsin expression, and the cells are positioned next to each other (Fig. 4). This expression pattern is apparent both at the time of hatching and at first feeding (Figs. 4–6). Expression of two different opsins in the pineal complex indicates that two parallel photoreceptor systems may be active in the pineal complex. The complex may be able to detect light with different wavelengths and thus expand the spectral information that it can obtain from the environment. In zebrafish, melanopsins are known to have a maximal sensitivity to blue light at 470 and 484 nm (Davies et al., 2011), whereas exorhodopsin has a maximal sensitivity toward the green light at 498 nm (Tarttelin et al., 2011). The flanking melanopsin-expressing cells may also function as a photoisomerase for the exorhodopsin-expressing pineal cells. Recent evidence show that only two of the zebrafish melanopsins display invertebrate bistability, whereas the others are monostable and function more like classical vertebrate-like photopigments (Davies et al., 2011), known to be dependent on dark isomerization to regenerate back to the 11-cis configuration (for review see Lamb, 2009). The same study has suggested that bistable melanopsins in the zebrafish retina may provide 11-cis retinal to the monostable melanopsins in the same retinal layer. Phylogenetic analysis branches the halibut melanopsin expressed around the pineal together with the bistable melanopsins of zebrafish (Fig. 2), whereas exorhodopsin branches together with the monostable visual opsins (Davies et al., 2010). This may indicate that the halibut melanopsin could provide 11-cis retinal to the presumably monostable exorhodopsin-expressing cell of the pineal, in addition to its role in photoreception. As with previous studies in halibut embryos, we show axonal projections from the pineal to the deep brain regions, indicating the presence of neural signaling pathways between the two structures (Forsell et al., 2001). In addition, we demonstrate that the projections descend from exorhodopsin-negative neurons medial in the pineal complex and that 5-HT-positive cells exist in the pineal complex at the same developmental stage (Fig. 4).
Differential melanopsin expression in the halibut brain
Prior to retinogenesis, the halibut melanopsins are differentially expressed in the brain and spinal cord (Fig. 3). Mammalian-like melanopsins are expressed dorsally and ventrally in diencephalon, presumably around the pineal (opn4m1) and in the prospective preoptic region (opn4m1/opn4m3). Expression of opn4m1 is also detected in the tegmentum and optic tectum, whereas both mammalian-like (opn4m1/opn4m3) and Xenopus-like (opn4x2) melanopsins are detected in the hindbrain and spinal cord. In the literature, early expression of melanopsin has received little attention, but zebrafish preretinal expression of melanopsin has also been reported (Matos-Cruz et al., 2011). In evaluating the early melanopsin expression between the two teleosts, the only consistent expression is around the pineal. However, in contrast to the mammalian-like melanopsin expressed around the pineal in halibut, a monostable Xenopus-like melanopsin is expressed in zebrafish. Although speculative, the potential bistable property of opn4m1 in halibut opens for melanopsin-driven photoisomerase activity in the pineal complex, whereas regeneration back to the 11-cis configuration in zebrafish has to be dependent on other systems.
This article contributes further information on the complexity of melanopsin expression in the brain of marine species and provides a possibility to compare the distribution of mammalian-like and Xenopus-like melanopsins in different species. In agreement with our previous findings in juvenile Atlantic cod (Drivenes et al., 2003) and juvenile Atlantic salmon (Sandbakken et al., 2012), melanopsin was detected in the habenula (opn4m1) and suprachiasmatic nucleus (opn4m1/opn4m3) of Atlantic halibut larvae (Figs. 5, 6). However, in contrast to the case in cod and salmon that express Xenopus-like melanopsins in these diencephalic brain structures, the mammalian-like melanopsins are expressed in the same structures in halibut. Moreover, the left asymmetry of melanopsin expression in the halibut habenula is consistent with findings in salmon, in which the asymmetric photoreceptive habenula was indicated to be linked to the photoreceptive function of the parapineal organ (Sandbakken et al., 2012). In accordance with our studies in salmon (Sandbakken et al., 2012), mammalian-like melanopsins are expressed in thalamus (opn4m1/opn4m3) and in the nucleus lateralis tuberis of the hypothalamus (opn4m1). The melanopsin expression detected in halibut larvae is highly comparable to that of juvenile cod and salmon, but we also found new brain regions expressing melanopsin in halibut. We discovered melanopsin-positive cells in several regions of the midbrain of halibut larvae. We found melanopsin expression presumably in nucleus pretectalis superficialis magnocellularis, in the dorsal tegmentum, in the optic tectum, and most likely in cells of the raphe nuclei. Melanopsin-positive cells were also detected in the hindbrain. We show that opn4m1 is expressed in the nucleus medialis octavolateralis and in the descending octaval nucleus, whereas opn4x2 was detected medially in the rostral hindbrain.
Melanopsin-positive cells in the brain may have a role in modulation
Recently it has been demonstrated that nonvisual opsins are expressed in interneurons of medaka and zebrafish (Fischer et al., 2013). According to the classification of photoreceptor cells based on distribution and neural identity (Ramirez et al., 2011), photosensitive interneurons represent high-order photoreceptor cells that are able to both receive and send electrical signals. With this refined and extensive halibut melanopsin expression in mind, one can speculate about the identity of the melanopsin-expressing cells. They may represent primary sensory neurons transducing external stimuli into electrical signals, or they may be photosensitive interneurons that modulate the incoming electrical signal before it is transmitted. Several of the brain regions expressing melanopsins, such as the preoptic area, habenula, dorsal thalamus, and tegmentum, have retinal and pineal innervations (Ekström and Meissl, 1997). One can further speculate on whether these melanopsin-positive cells are photosensitive interneurons having a role in the modulation of the signals from the retina and pineal. Interestingly, high-order photoreceptor cells have so far been identified only in aggregated cluster of cells (Ramirez et al., 2011). This article shows dispersed photoreceptor cells in the spinal cord of halibut larvae at the time of hatching, and one can speculate that these melanopsin-positive cells are primary sensory cells or interneurons representing the first example of dispersed high-order photoreceptor cells.
Melanopsin expression in the halibut retina
In halibut larvae, three of the melanopsins (opn4m1, opn4x1, and opn4x2) are differentially expressed in the retina at the time of first feeding, showing that melanopsins are extensively expressed in the pure-cone retina of halibut prior to rod development and dim-light vision. In accordance with the case of juvenile cod (Drivenes et al., 2003) and salmon (Sandbakken et al., 2012), the opn4x1 is expressed in ganglion cells and in the inner nuclear layer in cells resembling amacrine cells. Expression of opn4x2 is also consistent with previous findings in cod and salmon, showing melanopsin-positive horizontal cells and a diffuse expression pattern in bipolar cells. The mammalian-like melanopsin opn4m1 is detected in the inner nuclear layer, presumably in amacrine cells, as seen in salmon, but in addition opn4m1 is identified in horizontal cells of the inner nuclear layer and in the ganglion cell layer. In comparing our findings in brain and retina of marine teleosts, it is apparent that the expression of Xenopus-like and mammalian-like melanopsins is more conserved in the retina than in the brain. In zebrafish retina, the melanopsins are detected in all cell layers, including the photoreceptor cell layer with expression of the intronless opn4m2 in cones (Davies et al., 2011). Halibut have no melanopsin expression in the photoreceptor cell layer, and this may be due to lack of a functional orthologue of the intronless zebrafish opn4m2. Based on the expression of melanopsins in all cell layers of the adult zebrafish retina, it has been suggested that the melanopsins confer global photosensitivity to the teleost retina and may permit direct fine tuning of the retinal circuitry (Davies et al., 2011). Our study with halibut suggests that light influences fine tuning of retinal and brain circuitry early in development, expanding opsin's modulatory role in lower vertebrates.
ACKNOWLEDGMENTS
The authors thank Ragnfrid Mangor-Jensen at the Austevoll Aquaculture Research Station for providing Atlantic halibut eggs and larvae. We also thank Mari Sandbakken for degenerative primers and Sterling White Halibut for founding the Atlantic halibut genome sequencing.
Abbreviations
-
- D
-
- diencephalon
-
- DON
-
- descending octaval nucleus
-
- E
-
- ear
-
- Ey
-
- eye
-
- GCL
-
- ganglion cell layer
-
- H
-
- hindbrain
-
- Hb
-
- habenula
-
- Hyp
-
- hypothalamus
-
- INL
-
- inner nuclear layer
-
- IPL
-
- inner plexiform layer
-
- MHB
-
- midbrain-hindbrain boundary
-
- N
-
- neuromast
-
- NMO
-
- nucleus medialis octavolateralis
-
- NLT
-
- nucleus lateralis tuberis
-
- NPSM
-
- nucleus pretectalis superficialis magnocellularis
-
- ONL
-
- outer nuclear layer
-
- OPL
-
- outer plexiform layer
-
- OT
-
- optic tectum
-
- P
-
- pineal
-
- Po
-
- preoptic area
-
- RN
-
- raphe nuclei
-
- SCN
-
- suprachiasmatic nucleus
-
- T
-
- tegmentum
-
- Te
-
- telencephalon
-
- Thd
-
- dorsal thalamus
-
- Thv
-
- ventral thalamus
CONFLICT OF INTEREST STATEMENT
The authors certify that there are no conflicts of interest.
ROLE OF AUTHORS
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: ME, JVH. Acquisition of data: ME, JVH, ØD, CAB, RBE. Analysis and interpretation of data: ME, JVH, LOEE. Drafting the manuscript: ME, JVH. Critical revision of the manuscript for important intellectual content: ME, JVH, LOEE. Obtained funding: JVH. Study supervision: JVH, LOEE, ØD.