Organization of Kenyon cells in subdivisions of the mushroom bodies of a lepidopteran insect
Abstract
The mushroom bodies are paired structures in the insect brain involved in complex functions such as memory formation, sensory integration, and context recognition. In many insects these centers are elaborate, sometimes comprising several hundred thousand neurons. The present account describes the mushroom bodies of Spodoptera littoralis, a moth extensively used for studies of olfactory processing and conditioning. The mushroom bodies of Spodoptera consist of only about 4,000 large-diameter Kenyon cells. However, these neurons are recognizably similar to morphological classes of Kenyon cells identified in honey bees, Drosophila, and cockroaches. The spodopteran mushroom body is equipped with three major divisions of its vertical and medial lobe, one of which, the gamma lobe, is supplied by clawed class II Kenyon cells as in other described taxa. Of special interest is the presence of a discrete tract (the Y tract) of axons leading from the calyx, separate from the pedunculus, that innervates lobelets above and beneath the medial lobe, close to the latter's origin from the pedunculus. This tract is comparable to tracts and resultant lobelets identified in cockroaches and termites. The article discusses possible functional roles of the spodopteran mushroom body against the background of olfactory behaviors described from this taxon and discusses the possible functional relevance of mushroom body structure, emphasizing similarities and dissimilarities with mushroom bodies of other species, in particular the fruit fly, Drosophila melanogaster. J. Comp. Neurol. 491:290–304, 2005. © 2005 Wiley-Liss, Inc.
Mushroom bodies are paired centers in the protocerebra of all but one group of insects. Comparable centers having structural similarities have been identified in chelicerates, chilopods, and myriapods (Strausfeld et al., 1998). Mushroom bodies (MBs) of insect brains have attracted much attention, and continue to do so, originally because their folded calyces were once thought to be reminiscent of folds and sulci of the mammalian cortex (Dujardin, 1850), and their large size in social insects was thought to underlie “intelligence” in these species. MBs also have the distinction of being the first brain centers in insects to be analyzed using Golgi methods (Kenyon, 1896). More recently, at least since the 1960s, these centers have attracted special attention because of speculation, as well as hard evidence, that they support integrative functions that in mammals are ascribed to higher brain centers of association cortex and hippocampus. Experimental evidence that the MBs are involved in the analysis of sensory contexts and space comes from intracellular recordings of efferent multimodal neurons in the cockroach MBs (Li and Strausfeld, 1997, 1999) as well as from studies of place memory and the effects of MB lesioning (Mizunami et al., 1998b). Chronic recordings from the MBs of moving animals (Mizunami et al., 1998a) and the effects of MB mutants on the locomotion of fruit flies (Martin et al., 1998; Besson and Martin, 2005) further suggest a role for this center in motor actions. MBs clearly support different functions, as evidenced by studies of locusts, honey bees, and fruit flies showing these centers to be involved in odor coding (Laurent and Davidowitz, 1994) and in olfactory associative learning (Mauelshagen, 1993; Heisenberg, 1980; de Belle and Heisenberg, 1994; Zars et al., 2000), although recent studies on olfactory learning in honey bees using RNA interference also implicates the antennal lobes (Farooqui et al., 2003, 2004).
Studies focusing on the MB's possible roles in acquiring and retrieving memories suggest the importance of their structural integrity and the functionality of specific second-messenger pathways. Cooling different parts of the MBs in honey bees reversibly impairs odor memory (Erber et al., 1980). Fruit flies with chemically (de Belle and Heisenberg, 1994) or genetically (Heisenberg et al., 1985; Pasqual and Préat, 2001) disrupted or ablated MBs have either impaired or disabled learning. Similar effects are observed in genetically modified flies that lack functional cAMP-dependent signaling (Conolly et al., 1996; Zars et al., 2000), or certain CREB-dependent transcription and protein synthesis pathways (Tully et al., 1994; Dubnau et al., 2003).
Mushroom body shape, size, and connections can differ substantially between genera. However, certain features common to most taxa include a cap or cup-shaped structure (the calyx) situated posterodorsally in the brain. The calyx is connected by a forward-projecting pedunculus that divides into dorsal and medial lobes in front of (that is, ventral according to the neuraxis) the central complex. Commonalties among MBs of different species refer to the morphologies of intrinsic neurons (the Kenyon cells) that comprise the bulk of the MBs and which vary from several hundred thousand in some hymenopteran and blattid species (Neder, 1959; Witthöft, 1967) to a few thousand or even hundreds, as in fruit flies or apterygote insects (Technau and Heisenberg, 1982; Farris, 2005).
In neopteran insects, three classes of Kenyon cells provide discrete subdivision of the MB lobes. It is organization within these divisions that confers major differences between taxa. For example, in Blattaria, Isoptera, and Mantodea class I and II Kenyon cells contribute to contiguous subdivisions that are organized as adjoining layers that can share the dendrites of efferent neurons. In contrast, class III Kenyon cells in these species provide a separate system of small vertical and median components called lobelets (Farris and Strausfeld, 2003; Farris et al., 2004; Strausfeld, 2002). In honey bees, discrete divisions of the lobes are denoted by their affinities to antisera raised against modulatory peptides (Strausfeld, 2002). Each division is further subdivided into laminae, each of which represents a discrete zone in the calyces and provides its own system of efferent neurons. Brachyceran flies, such as Phaenicia sericata and Drosophila melanogaster, and at least one coleopteran species (Larsson et al., 2004) have class I and II Kenyon cells that supply discrete but adjacent subdivisions of the medial and vertical lobes. However, in some taxa the divisions of the lobes adopt the form of separate neuropils that diverge from their common origin at the pedunculus.
In terrestrial neopterans, each MB possesses a pair of distal structures, called the calyces, that comprise the dendrites of class I and II Kenyon cells. These are supplied by afferents originating from sensory neuropils. Inhibitory neurons that reciprocally connect the lobes to the calyces or which originate from within the protocerebrum also terminate in these peripheral neuropils (Strausfeld and Li, 1999a; Grünewald, 1999). The shape of the calyx varies among different genera (Strausfeld et al., 1998), a variation sometimes reflecting the type of sensory input, as in the Orthoptera (Schürmann, 1974) and Apidae (Ehmer and Gronenberg, 2002; Strausfeld, 2002) or relating to the very large number of Kenyon cells comprising some MBs, as in many hymenopteran genera and Blattaria.
One intriguing aspect of the MBs is that these taxonomic differences appear to characterize specific orders of insects. What might such differences suggest about the functional properties of the MBs? And what is the significance of organization in the subdivided lobes (Strausfeld, 2002; Strausfeld et al., 2003)? Do different subdivisions of the MBs support distinct functions, as suggested by studies of short and long-term memory in Drosophila (Zars et al., 2000; Pascual and Préat, 2001)? One approach to these questions is to select a taxon in which the MBs are pronounced but have relatively few Kenyon cells contributing to divisions that are arranged as discrete and separate entities. The noctuid moth Spodoptera littoralis provides such an organization, yet is similar enough to the MBs of Drosophila as to provide an important comparative species. A taxon indigenous to the Mediterranean area (the larva is considered a major pest on several crops), Spodoptera is nocturnal, relying on odors for finding resources, which for the females are primarily food and oviposition sites and for the males are nectar sources and females. The adults are short-lived and die after about a week. Although this lifestyle suggests a limited need for elaborate memory functions, conditioning experiments have shown that S. littoralis can indeed learn to associate odors with a food reward (Fan et al., 1997; Hartlieb et al., 1999). The structural arrangement of the MBs reveals widely separated lobes, the Kenyon cells of which are large enough to accommodate penetration by dye-filled recording electrodes. In the present article we describe the small yet structurally elaborate MBs of this moth. We identify four different morphological species of Kenyon cells that provide branches to discrete subdivision of the lobes and we show a clear segregation of inputs to the calyx that relate to these divisions. The present data reveal specific differences as well as similarities between the structural organization of the MBs of Spodoptera and other taxa.
MATERIAL AND METHODS
Experimental animals
Male Spodoptera littoralis were obtained from a laboratory culture at Chemical Ecology, SLU, Alnarp Sweden. To maintain genetic vigor, wild-collected moths were periodically introduced to the culture. Larvae were kept on a semisynthetic diet (Hinks and Byers, 1976), at 25°C, 65% relative humidity, and on a 16:8 light/dark photoperiod. The pupae were sexed and separated and emerged moths were kept separated without food but with access to water ad libitum. Males were used 2–4 days after emergence.
Mass injections
The moth was mounted in a 1-ml plastic pipette tip with the narrow end cut so that the head of the moth projected from the opening. The head was immobilized with wax and the scales were gently removed with a fine brush. The head capsule was then opened and the brain exposed. A thin glass pipette was used to penetrate the neural sheath and to sever the neurons in the desired area. A small crystal of tetramethylrhodamine dextran (Molecular Probes, Eugene, OR; D-3308) was placed where the sheath had been ruptured. The head was sealed with Vaseline and the moth was left at 4°C overnight in a damp chamber. The following day the moth was decapitated and the brain immersed in fixative (4% formaldehyde in 0.01 M PBS (phosphate-buffered saline), pH 7.4) where it was left overnight at 4°C. The brain was next washed, dehydrated, and cleared for subsequent viewing in a confocal laser microscope (see below). After a first scan in the confocal to confirm that the wanted neurons were stained, the brains were embedded in Durcupan plastic (Fluka, Heidelberg, Germany), serially sectioned, and scanned again for detail resolution.
Single Kenyon cell injections
The moths were treated as above with the exception that after opening the head capsule and desheathing the caudal part of the brain the moth was decapitated and the head was transferred to a specially designed Perspex chamber filled with Ringer saline. The chamber was placed under a fixed stage microscope fitted with a water immersion 20× objective. The Kenyon cell somata, which lie above the calyces, were identified and a wide tip glass electrode mounted on a micromanipulator was brought in contact with the cell membrane. The membrane was ruptured using mild suction through the electrode and the neuron was filled by passive diffusion with 1% Neurobiotin tracer (Vector Laboratories, Burlingame, CA; SP 1120) in 0.25 M KCl. To facilitate rupturing cell body membranes, the desheathed brain was treated for 5 minutes before penetration with a mild enzyme solution (0.01% papain (Sigma, St. Louis, MO, P4762) and 0.1% L-cysteine (Sigma, C7755) in Ringer saline). After filling the neuron the brain was placed in fixative (4% paraformaldehyde in Millonig's buffer, pH 7.2) overnight at 4°C. The next day the brain was washed in Millonig's solution, then dehydrated and permeabilized in propylene oxide. After 10 minutes the brain was washed in absolute ethanol and then rehydrated and returned to Millonig's buffer. The whole brain was then incubated with NeutrAvidin-Alexa488 (Molecular Probes, A-11232) conjugate (0.025 mg/ml Millonig's buffer containing 0.25% Triton X-100 and 1% BSA) overnight at 4°C. Finally, the brain was washed in Millonig's buffer and prepared for viewing with confocal microscopy.
Phalloidin staining
Phalloidin is a fungal toxin with affinity for filamentous actin, which is abundant in synapse-dense nerve tissue and therefore specifically stains neuropil (Rössler et al., 2002). Brains for phalloidin staining were dissected from the head and fixed in 4% formaldehyde overnight in 4°C. They were then washed in PBS and embedded in 8% agarose gel (Sigma, A-0169). Blocks were cut and sectioned on a Vibratome (Leica, VT1000S) at 60–100 μm. The sections were preincubated in 1% Triton X-100 in 0.01 M PBS and subsequently incubated with Phalloidin AlexaFluor488 (Molecular Probes, A-12379) at a 1:200 concentration in PBS with 2% NGS and 1% Triton X-100 for three nights at 4°C. They were then washed in PBS, cleared in 80% glycerol, and mounted. The sections were then scanned in a confocal microscope and used for 3D reconstruction of the MBs (see below).
Golgi impregnations
Brains were dissected under a 2.5% potassium dichromate solution containing 1.3% sucrose. They were then kept for 5 days in the dark at 4°C immersed in four parts 2.5% potassium dichromate with one part 25% glutaraldehyde. Next, brains were washed in 2.5% potassium dichromate solution and kept for 5 days in 99 parts potassium dichromate with 1 part of 1% osmium tetroxide in water. After rinsing in 0.75% silver nitrate, brains were kept in this solution for 3 days, then washed in distilled water, dehydrated, and embedded in Durcupan plastic (Fluka). Polymerized blocks were serially sectioned at 25 μm. Buffered silver nitrate, using borax-borate buffer at pH 7.0, was sometimes used instead of the aqueous solution. Some brains were prefixed in 2.5% glutaraldehyde in 0.16 M cacodylate buffer (pH 7.0) with 1.3% sucrose or in glutaraldehyde carried in Millonig's phosphate buffer. Osmium concentrations were sometimes doubled to select for wide-diameter neurons and 1% chloral hydrate was added during the second chromation stage to promote mass-impregnation of Kenyon cells.
Bodian preparations
Moths were cooled to immobility and then decapitated. Heads were opened under AAF fixative (85 volumes absolute ethanol, 10 volumes of 37% formaldehyde solution, and 5 volumes of glacial acetic acid) and kept overnight at room temperature. After washing in 70% ethanol, brains were dissected out from the head capsule, dehydrated, and cleared in terpineol before embedding in Paraplast Plus (Corning, Corning, NY). Series of 12 μm thick sections were deparaffinized, hydrated, and then silver-impregnated with Bodian's (1937) original procedure, using 2 g of silver protein (Merck, Darmstadt, Germany)/100 ml distilled water containing 1 g of clean copper shot. Slides were developed in 1% hydroquinone with 2% sodium sulfite for 5 minutes, toned in 1% gold chloride, and differentiated in 2% oxalic acid before fixing 5% sodium thiosulphate. Dehydrated sections were mounted under glass in Entellan.
Image processing
Confocal microscopy was performed with a Zeiss LSM META microscope equipped with Plan Neofluar 20×, 40×, and 63× oil immersion objectives viewed at 543 nm and 488 nm excitation, depending on the fluorophores used. Golgi-impregnated and Bodian-stained material was photographed using a CCD camera (Olympus DP70) mounted on an Olympus AX70 microscope fitted with Uplan Apo 20×, 40×, and 100× oil immersion objectives. The camera was linked to a personal computer equipped with graphics software. Confocal and light micrographs were transferred to an Apple Power Mac G4 Dual personal computer (Apple Computer, Cupertino, CA), where they were edited in Adobe Photoshop and Adobe Illustrator (Adobe Systems, San Jose, CA). For illustrating Golgi impregnations, optical images were captured at ∼1-μm intervals, stacked in Photoshop, and made transparent using the darkening function. Shadows of out of focus profiles were removed manually from each layer and the picture then flattened. For the 3D reconstructions, stacks of serial optical sections saved as Zeiss LSM files were transferred to a personal computer (Dell Computer, Austin, TX) equipped with AMIRA software (TGS, San Diego, CA). The physical sections were 3D-rendered individually and subsequently montaged in Photoshop to create a complete 3D image of the entire mushroom body. Where phalloidin staining was insufficient, Bodian preparations were used as a guide. Some Golgi impregnations were used for camera lucida drawings. These were made under a 100× oil immersion Achromat objective using an Olympus BX40 microscope fitted with a camera lucida arm. The drawings were montaged on translucent paper and digitized in a scanner (HP Scanjet 7400c). The drawings were further improved in Photoshop and combined with the outline of the surrounding neuropil obtained from serial light micrographs of the same preparation, using Adobe Illustrator software.
Terminology
When describing the entire vertical or medial aspect of the MBs the terms vertical and medial lobe are used. The terms α and β division are adopted from Heisenberg's (1980) study of the Drosophila MBs and denote the posterior divisions of the vertical and medial lobes, respectively. The terms α′ and β′ divisions denote the divisions lying anterior to the α and β divisions (Crittenden et al., 1998; Strausfeld et al., 2003). The term γ lobe was originally adopted from Pearson (1971) and describes here the anteriormost division of the vertical and medial lobes containing class II Kenyon cells. The name Y tract is also adopted from Pearson's description of the MBs in Sphinx ligustri (1971). The terms dorsal and ventral lobelet are taken from a study on cockroaches and termites describing class III Kenyon cells (Farris and Strausfeld, 2003). The term “division” thus refers to discrete components of the vertical and medial lobes.
RESULTS
The paired MBs occupy a substantial volume of the protocerebrum (Fig. 1A). Their calyces consist of paired cups that are completely fused (Fig. 1A–D). 3D reconstructions and phalloidin staining reveal that each cup consists of a thick wall, the inner margin of which gives rise to a discrete rim that originates from an inner concentric layer (Fig. 1D,G). As described below, this layer receives a separate afferent supply from the visual system, whereas the bulk of the calyces are supplied by afferents originating in the antennal lobes. The calyx neuropil comprises many thousands of microglomeruli, reminiscent of those described from observations of Drosophila melanogaster, the cricket Gryllus bimaculatus, and honey bee (Yusuyama et al., 2002; Frambach et al., 2004; Groh et al., 2004), where presynaptic boutons interact with postsynaptic specializations of the dendrites of Kenyon cells, the intrinsic interneurons of the MBs. Cell bodies belonging to Kenyon cells surround the calyces on all sides and are also present in the cavities of each cup (Fig. 1B). Kenyon cell bodies are uniformly round, with diameters ranging from 5–8 μm. Primary neurites project from these cell bodies to the calyx neuropil, extending along the inner layer of the cups where they form a dense stratum of fibers interposed between the cell bodies and synaptic neuropil (Fig. 1G).
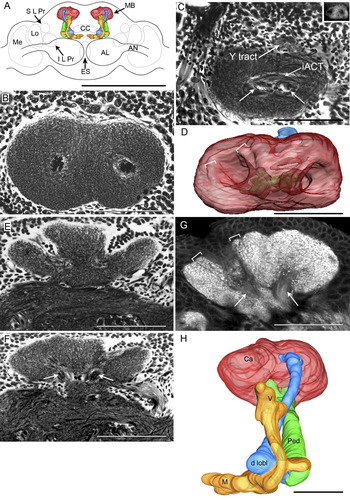
Organization of the calyces and pedunculus. A: Computer reconstruction of phalloidin-stained optical sections showing a frontal view of the brain of S. littoralis to illustrate the size and arrangements of the mushroom bodies (MB) in relation to other brain structures: the antennal lobes (AL); antennal nerves (AN); central body complex (CC); lobula (Lo); superior and inferior lateral protocerebrum (S L Pr, I L Pr); medulla (Me); esophageal foramen (ES). B: Top-down view of the two fused calyx cups surrounded by Kenyon cell somata stained by Bodian's method. C: Top-down view of the calyx, sectioned at exit points of the pedunculus (arrows) and Y tract shown in the insect from a Bodian-stained section. Inner antennocerebral tract (iACT). Inset: Ethyl gallate-stained preparation showing the two roots of the Y tract. D: Top-down view of the 3D-reconstructed calyx (red) showing the orientation of the cups, their inner layer (white brackets) and the exit regions of the pedunculus necks (green) and Y tract (blue). E: Transverse section of a Bodian-stained calyx and surrounding perikarya. F: Transverse section taken at the level of the exit of the two roots of the pedunculus necks (arrows). G: Transverse section of a phalloidin-stained calyx showing microglomeruli, the two converging necks of the pedunculus (arrows), and the inner layer of the calyces (white brackets). H: Frontal view of the 3D reconstruction of the complete MB showing the calyx (Ca; red), pedunculus (Ped; green), Y tract (blue), and the dorsal lobelet (d lobl), and the vertical (V) and medial (M) lobes (yellow). Scale bars = 1 mm in A; 100 μm in B–H.
Two major tracts originate from the calyces, the larger of which emerge as two roots from the base of each cup (Fig. 1C,F,G). These roots then merge to form the stalk-like pedunculus that extends anteriorly and slightly ventrally through the protocerebrum, reaching almost to the front of the brain. After a distance of about 300 μm, the pedunculus bifurcates to provide two clusters of neuropils that constitute the vertical and medial lobes. The vertical lobe extends upwards, curving back through the surrounding neuropil, reaching up to the dorsal brain surface. The medial lobe projects towards the brain's midline, curving downwards. At the midline, just anterior to the central complex, its tip meets that of its contralateral counterpart (Fig. 1A,H). A second tract, called the Y tract, which is thinner than the pedunculus, originates from the dorsolateral calyces (Fig. 1C,D). It also has two roots (inset in Fig. 1C) emerging from each cup that then coalesce. The resulting tract extends frontally for a short distance to then project downwards and forwards towards the brain's midline. As shown in the reconstruction (Fig. 1H), this tract crosses in front of the pedunculus and then curves behind the vertical lobe before bifurcating into two components. One of these forms a narrow club-like lobelet after protruding through the medial lobe and penetrating beyond its anterior surface. The other component forms a broad oval lobelet that lies dorsal to the medial lobe (Figs. 1H, 2A–F). These two components are here termed dorsal and ventral Y lobelets.
The organization of mushroom body lobes and their subdivisions. A–E: Selected images from a series of reduced silver-stained frontal sections, from anterior to posterior in the brain showing the different divisions of the MB lobes and their 3D organization. α/β, α′/β′, γ lobes, dorsal and ventral lobelets (d lobl, v lobl), and Y tract (Y) are indicated in each section. F: Posterior view of a 3D reconstruction of a phalloidin-stained mushroom body to illustrate the relative locations of mushroom body subdivisions; pedunculus (Ped; green), Y tract (Y; blue). Scale bars = 100 μm.
The vertical and medial lobes proper, which arise from the pedunculus, are in turn divided into three finger-like and adjacent divisions that end at different distances from their origin from the pedunculus (Fig. 2A–F). The most anterior division is the γ lobe, which in S. littoralis consists of both a vertical and medial element. The vertical element is slender, extending up the lateral side of the vertical component of the Y lobelet, after which it twists slightly toward the midline, thereafter reaching the brain's surface, where it terminates as a distinct swelling. The medial element of the γ lobe lies anterior and dorsal to other medial lobe divisions and beneath the dorsal component of the Y lobelet. The tip of the medial γ lobe is swollen, as is a zone about halfway along its length. These swellings are regions in which its constituent Kenyon cell “axons” give rise to numerous collaterals (see below).
The most posterior components of the vertical and medial lobes consist of the α and α′ and the β and β′ divisions, respectively. The β and β′ divisions are both shorter than the γ division and characteristically bend downwards toward the midline. Likewise, the α and α′ divisions are also shorter than their adjacent γ component and both bend backwards to reach into the protocerebrum. All the divisions end in swellings, again reflecting the branching of their constituent intrinsic neurons. A large swelling in the spur or heal area of the mushroom body, lateral to where the pedunculus divides into its subsequent lobes, is mainly provided by the most distal part of the α′ and β′ divisions, at their origin from the pedunculus.
Intrinsic elements
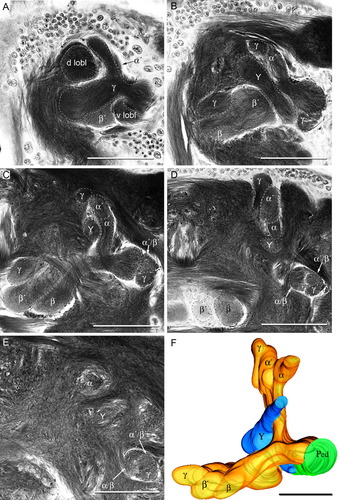
We identified four main types of Kenyon cell, each of which contributes to one of the lobe's γ, α/β, α′/β′ divisions and Y lobelets (Figs. 3, 4). These cell types are best distinguished from their morphologies in the lobes. However, in the pedunculus their fibers form entangled bundles that cross each other's paths and run in a seemingly disorderly fashion. Some of these processes in the pedunculus have short beaded branches (Fig. 3K), but these are not characteristic of any Kenyon cell type in particular. Thus, in contrast to coleopteran or dipteran mushroom bodies, other than for the first cell type described below, there is no obvious representation in the pedunculus of organization in the calyx or the subsequent segregation of cell types into their medial and vertical lobe divisions.
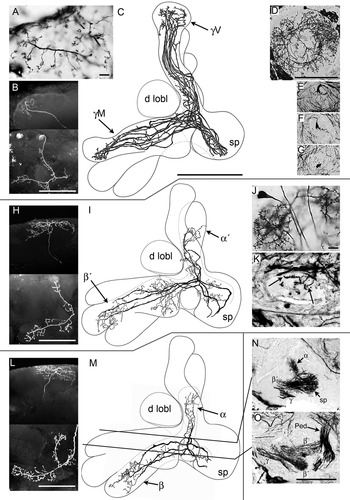
Segregation of Kenyon cells to the divisions of the lobes. A: Golgi impregnation of clawed class II dendritic specializations in the calyx. B: Neurobiotin-filled γ lobe neuron. Top panel: dendritic arborizations in the calyx. Lower panel: branches in the lobes in the lower. C: Camera lucida reconstruction of Golgi-impregnated clawed Kenyon cells invading both the medial and vertical components of the γ division of the lobes. γ lobe vertical branch (γV), γ lobe medial branch (γM), spur (sp), dorsal lobelet (d lobl). D–G: Serial sections of class II Kenyon cells reconstructed in C, from their posterior origin in the calyces (D), through the pedunculus and lobes (E–G). H: Neurobiotin-filled spiny class I Kenyon cell. Upper panel shows the dendritic tree, lower panel the axon and its branches. I: Camera lucida drawing of Golgi-impregnated spiny class I Kenyon cells invading the α′/β′ division of the lobes. Note their collateral branches localized to swellings of this subdivision. Spur (sp), dorsal lobelet (d lobl). J: Detail showing the large Kenyon cell body and spiny dendritic specializations in the calyx of cells reconstructed in I. K: Transverse section of the pedunculus showing short blebbed branches (arrows) on the axons of the Kenyon cells in I. L: Neurobiotin-filled spiny class I Kenyon cell supplying α/β divisions. Upper panel shows its dendritic tree; the lower panel its axon and axons collaterals. M: Camera lucida drawing of class I Kenyon cells in the posterior α/β division of the lobes. The reconstructions in I and M are from the same preparation, but in I only the α′/β′ division is fully outlined and not the α/β divisions. The opposite is the case in M. The γ division is shown in both I and M. Spur (sp), dorsal lobelet (d lobl). N: Golgi impregnation sectioned horizontally at the level of the top line in M showing the basal part of the α lobe, the spur region (sp), the proximal part of the β′ lobe, and part of the medial γ lobe. O: Section at the level of the lower line in M showing the distal end of the pedunculus, the entire β division, the distal part of the β′ division with the bulbous swelling and the distal part of the medial γ division. Scale bars = 10 μm in A,J (applies to J,K); 100 μm in B,C (applies to C,I,M), D (applies to D–G), H,L,O.
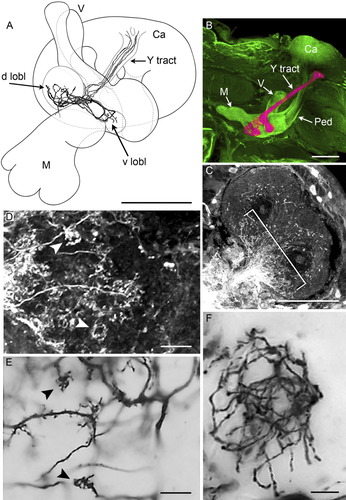
Organization of the Y tract and lobelets. A: Camera lucida drawing of Golgi impregnated Y tract neurons showing the disposition of the dorsal and ventral lobelets (d lobl, v lobl) in relation to the medial and vertical lobes (M, V). Calyx (Ca). B: False-color image of a dextran-filled MB, showing the Y tract and its lobelets in purple and the calyx (Ca) and the vertical (V) and medial (M) lobes in green. C: Y tract neurons (bracketed) in the calyx filled retrogradely from the lobelets with dextran. D: Detail from C showing the condensed claw-like dendritic specializations (arrowheads). E: Golgi impregnation showing condensed claw-like dendritic specializations (arrowheads) of Y tract neurons. F: Y tract neuron branches in the dorsal lobelet. E and F were digitally sharpened in PhotoShop. Scale bars = 100 μm in A–C; 10 μm in D–F.
Three distinctive dendritic morphologies were found in the calyces. These are spiny dendrites (Fig. 3J), open clawed dendrites (Fig. 3A), and dense clawed dendrites (Fig. 4E). The most commonly impregnated neurons were those with spiny dendrites, having their cell bodies posterior and above the calyces. Their primary neurites give rise to numerous dendritic branches that provide umbrella-shaped dendritic trees that in most cases arborize through large volumes of the calyx (Fig. 3H,L).
Kenyon cells supplying the γ lobes.
Kenyon cells supplying the γ division have thick axon-like processes that after their bifurcation from the pedunculus extend to the tips of both the medial and vertical γ divisions. In the lobes and pedunculus, these processes have sparse, short side branches but have pronounced branches in the swollen mid-zone and endings of their lobes (Fig. 3B,C). Kenyon cell axons supplying the γ divisions emerge from each calyx as a ring-like sheath surrounding the neck of the pedunculus. The axons then converge to form a tight bundle before their bifurcation from the pedunculus into the medial and vertical lobes (Fig. 3D–G). In the calyx, Kenyon cells supplying the γ division have long and narrow dendritic trees that are sparsely equipped with short branches that extend radially cross the calyx (Fig. 3D). Their short branches are equipped with open-clawed specializations (Fig. 3A), denoting these as class II Kenyon cells.
Kenyon cells supplying α′/β′ divisions.
The most obvious differences between Kenyon cells supplying the γ lobe and the α′/β′ divisions are found in the calyx. Whereas γ division Kenyon cells have narrow dendritic arborizations, the α′/β′ neurons have extensive dendritic trees richly decorated with short stubby spines (Fig. 3H,J). An axon-like process originates from the center of each dendritic tree to extend throughout the length of the pedunculus. About two-thirds along their length some of these “axons” give rise to short unbranched collaterals that are equipped with swellings (Fig. 3K). Just before their bifurcation into the medial and vertical lobes they provide numerous short and sometimes branched collaterals that extend into the spur of the pedunculus (Fig. 3I,N). Each of their axons thereafter divides in two main tributaries extending into the α′ and β′ divisions. These tributaries are the same thickness as those of α/β division Kenyon cells, but they give rise to numerous, thin second-order collaterals that are evenly decorated with small varicosities. The β′ division tributaries provide additional branches in a medial swelling (Fig. 3I,O) and at the bulbous tip.
Kenyon cells supplying α/β divisions.
Kenyon cells supplying these divisions have spiny dendritic specializations in the calyces and similar morphologies to Kenyon cells supplying the α′/β′ divisions. Their dendritic trees are large and umbrella-shaped (Fig. 3L). Their axon-like processes in the pedunculus occasionally give rise to short side branches, as described above. Some collaterals extend into the spur, but these are not as extensive as from Kenyon cells supplying the adjacent α′/β′ divisions. Tributaries supplying the β division do not send collaterals into the medial bulb, but have abundant side branches almost along their entire length. Tributaries supplying the α division give rise to thin varicose side branches (Fig. 3M).
Kenyon cells supplying the Y tract and its lobelets.
The cell bodies of these Kenyon cells lie in a cluster displaced dorsolaterally from the calyx neuropil. Their primary neurites extend along the surface of the calyx for a short distance before dividing, one tributary entering the neuropil, where it then further divides into several branches. These are equipped with compact and clenched claw-like specializations (Fig. 4D,E). The dendritic branches of these Kenyon cells are sparse but spread out widely, although each tree extending through less volume of the calyx area than do those of the class I Kenyon cells described above (Fig. 4C). The second tributary extends to the dorsolateral surface of the calyx to then enter the Y tract. Each of these axon-like processes project to just behind the base of the vertical lobe, after which they divide at least once to send tributaries into the dorsal and ventral components the Y lobelets (Fig. 4A,B). The projection of these tributaries is less orderly than those of the vertical and medial lobes, and the same tributary may extend back and forth between the two Y lobelets before ending abruptly. The tributaries appear “naked,” lacking any obvious terminal specializations, being instead heterogeneously swollen along their lengths (Fig. 4F). Thus, tributaries supplying the Y lobelets are thicker than their axons in the Y tract and Y tract axons are about twice as thick as axons in the pedunculus.
Extrinsic elements
Afferent supply to the calyces from the antennal lobes.
The major afferent input to the calyces of S. littoralis is supplied by antennal lobe projection neurons that send their axons to the calyx and the lateral protocerebrum on the same side of the brain via the inner (iACT; Fig. 5A) and outer antennocerebral tract (oACT). Some axons from the antennal lobe enter three additional tracts, but it is not clear if any of these reach the calyx. Axons in the iACT pass in front of the calyces and then terminate in the lateral horn of the lateral protocerebrum. They give rise to systems of collaterals that penetrate the calycal neuropil, where they provide large and irregularly shaped boutons, each 2–3 μm in diameter (Fig. 5C). Projection neuron axons in the oACT first give rise to tangled collaterals in the lateral protocerebrum and then terminate in the calyces, where they provide similar oval specializations. The entire calyx is densely innervated by these endings, with the exception of the narrow layer of neuropil lining the cup cavities, corresponding to the thinner inner layer shown in Figures 1D,G and 5D. Double labeling of antennal lobe projection neurons and single Kenyon cells show that boutons of the projection neurons are closely apposed to the dendritic spines of Kenyon cells (Fig. 5F), in some cases the same bouton contacting several spines of the same Kenyon cell.
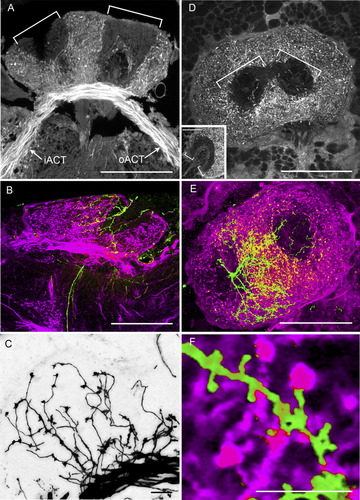
Antennal lobe projection neurons in the calyx. A: Transverse section of the calyx showing the trajectory of the inner and outer antennocerebral tracts (iACT, oACT) comprising axons of antennal lobe projection neurons providing collateral terminals in the calyces. The mass fill clearly shows the division of the calyx into concentric zones, one of which is not invaded by olfactory afferents (white brackets). B: Double-stained preparation showing dextran-filled projection neurons (purple) and one neurobiotin-filled Kenyon cell (green). C: Golgi impregnation illustrating the characteristic oval boutons that decorate collateral endings from antennal lobe projection neurons. D: Top-down view of dextran-filled projection neuron collaterals in the calyx and noninnervated areas (brackets). Inset: Deeper section showing the inner layer of the calyx (brackets). E: Top-down view of the same preparation as in B showing the extent of spread of Kenyon cell dendrites across the olfactory afferent supply. F: Detail showing projection neuron boutons (purple) making contacts with the spines of a class I Kenyon cell (green). Scale bars = 100 μm in A,B,D,E; 50 μm in inset; 10 μm in C,F.
Other afferent elements of the lobes and zones of the calyces.
Golgi impregnation shows the MB lobes to be extensively invaded by dense arborizations of small-diameter processes that are decorated with small bead-like swellings suggestive of presynaptic specializations. These arborizations are particularly dense in the γ division, the Y lobelets, parts of the α′/β′ divisions, and the MB spur (Fig. 6A,B). Afferent arborizations in the medial lobes have a columnar or laminar appearances (Fig. 6C). Diffuse beaded processes were also identified in the calyx (Fig. 6D). Because Kenyon cells in the preparation showing these features were unimpregnated, these blebbed processes are interpreted as representing a ubiquitous afferent supply that originates from the protocerebrum.
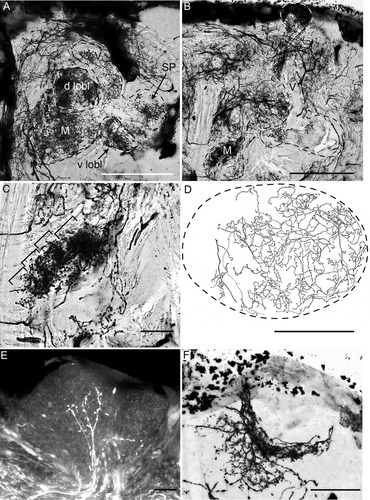
“Extrinsic” elements in the MBs. A,B: Golgi impregnations showing extrinsic neurons in the lobes in two different sections of the same preparation. A dense arborization of beaded fibers invades the medial (M) lobes (V) and lobelets (d lobl, v lobl) and the spur (SP). C: Detail from B showing laminar organization (brackets) of beaded fibers in the β′ division of the medial lobe. D: Camera lucida drawing of beaded arborizations invading the calyx from the protocerebrum. Dashed line indicates the calyx outline. E: Terminals branching in the inner layer of the calyx revealed by dextran injection into the ipsilateral optic lobe. F: Golgi impregnation of the inner layer of the calyx showing it occupied by processes of a population of neurons invading the calyx from the protocerebrum. The invaded area is equivalent to the unstained area in 5D. Scale bars = 100 μm in A,B,D; 50 μm in C,F; 10 μm in E.
Additional supply to the MBs has been resolved by dextran fills into the optic lobes that resulted in the resolution of a single type of neuron that sparsely branches in the inner layer of the calyx cup (Fig. 6E). This observation suggests that the inner layer of the calyx, which lacks antennal lobe innervation, may represent a discrete zone of neuropil supplied by visual system afferents. In addition, a system of additional processes that principally invade the inner calyx layer (Fig. 6F) further suggest the special nature of this calycal zone. Although these processes could not be traced to a parent cell body, their distribution is reminiscent of elaborate calycal intrinsic neurons identified in honey bees and in the scarab beetle Pachnoda marginata (Strausfeld, 2002; Larsson et al., 2004).
DISCUSSION
Each of the paired mushroom bodies of Spodoptera littoralis contains about 4,000 Kenyon cells, compared to the ∼200,000 cells in each MB of the cockroach Periplaneta americana, 170,000 in an MB of the worker honey bee, 50,000 in locust's MB, and 2,000 in the MB of the fruit fly Drosophila melanogaster (Neder, 1959; Witthöft, 1967; Laurent, 2002; Technau and Heisenberg, 1982). The volume of the supraesophageal ganglion of Spodoptera is about four times that of the fruit fly, and the diameter of Kenyon cell axons range from between 3 and 6 times those of Kenyon cells in the honey bee's MBs. Thus, in this moth, which is about the same size as a honey bee, the mushroom bodies display some unusual features. The Kenyon cell complement is rather small, and its cells are unusually large, there being no obvious distinction between the size of Kenyon cell bodies and those of other interneurons of the protocerebrum. Yet the overall organization of the MBs does not depart from that expected from descriptions of other taxa, particularly D. melanogaster. As in Drosophila, the calyces consist of two fused elements, which in Apis and Periplaneta are represented by completely separated cup-like structures. Also, as in fruit flies, honey bees, and cockroaches, the spodopteran MB lobes are divided into distinct divisions, each supplied by a morphological class of Kenyon cell. The present study identifies three such classes based on their dendritic anatomy. Together, Kenyon cells project into four discrete lobular subdivisions of the MBs. Spiny class I Kenyon cells supply the α/β and α′/β′ subdivisions of, respectively, the vertical and medial lobes. Class II (clawed) Kenyon cells supply the vertical and medial components of the γ lobes; bunched clawed class III Kenyon cells supply the Y tract and its lobelets.
As in fruit flies, honey bees, and cockroaches, the γ division is the most anterior division of the vertical and medial lobes in Spodoptera. In adult honey bees, the γ division is ascribed to the vertical lobe (Strausfeld, 2002), whereas in adult fruit flies this division belongs to the medial lobe, although in larval stages the γ division is represented both in the vertical and medial lobes (Lee et al., 1999), as is the case in early pupae of honey bees (Farris et al., 2004). Although the γ lobe of Spodoptera is supplied by clawed class II Kenyon cells, as it is in Diptera, Hymenoptera, and Blattidae, to ascertain homology between divisions ascribed to the MBs of this moth and other taxa still requires additional cytological tests, such as using antisera that identify comparable divisions in the fruit fly and cockroach (Sinakevitch et al., 2001; Strausfeld et al., 2003).
The present article is not the first to describe lepidopteran MBs. Pearson's (1971) Golgi study on the privet moth Sphinx ligustri identified some of the cell types described here. The spiny intrinsic cells of Sphinx correspond to Kenyon cells supplying the α/β and α′/β′ divisions of Spodoptera. The bunched intrinsic cells of S. ligustri correspond to clawed Kenyon cells supplying the γ division. Pearson's bunched accessory cells correspond to the dense clawed Kenyon cells in Spodoptera supplying the Y tract and its lobelets, a structure also identified in the hawkmoth Manduca sexta (Homberg et al., 1987). This tract, which was originally identified in S. ligustri, is comparable to a tract, seen in cockroaches and termites, that originates from class III clawed Kenyon cells, the dendrites of which form a discrete accessory calyx. Axons from these dendrites supply two lobelets that are separate from the main lobes of the MB, but lie alongside them (Farris and Strausfeld, 2003). Developmental studies suggest that class III cells are generated before any of the other types of Kenyon cells, resulting in their cell bodies being most distant from the center of the calyx where Kenyon cells are generated from clusters of ganglion mother cells (Farris and Sinakevitch, 2003). In Spodoptera, the distinctive cluster of Kenyon cell bodies supplying the Y tract are displaced laterally to one side of the calyces, suggesting their early differentiation. Similarly, Kenyon cells identified in the scarab beetle P. marginata and ascribed to class III also have their cell bodies furthest from the calyx, and in that species they contribute to about half of the total MB volume (Larsson et al., 2004). Although the adult honey bee MB lacks any structure equivalent to the Y tract, Farris et al. (2004) discussed whether axons enwrapping the pedunculus and lobes during postembryonic development represent class III Kenyon cells that are transitorily expressed in the MB. Whereas class III Kenyon cells in Isoptera and Blattidae provide a satellite accessory calyx that is distinct from the calyces, in Spodoptera class III Kenyon cells providing the Y tract and its lobelets have their dendrites integrated into calycal neuropil.
In male moths the antennal lobes are equipped with specialized macroglomerular complexes in which single and uniquely identifiable glomeruli are exclusively tuned to components of the female sex pheromone. Projection neurons originating from these glomeruli send their axons into the iACT, as do many projection neurons serving sexually isomorphic glomeruli that are tuned to plant volatiles. Collaterals from these axons invade the calyces (Anton and Hansson, 1994, 1995). Interestingly, conditioning experiments in S. littoralis have shown that female pheromone and plant odors elicit conditioned responses differentially. Single components of the female pheromone blend and plant odor components, as well as plant odor blends, can all be associated with a food reward. However, the complete conspecific pheromone blend cannot be learned (Hartlieb et al., 1999). This suggests that pheromone information and plant odors are processed differently, whereby Kenyon cells receiving information about the full pheromone blend supply a discrete subsystem of the MBs incapable of forming memories.
Our study raises questions about how organizational complexity, Kenyon cell number, and MB size relate to MB functions. The number of Kenyon cells in the honey bee's MBs is roughly 50 times greater than in the moth, even though they have the same body size. Is it reasonable to assume that long-lived, behaviorally complex generalists with exploratory foraging strategies, like the cockroach or the honey bee, require larger MBs with more neurons because they need to process more information than, say, a small moth that lives but a week? Might MBs that support long-term memory, compute positional information, and store space memory require greater cellular complexity than a homologous structure in a moth in which spatial orientation relies on pheromone detection? Is a short-lived, behaviorally relatively simple insect like Spodoptera less dependent on learning than, say, a honey bee, even though it is capable of forming an association between an odor and food (Fan et al., 2001)?
It should be cautioned that a possible role in learning and memory need not at all relate to the size or structural elaboration of the MBs, particularly if some of its divisions serve other functions. One factor that might be thought to influence the size and cellular organization of the MBs is the sensory diversity of afferent supply to its calyces, as exemplified by honey bees, in which calyces receive a major input from the optic lobes (Ehmer and Gronenberg, 2002). However, comparisons between ants equipped with compound eyes and species in which the optic lobes are diminutive (Gronenberg, 1999) suggest that, although multimodal inputs to the calyces reflect their structural elaboration, the volume of calycal neuropil reflects more the density of afferent axons supplying it rather than their sensory identity.
Despite differences of calycal organization and size, the principal organization of Kenyon cells and their segregation to different divisions of the lobes appears to be shared across taxa. Thus, a small MB with relatively few neurons does not a priori suggest that it is fundamentally simpler than a larger one with many neurons. As shown recently for Drosophila, a taxon that lives only a few weeks longer than the moth and possesses only about 2,000 Kenyon cells in each of its MBs, this animal can associate and generalize different cues independent of context, can form long-term memories, and requires its MBs for spatial orientation (Martin et al., 1998). Thus, to appreciate the significance of differences between taxa it is essential to first determine the commonalties of this system among taxa. Here we have shown that the intrinsic organization of Kenyon cells in a lepidopteran provides subsystems within the lobes that are characteristic of many other genera. Evidence has been provided that suggest the calyces are not uniform structures, but that afferent supply is parsed into discrete zones and that these are likely to be subtended by systems of Kenyon cells supplying discrete divisions in the lobes. Such data suggests fundamental similarities of MB organization among evolutionary diverse genera. And because the most recent common origin of these taxa precedes the Permian era, we suggest that subdivisions of the MBs are ancient attributes, serving functions that are fundamental to life on land.




