Differential distribution of voltage-gated calcium channel alpha-2 delta (α2δ) subunit mRNA-containing cells in the rat central nervous system and the dorsal root ganglia
Abstract
Voltage-gated calcium channels (VGCCs) play an essential role in controlling neurotransmitter release, neuronal excitability, and gene expression in the nervous system. The distribution of cells that contain mRNAs encoding the auxiliary α2δ-1, α2δ-2, and α2δ-3 subunits of the VGCCs in the central nervous system (CNS) and the dorsal root ganglia (DRG) was examined in rats by using in situ hybridization. Specific labeling of α2δ-1, α2δ-2, and α2δ-3 mRNAs appeared to be largely confined to neurons and was widely, although differentially, distributed in the brain, the spinal cord, and the DRG. Importantly, α2δ-2 mRNA was found to be expressed in interneurons in the cortex, the hippocampus, the striatum, and in regions that contain dense cholinergic neurons. Our results suggest that different α2δ subunits may exert distinctive functions in the CNS. The α2δ-1 subunit mRNA is localized in brain regions known to be involved in cortical processing, learning and memory, defensive behavior, neuroendocrine secretion, autonomic activation, primary sensory transmission, and general arousal. The α2δ-2 subunit mRNA is present in brain regions known to modulate the overall activities of the cortex, the hippocampus, and the thalamus. The α2δ-2 subunit is also found in brain regions known to be involved in olfaction, somatic motor control, fluid homeostasis, ingestive and defensive behaviors, neuroendocrine functions, and circadian rhythm. In addition to being localized in brain regions that express α2δ-1 and α2δ-2 subunit mRNAs, α2δ-3 subunit mRNA is highly expressed in regions involved in auditory information processing and somatic movement. J. Comp. Neurol. 491:246–269, 2005. © 2005 Wiley-Liss, Inc.
Calcium (Ca++) plays an essential role in cell excitability and intracellular signaling in the nervous system. Ca++ binds to intracellular molecules, resulting in a variety of significant cellular changes. Such changes include the triggering of neurotransmitter release from nerve terminals and the activation of second messenger systems that may alter gene expression and initiate apoptosis (Berridge et al.,1998; Berridge,1998; Nestler,2001).
The opening of Ca++ channels is the critical link between cell depolarization and Ca++ entry. Increased intracellular potential triggers the opening of voltage-gated calcium channels (VGCCs) and, as a result, Ca++ enters the cell. Based on their voltage dependence, kinetics, and pharmacology, VGCCs have been categorized into L-, N-, P/Q-, R-, and T-types. Mutations of VGCCs have been found to underlie some neurological diseases (Burgess and Noebels,1999).
VGCCs are heteromultimeric complexes composed of several different subunits including α1, β, α2δ, and γ (Hofmann et al.,1999; Catterall,2000). The α1 subunit is a large transmembrane protein that forms the ion-conducting pore of the channel and functions as the voltage-sensor (Mikami et al.,1989; Biel et al.,1990; Varadi et al.,1991). The β, α2δ and γ are auxiliary subunits that play a role in facilitating targeting and assembly of channels on the cell surface as well as modulating the biophysical characteristics of the channel (Klugbauer et al.,2003). The α2δ subunits are transcribed as a single mRNA from the same gene. The translated protein is cleaved to yield α2 and δ proteins, which are then disulfide-linked and heavily glycosylated to yield functional α2δ subunits (De Jongh et al.,1990; Jay et al.,1991). Four different α2δ subunits have been described. α2δ-1 was the first subunit cloned (Ellis et al.,1988), followed by the cloning of α2δ-2 and α2δ-3 (Klugbauer et al.,1999; Gao et al.,2000). The α2δ-2 and α2δ-3 subunits share 55.6 and 30.3% sequence identity with the α2δ-1 subunit, respectively (Klugbauer et al.,1999). Another subunit, α2δ-4, which is not expressed in the brain, was more recently cloned and characterized (Qin et al.,2002).
The α2δ-1 and α2δ-2 VGCC subunits have gained considerable attention in recent years because they serve as a high-affinity binding site for gabapentin [1-(aminomethyl) cyclohexaneacetic acid] (Gee et al.,1996; Gong et al.,2001). Gabapentin was originally developed as an anticonvulsant, but it is also effective clinically for other indications, including neuropathic pain and affective disorders (Singh et al.,1996, Harden,1999; Singh and Kennedy,2003). Binding with high affinity to α2δ-1 and α2δ-2 VGCC subunits is believed to be related to the analgesic effect of gabapentin on neuropathic pain (Gee et al.,1996) and to the antiepileptic effect of gabapentin on epilepsy (Rogawski and Loscher,2004), but the exact mechanism of action of these effects remains to be determined.
The earliest evidence for the expression of VGCC subunits in the CNS relied on molecular cloning and in situ hybridization studies (Snutch et al.,1990; Tanaka et al.,1995). While α2δ-2 mRNA is found to be nearly ubiquitously expressed, the α2δ-3 subunit is only found in the brain (Hobom et al.,2000). General distribution of α2δ subunit mRNA has been described in horizontal and sagittal sections throughout the mouse brain (Klugbauer et al.,1999; Hobom et al.,2000). α2δ-1, -2, and -3 subunits were found to be expressed in a discrete pattern, with α2δ-1 mRNA being expressed highly in the hippocampus, the cerebellum, the cortex, and the olfactory bulb (Klugbauer et al.,1999), α2δ-2 mRNA being expressed at high levels in cerebellar Purkinje cells, the habenulae, the septum, and the reticular thalamic nucleus (Hobom et al.,2000; Barclay et al.,2001), and α2δ-3 mRNA being expressed in the hippocampus, the cerebral cortex, the striatum, and the thalamus (Klugbauer et al.,1999).
To further the understanding of the function of α2δ subunits and their potential role in neurologic and psychiatric disorders, a detailed comprehensive study aimed to map the α2δ-1, 2, and 3 subunits on coronal sections through the rat CNS were carried out using in situ hybridization. Because of the unique distribution pattern of the α2δ-2 subunit in various brain regions, the neurochemical identity of α2δ-2-positive cells was examined using a double-labeling method. Our mapping results in rats confirm and greatly extend previous findings in the mouse. A preliminary account of this work has been reported elsewhere (Cole et al.,2003).
Abbreviations
-
- AAA
-
anterior amygdaloid area
-
- ACB
-
nucleus accumbens
-
- AD
-
anterodorsal nucleus thalamus
-
- ADP
-
anterodorsal preoptic nucleus
-
- AHN
-
anterior hypothalamic nucleus
-
- AON
-
anterior olfactory nucleus
-
- AP
-
area postrema
-
- ARH
-
arcuate nucleus hypothalamus
-
- AVPV
-
anteroventral periventricular nucleus hypothalamus
-
- B
-
Barrington's nucleus
-
- BLAa
-
basolateral nucleus amygdala
-
- BMA
-
basomedial nucleus amygdala
-
- BSTov
-
bed nuclei stria terminalis, anterior division, oval nucleus
-
- c
-
central canal, spinal cord/medulla
-
- CA1
-
field CA1, Ammon's horn
-
- CA2
-
field CA2, Ammon's horn
-
- CA3
-
field CA3, Ammon's horn
-
- cc
-
corpus callosum
-
- CEA
-
central nucleus amygdala
-
- CL
-
central lateral nucleus thalamus
-
- CLA
-
claustrum
-
- COA
-
cortical nucleus amygdala
-
- CP
-
caudoputamen
-
- CUN
-
cuneiform nucleus
-
- DG
-
dentate gyrus
-
- DMHp
-
dorsomedial nucleus hypothalamus, posterior part
-
- DMX
-
dorsal motor nucleus vagus nerve
-
- DR
-
dorsal nucleus raphe
-
- DTN
-
dorsal tegmental nucleus
-
- FS
-
fundus striatum
-
- fx
-
columns of the fornix
-
- GPI
-
globus pallidus, lateral segment
-
- IA
-
intercollated nuclei amygdale
-
- Ic
-
internal capsule
-
- IG
-
induseum griseum
-
- INC
-
interstitial nucleus [Cajal]
-
- isl
-
major island of Calleja (olfactory tubercle)
-
- KF
-
Kolliker-Fuse subnucleus
-
- LA
-
lateral nucleus amygdala
-
- LC
-
locus coeruleus
-
- LD
-
lateral dorsal nucleus thalamus
-
- LDT
-
laterodorsal tegmental nucleus
-
- LG
-
lateral geniculate complex
-
- LGd
-
lateral geniculate complex, dorsal part
-
- LRNm
-
lateral reticular nucleus, magnocellular part
-
- LSc
-
lateral septal nucleus, caudal part
-
- LSr
-
lateral septal nucleus, rostral part
-
- LSv
-
lateral septal nucleus, ventral part
-
- MA
-
magnocellular preoptic nucleus
-
- MDRN
-
medullary reticular nucleus
-
- MEA
-
medial nucleus amygdala
-
- MEPO
-
medial preoptic nucleus
-
- MGd
-
medial geniculate complex, dorsal part
-
- MGv
-
medial geniculate complex, ventral part
-
- MH
-
medial habenula
-
- MS
-
medial septal nucleus
-
- ND
-
nucleus of Darkschewitsch
-
- NDB
-
nucleus of the diagonal band [Broca]
-
- NLOT
-
nucleus of the lateral olfactory tract
-
- NTB
-
nucleus of the trapezoid body
-
- NTS
-
nucleus of the solitary tract
-
- OT
-
olfactory tubercle
-
- PAG
-
periaqueductal gray
-
- PBIe
-
parabrachial nucleus, external lateral part
-
- PBmm
-
parabrachial nucleus, medial part
-
- PF
-
parafascicular nucleus
-
- PH
-
posterior hypothalamic nucleus
-
- PIR
-
piriform area
-
- PMd
-
dorsal premammillary nucleus
-
- PMv
-
ventral premammillary nucleus
-
- PPN
-
pedunculopontine nucleus
-
- PRNr
-
Pontine reticular nucleus, rostral part
-
- PS
-
parastrial nucleus
-
- PVH
-
paraventricular nucleus hypothalamus
-
- PVT
-
paraventricular nucleus thalamus
-
- RE
-
nucleus reuniens
-
- RH
-
rhomboid nucleus
-
- RT
-
reticular nucleus thalamus
-
- SAG
-
nucleus sagulum
-
- SC
-
superior colliculus
-
- SCdg
-
superior colliculus, deep gray layer
-
- SCN
-
suprachiasmatic nucleus
-
- SF
-
septofimbrial nucleus
-
- SFO
-
subfornical organ
-
- SI
-
substantia innominata [Reichert]
-
- SNc
-
substantia nigra, compact part
-
- SNr
-
substantia nigra, reticular part
-
- SPVC
-
spinal nucleus of the trigeminal, caudal part
-
- STN
-
subthalamic nucleus
-
- TRN
-
tegmental reticular nucleus, pontine gray
-
- TTd
-
taenia tecta, dorsal part
-
- TTv
-
taenia tecta, ventral part
-
- V
-
motor nucleus of the trigeminal nerve
-
- V3
-
third ventricle
-
- VL
-
lateral ventricle
-
- VMH
-
ventromedial nucleus hypothalamus
-
- VTA
-
ventral tegmental area [Tsai]
-
- VTN
-
ventral tegmental nucleus
-
- XII
-
hypoglossal nucleus
-
- ZI
-
zona incerta
MATERIALS AND METHODS
Animals
The protocols performed in the present study were approved by the Institutional Animal Care and Use Committee of Merck Research Laboratories, in accordance with the guidelines of the National Institutes of Health and the US Department of Agriculture. Adult male Sprague-Dawley albino rats (250–300 g) were obtained from Harlan (Indianapolis, IN) and housed in individual cages under controlled temperature and humidity on a 12:12 light/dark cycle (lights on at 07:00). Food and water were available ad libitum.
Perfusion and tissue processing
Animals were deeply anesthetized with pentobarbital anesthesia (65 mg/kg, i.p.). Each rat was perfused transcardially with normal saline (50–100 ml), followed by ice-cold 4% paraformaldehyde (Electron Microscopy Sciences, Fort Washington, PA) in 0.1 M borate buffer at pH 9.5 for 20 minutes. The brains were quickly removed, postfixed for 2 hours in the same fixative, then placed overnight in 20% sucrose in 0.02 M potassium PBS (KPBS) for cryoprotection. Five series of 30-μm thick frozen sections through the brain and the lumbar enlargement of the spinal cord were cut using a sliding microtome. Sections were collected at 150-μm intervals into an antifreeze solution and stored at –20°C until use. The L4 or L5 dorsal root ganglia (DRG) were cut at 20 μm on a cryostat, thaw-mounted onto poly-L-lysine-coated slides, and stored at –80°C until use.
In situ hybridization
Sections were mounted onto gelatin-subbed, poly-L-lysine-coated microscopic slides, postfixed with 10% formalin for 30 minutes, and processed for in situ hybridization as described previously (Simmons et al.,1989). To minimize procedural artifacts, all tissue hybridized with each probe was processed together in a single in situ hybridization histochemistry experiment. After a 30-minute proteinase K digestion (10 μg/ml at 37°C; Boehringer Mannheim, Indianapolis, IN) and acetylation (0.0025% acetic anhydride at room temperature), the sections were dehydrated in ascending alcohols and dried under vacuum overnight. T7 polymerase (Promega, Madison, WI) was used to transcribe 35S-labeled antisense cRNA probes. To prepare probes, first-strand cDNA was synthesized from rat DRG total RNA using the RETROscript kit (Ambion, Austin, TX). α2δ-specific DNA fragments were generated by PCR using PfuTurbo DNA polymerase (Stratagene, La Jolla, CA). For the α2δ-1 specific probe, a α2δ-1-specific sense strand 25-mer, 5′-GCACCAAGGGAATACTGCAATGACC-3′, and an antisense strand 27-mer, 5′-CCACCATCATCTAGAATGACACAGTCC-3′ were used to generate a 587-bp fragment (nucleotides 1948–2534 of rat coding sequence, Access. no. AF286488). For the α2δ-2-specific probe, a α2δ-2-specific sense strand 30-mer, 5′-GTCTGATGATGACTATGTGAATGTGGCCTC-3′, and an antisense strand 24-mer, 5′-CCAGGGCCATCCTGTGTCAGGTTG-3′ were used to generate a 579-bp fragment (nts 972–1550 of rat coding sequence, Access. no. AF486277). For the α2δ-3-specific probe, a α2δ-3 specific sense strand 35-mer, 5′-CCATCGCTGCCAAGTACTCGGGCTCCCAGCTTCTG-3′, and an antisense strand 28-mer, 5′-GCACTTCCAAAGTACTGCCATATGACAG-3′ were used to generate a 487-bp fragment (nts 164–650 of rat coding sequence, Access. no. AF486278). All PCR was performed under the following conditions: 5 minutes at 95°C followed by 35 cycles of 20 seconds at 95°C, 20 seconds at 50°C, and 1 minute at 72°C. The PCR products were cloned into the pCR-Blunt II-TOPO vector (Invitrogen, La Jolla, CA) and linearized at the 5′ or 3′ end of the cloned fragment for use as an in vitro transcription template. The identity of each probe was confirmed by sequence analysis, and each yielded hybridization patterns in accord with available evidence. The specificity of the hybridization was examined by hybridizing the sections with 35S-labeled sense probes with similar specific activities. The radiolabeled cRNA probe was purified by passing the transcription reaction solution over a Sephadex G-50 Nick column (Pharmacia, Piscataway, NJ), and four 100-μl fractions were collected and counted by using a scintillation counter (Packard, Meridian, CT). The leading fraction was heated at 65°C for 5 minutes with 500 μg/ml yeast tRNA (Sigma, St. Louis, MO) and 50 μM dithiothreitol (DTT) (Stratagene) in DEPC (Sigma) water and then diluted to an activity of ∼107 with hybridization buffer containing 50% formamide (Boehringer Mannheim), 0.25 M sodium chloride, 1× Denhardt's solution (Sigma), and 10% dextran sulfate (Pharmacia). This hybridization solution was pipetted onto the sections (80 μl/slide), which were covered with a glass coverslip and sealed with DPX (Electron Microscopy Sciences) before incubation for 20 hours at 58°C. After hybridization, the slides were washed four times (5 minutes each) in 4× SSC before RNase digestion (20 μg/ml for 30 minutes at 37°C; Sigma) and rinsed at room temperature in decreasing concentrations of SSC that contained 1 mM DTT (2×, 1×, 0.5× for 10 minutes each) to final stringency of 0.1× SSC at 75°C for 30 minutes. After dehydration in ascending alcohols, the sections were exposed to X-ray films (BioMax MS; Eastman Kodak, Rochester, NY) for 2–3 days, together with autoradiographic 14C microscales (Amersham, Arlington Heights, IL), before being dipped in NBT-2 liquid emulsion (Eastman Kodak). The dipped autoradiograms were developed 21 days later with Kodak D-19 developer and the sections were counterstained with thionin.
Combined immunohistochemistry and hybridization histochemistry
Colocalization studies were performed to examine the neurochemical identity of the scattered α2δ-1 and α2δ-2 mRNA-containing cells in the hippocampus, the cerebral cortex, the caudoputamen, and some other selected regions. In the hippocampus and cortex the scattered distribution pattern of α2δ-2 mRNA appeared to be reminiscent of GABAergic interneurons and it was hypothesized that α2δ-2, but not α2δ-1, might be selectively expressed in GABAergic interneurons. To address this hypothesis, colocalization of α2δ-2 or α2δ-1 with parvalbumin (PV) was performed. PV is a calcium-binding protein that is known to be expressed in a subpopulation of GABAergic interneurons in the hippocampus and cortex, and therefore has been widely used as a surrogate marker for GABAergic interneurons (Miettinen et al.,1996; Sloviter et al.,2001). Furthermore, because α2δ-2 mRNA was expressed in scattered cells in the caudoputamen and appeared to be reminiscent of the pattern of cholinergic interneurons, colocalization of α2δ-2 or α2δ-1 with choline acetyltransferase (ChAT), a marker for cholinergic neurons, was performed. Both ChAT and PV antibodies were obtained from Chemicon (Temecula, CA, nos. AB5042 and MAB1572, respectively). The ChAT polyclonal antibody was raised against a synthetic peptide (H-GLFSSYRLPGHTQDTLVAQKSS-NH2) from porcine ChAT, coupled to KLH. The PV monoclonal antibody was raised against PV protein purified from frog muscle, and reacts with PV from both brain and muscle. The antibody specifically stains the Ca++ bound form of PV. The staining of PV and ChAT immunoreactive cells in this study using these two antibodies was identical to previous reports (Armstrong et al.,1983; Celio,1990) and no extraneous objects were stained. Immunolocalization of PV (1:2,000), or ChAT (1:1,000) was combined with hybridization histochemical detection of α2δ-1 or α2δ-2 subunit mRNAs. The protocol for combining immunoperoxidase labeling with isotopic in situ hybridization involves minor modifications (Chan et al., 1993) of the procedure described by Watts and Swanson (1989). Immunohistochemistry was carried out first, using a 48-hour at 4°C primary antibody incubation. A nonimmune (blocking) serum, a potential source of RNAse contamination, was replaced with 2% heparin sulfate and 2% BSA. A conventional avidin-biotin immunoperoxidase protocol (Sawchenko et al., 1990), Vectastain Elite reagents (Vector Laboratories, Burlingame, CA), and a diaminobenzidine reaction were used to visualize PV or ChAT immunoreactivities.
Data collection and analysis
The optical density of the autoradiographic images of α2δ-1, α2δ-2, or α2δ-3 mRNA in different brain regions was measured from x-ray films by using a microcomputer-assisted video imaging densitometer (MCID system, Imaging Research, St. Catherines, Ontario, Canada). The boundaries of different brain regions were determined from observation of the corresponding Nissl-stained sections. The mean optical density over a large irregularly shaped region of the lateral and the third ventricles was also measured and used to calculate the mean background density, which was subtracted from the optical density measurement of signals over different brain regions. Although these mean optical density measurements do not correspond to absolute optical density units, they reflect relative mRNA levels. As mentioned above, commercially available 14C autoradiographic standards were exposed to each x-ray film along with the experimental material. The mean optical density of an interactively defined region over each standard was measured. The mean optical densities of the autoradiographic images recorded over different brain regions all fell within the linear range of the standard values.
Clustering analysis of the numerical optical density measurements of autoradiographic images of each channel subunit recorded over different brain regions was performed with SciTegic Pipeline Pilot software using a maximal dissimilarity-based partitioning method (Abercrombie,1946; Kaufman and Rousseeuw,1990). Untransformed α2δ abundance measurements for each subunit were clustered to assign brain regions to qualitative abundance bins. A five-scale rating method was adopted, in which “+” represents close to background, “++” represents low, “+++” represents moderate, “++++” represents high, and “+++++” represents very high optical densities. Ratings are relative within each probe, and because of differences in size, sequence, and isotope-labeling efficiency among probes, the exact quantitative comparisons of subunit expression across probes are treacherous. Thus, comparisons of the expression pattern of different subunits were made based on the relative ratings.
Analysis of cells double-labeled for PV and α2δ-1 or -2 in the hippocampus was made by standard counting methods. This involved counting the number of cells in the CA1, CA3, and DG of the hippocampus that contained clusters of silver grains whose density was >5 times background. Data was collected from the hippocampal regions as the number of cells that were PV-positive but did not express an α2δ subunit, those which expressed both PV and an α2δ subunit, as well as those that were PV-negative, but expressed an α2δ subunit. Average counts per section were generated from five sections through the hippocampal region of interest in each animal, then averaged again to obtain group means. Abercrombie's (1946) method was used to correct for double-counting errors. Double-labeling in other brain regions were estimated by counting single and double-labeled cells under 20× or 40× objectives at representative levels in a complete series.
Images from representative levels of the brain were scanned from the x-ray film using a desktop scanner and photomicrographs taken using an Olympus microscope. All images were processed with Adobe PhotoShop software (San Jose, CA) for optimal sharpness, contrast, and brightness, and plotted using Freehand software (Macromedia, San Francisco, CA).
RESULTS
Expression of α2δ subunit mRNA in the rat CNS and DRG
The α2δ-1, α2δ-2, and α2δ-3 mRNA-containing cells were found to be widely distributed throughout the rat brain, the spinal cord, and the DRG (Figs. 1-3, 8, 9), including areas involved in somatosensory, motor, autonomic, and neuroendocrine functions. The three subunits showed unique distribution patterns within each major subdivision of the brain as well as the spinal cord (Table 1), and appeared to be expressed in the majority of, if not all, neurons because each cell examined at high power showed typical neuronal morphology in Nissl counterstaining, and no significant labeling was found over fiber tracts. Similarly, in the DRG all labeling was localized to neurons and no satellite cells appeared to express α2δ mRNA. Specific signals were obtained in all experiments, as silver grains were not detected over cells when sections were hybridized with isotope-labeled sense strand probes (data not shown). Moreover, the specificity of the labeling in the present study is also supported by our results in mouse brain using the same probes (data not shown), which is in good agreement with data previously reported in the literature (Hobom et al.,2000).
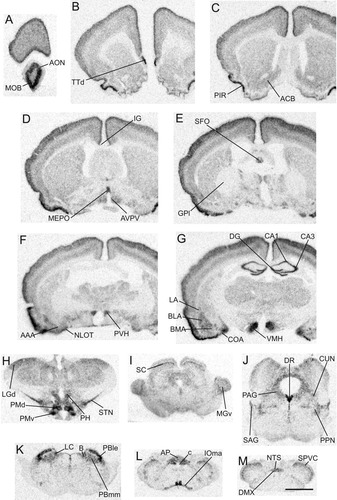
Distribution of α2δ-1 mRNA in the rat brain. Series of low-power images of film autoradiograms arranged from rostral (A) to caudal (M) to show the overall distribution of α2δ-1 mRNA expression in the rat brain. Scale bar = 2 mm in M (applies to A–M).
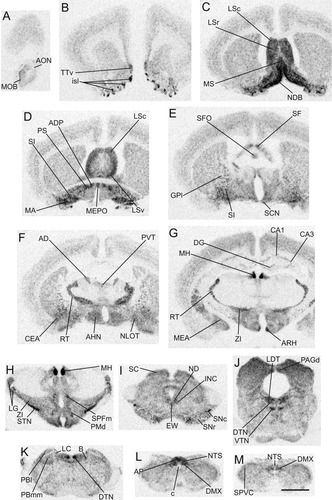
Distribution of α2δ-2 mRNA in the rat brain. Series of low-power images of film autoradiograms at comparable levels to those in Figure 1 arranged from rostral (A) to caudal (M) to show the overall distribution of α2δ-2 mRNA expression in the rat brain. Scale bar = 2 mm in M (applies to A–M).
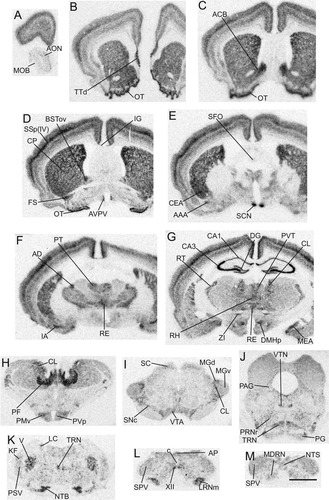
Distribution of α2δ-3 mRNA in the rat brain. Series of low-power images of film autoradiograms at comparable levels to those in Figure 2 arranged from rostral (A) to caudal (M) to show the overall distribution of α2δ-3 mRNA expression in the rat brain. Scale bar = 2 mm in M (applies to A–M).
| Cell group | Alpha2d1 | Alpha2d2 | Alpha2d3 |
|---|---|---|---|
| I. Forebrain | |||
| A. Isocortex | |||
| I | + | + | ++ |
| II | +++ | + | +++ |
| III | +++ | + | +++ |
| IV | +++ | ++ | ++++ |
| V | ++ | ++ | +++ |
| VI | ++ | ++ | ++ |
| Claustrum | ++ | ++ | ++ |
| B. Olfactory regions | |||
| 1 Piriform cortex | |||
| I | + | + | + |
| II | +++++ | + | ++ |
| III | ++ | ++ | ++ |
| 2 Endopiriform nucleus | ++ | ++ | +++ |
| C. Hippocampal formation (cortex) | |||
| 1 Entorhinal area (lateral and medial) | |||
| I | + | + | +++ |
| II | +++++ | + | ++ |
| III | ++++ | + | ++ |
| IV-VI | ++ | + | ++ |
| 2 Subiculum (dorsal) | ++ | + | + |
| Subiculum (ventral) | ++ | + | +++ |
| 3 CA1 | |||
| S. lacunosum-moleculare | + | + | + |
| Stratum radiatum | + | + | + |
| Pyramidal layer | ++++ | + | +++ |
| Stratum oriens | + | + | ++ |
| 4 CA3 | |||
| Stratum radiatum | + | + | + |
| Pyramidal layer | ++++ | + | +++++ |
| Stratum oriens | + | + | + |
| 5 Dentate gyrus | |||
| Molecular layer | + | + | + |
| Granular layer | +++++ | + | ++++ |
| Polymorph layer | ++ | + | +++ |
| 6 Induseum griseum/fasciola cinerea | ++ | + | +++ |
| D. Amygdala | |||
| 1 Medial nucleus | |||
| Anterior part | ++ | ++ | +++ |
| Posterodorsal part | ++ | ++ | +++ |
| 2 Amygdalohippocampal area | ++++ | + | ++++ |
| 3 Cortical nucleus | |||
| Anterior part | +++ | + | ++ |
| Posterior part | +++ | + | ++ |
| 4 N. lat. olfactory tract | ++ | ++++ | +++ |
| 5 Anterior amygdaloid area | ++ | +++ | +++ |
| 6 Central nucleus | ++ | +++ | +++ |
| 7 Lateral nucleus | ++ | + | ++ |
| 8 Basolateral nucleus | +++ | + | ++ |
| 9 Basomedial nucleus | ++++ | + | ++ |
| 10 Intercalated nuclei | +++ | ++ | ++++ |
| E. Septum | |||
| 1 Lateral nucleus | |||
| Dorsal part | + | +++++ | + |
| Intermediate part | + | +++++ | + |
| Ventral part | + | +++++ | + |
| 2 Medial nucleus | + | +++ | + |
| Nucleus of diagonal band | + | +++++ | ++ |
| 3 Bed n. Stria terminalis | |||
| Rostromedial region | + | ++ | ++ |
| Rostrolateral region | + | +++ | ++++ |
| Posterodorsal region | + | ++ | ++++ |
| Posteroventral region | + | ++ | +++ |
| 4 Bed n. anterior commissure | ++ | ++ | + |
| 5 Septofimbrial nucleus | + | +++++ | + |
| 6 Triangular nucleus | ++ | +++++ | ++ |
| 7 Subfornical organ | +++ | +++++ | + |
| F. Basal ganglia | |||
| 1 Caudoputamen | |||
| Posteroventral part | ++ | ++ | +++ |
| Nucleus accumbens | +++ | ++ | ++ |
| Fundus of striatum | ++ | ++ | +++ |
| 2 Globus pallidus | |||
| Entopeduncular n. | + | ++ | + |
| GPI | + | ++ | + |
| Substantia innominata | + | ++++ | ++ |
| Magnocellular preoptic nucleus | ++ | +++++ | ++ |
| 3 Subthalamic nucleus | ++++ | +++ | ++ |
| 4 Substantia nigra | |||
| Compact part | ++ | +++ | ++ |
| Reticular, latertal parts | + | ++ | + |
| Ventral tegmental area | ++ | ++ | ++ |
| G. Thalamus | |||
| 1 Medial habenula | + | +++++ | + |
| 2 Lateral habenula | ++ | ++ | + |
| 3 Anterior group | |||
| Anteroventral n. | ++ | + | ++ |
| Anteromedial n. | ++ | + | ++ |
| Anterodorsal n. | ++ | +++ | ++ |
| 4 Mediodorsal nucleus | |||
| Medial part | ++ | + | +++ |
| Central part | ++ | + | +++ |
| Lateral part | ++ | + | +++ |
| 5 Lateral group | |||
| Lateral dorsal n. | ++ | + | ++ |
| Lateral posterior n. | + | + | ++ |
| 6 Midline group | |||
| Paraventricular n. | ++ | ++ | +++ |
| Parataenial n. | + | + | ++++ |
| N. reuniens | ++ | ++ | +++ |
| Rhomboid n. | ++ | + | ++++ |
| N. gelatinosa | ++ | + | ++ |
| 7 Ventral group | |||
| Ventral anterior/v. lat. | ++ | + | +++ |
| Ventral medial | + | + | ++ |
| Ventral posterior | ++ | + | ++ |
| Gustatory nucleus | ++ | + | ++ |
| 8 Posterior complex | ++ | + | ++ |
| 9 Medial geniculate n. | |||
| Dorsal part | ++ | + | ++ |
| Ventral part | ++ | + | ++ |
| Medial part | ++ | ++ | ++ |
| 10 Lateral geniculate n. | |||
| Dorsal part | ++ | + | ++ |
| Intergeniculate leaflet | + | + | ++ |
| Ventral part | + | + | ++ |
| 11 Intralaminar nuclei | |||
| Central medial n. | ++ | + | ++ |
| Paracentral n. | + | + | ++ |
| Central lateral n. | ++ | + | ++ |
| Parafascicular n. | ++ | ++ | ++++ |
| 12 Reticular nucleus | + | ++++ | ++++ |
| 13 Zona incerta | |||
| Rostral | + | +++ | + |
| Caudal | + | +++ | ++ |
| 14 N. fields of Forel | + | ++ | ++ |
| H. Hypothalamus | |||
| 1 Periventricular zone | |||
| Median preoptic n. | ++++ | +++++ | + |
| Anteroventral periventricular n. | +++ | ++ | +++ |
| Preoptic periventricular nucleus | + | +++ | ++ |
| Suprachiasmatic n. | ++ | ++++ | +++++ |
| Supraoptic nucleus | ++++ | ++ | ++ |
| Paraventricular n. | |||
| Autonomic part | ++++ | ++ | ++ |
| Parvicellular part | ++++ | +++ | ++ |
| Magnocellular part | +++++ | ++ | ++ |
| Anterior periventricular nucleus | ++ | ++ | ++ |
| Arcuate nucleus | ++ | +++++ | ++ |
| Posterior periventricular n. | ++ | +++ | ++ |
| 2 Medial zone | |||
| Medial preoptic area | + | +++ | ++ |
| Medial preoptic n. | |||
| Lateral part | ++ | +++ | ++ |
| Medial part | ++ | ++ | ++ |
| Central part | +++ | ++ | +++ |
| Anterior hypo. n. | |||
| Anterior part | ++ | +++ | ++ |
| Central, posterior parts | + | +++++ | ++ |
| Retrochiasmatic area | + | ++ | ++ |
| Ventromedial n. | +++++ | ++ | ++ |
| Dorsomedial n. | ++++ | ++ | ++++ |
| Tuberomammillary n. | ++++ | ++ | +++ |
| Premammillary n. | |||
| Dorsal part | +++++ | +++++ | ++ |
| Ventral part | ++++ | +++ | +++ |
| Supramammillary n. | |||
| Lateral part | ++++ | ++ | ++ |
| Medial part | ++++ | ++ | ++ |
| Lateral mammillary n. | ++ | ++ | +++ |
| Medial mammillary n. | ++ | ++++ | ++ |
| 3 Lateral zone | |||
| Lateral preoptic area | + | ++++ | ++ |
| Lateral hypothalamic a. | ++ | ++++ | ++ |
| Posterior hypo. area | +++ | +++ | ++ |
| II. Brainstem | |||
| A. Sensory | |||
| 1 Visual | |||
| Superior colliculus | |||
| I | + | ++++ | ++ |
| II | ++ | +++++ | ++ |
| III | ++ | +++++ | ++ |
| IV | ++++ | +++++ | +++ |
| V-VI | + | +++ | ++ |
| Parabigeminal n. | ++ | ++++ | ++ |
| Pretectal region | |||
| Olivary n. | +++ | +++ | ++ |
| N. optic tract | + | +++ | ++ |
| Anterior n. | ++ | ++++ | +++ |
| Posterior n. | ++ | +++ | ++ |
| Medial pretectal a. | +++ | +++++ | ++ |
| N. posterior commissu | + | +++++ | ++ |
| Medial terminal n. | ++ | +++++ | ++ |
| 2 Somatosensory | |||
| Mesencephalic n. V | ++ | ++ | ++ |
| Principal sensory n. V | + | ++ | ++ |
| Spinal n. V | |||
| Oral part | + | ++ | ++ |
| Interpolar part | + | +++ | ++ |
| Caudal part | ++ | ++ | +++ |
| External cuneate n. | + | ++ | +++ |
| 3 Auditory | |||
| Cochlear nuclei | |||
| Dorsal | + | ++ | ++ |
| Ventral | + | ++ | ++++ |
| N. trapezoid body | + | ++ | ++++ |
| Superior olive | + | ++ | ++ |
| N. lateral lemniscus | |||
| Ventral | + | ++ | ++ |
| Dorsal | + | ++ | ++ |
| Inferior colliculus | |||
| External | ++ | ++++ | ++ |
| Dorsal | ++ | ++++ | ++ |
| Central | + | +++ | ++ |
| N. brachium inf. coll. | ++ | ++ | ++ |
| N. saguluum | ++ | ++ | ++ |
| 4 Vestibular | |||
| Medial n. | ++ | +++++ | ++ |
| Lateral n. | + | + | + |
| Superior n. | + | +++ | ++ |
| Spinal n. | + | ++ | +++ |
| 5 Gustatory | |||
| N. solitary tract, ant. | ++ | ++ | ++ |
| 6 Visceral | |||
| N. solitary tract | |||
| Medial part | ++++ | +++++ | ++ |
| Commissural part | ++++ | +++++ | ++ |
| Lateral part | ++++ | ++++ | ++ |
| Area postrema | +++ | +++++ | ++ |
| Parabrachial n. | |||
| Lateral | ++++ | +++ | + |
| Medial | +++ | ++ | + |
| Kölliker-Fuse n. | ++ | +++++ | ++ |
| B. Motor | |||
| 1 Eye | |||
| Oculomotor (III) | + | +++ | ++ |
| Trochlear (IV) | + | +++ | ++ |
| Abducens (VI) | + | ++ | ++ |
| 2 Jaw | |||
| Motor n. V | + | ++ | +++ |
| 3 Face | |||
| Facial n. (VII) | + | ++ | +++ |
| 4 Pharynx/larynx | |||
| N. ambiguus | +++ | ++ | +++ |
| 5 Tongue | |||
| Hypoglossal n. (XII) | ++ | ++ | +++ |
| 6 Viscera | |||
| Dorsal motor n. X | ++++ | +++++ | ++ |
| C. Reticular core (including central, gray and raphé) | |||
| 1 Periaqueductal gray - assoc. w/PAG | |||
| Interstitial nucleus of Cajal | + | ++ | ++ |
| N. Darkschewitsch | ++ | +++++ | ++ |
| Dorsal tegmental n. | + | +++++ | ++ |
| Ventral tegmental n. | + | ++++ | +++ |
| N. incertus | + | +++++ | ++ |
| Laterodorsal teg. n. | ++ | +++++ | ++ |
| Barrington's n. | +++ | +++ | + |
| Locus coeruleus | ++++ | ++++ | ++ |
| 2 Raphé | |||
| Interfascicular n. | + | ++ | ++ |
| Rostral linear n. | + | ++ | ++ |
| Dorsal raphé | +++++ | +++ | ++ |
| N. raphé pontis | ++ | +++ | ++ |
| N. raphé magnus | + | ++ | ++ |
| N. raphé obscurus | + | ++ | + |
| N. raphé pallidus | + | + | ++ |
| 3 Interpeduncular n. | |||
| Rostral subnucleus | + | +++++ | +++ |
| Apical subnucleus | + | ++++ | ++ |
| Dorsomedial subnucleus | + | +++++ | ++ |
| Lateral subnucleus | + | ++++ | +++ |
| Intermediate subnucleus | + | +++++ | +++ |
| Central subnucleus | + | ++++ | ++ |
| 4 Reticular formation | |||
| Central teg. field | |||
| Subcuneiform part | + | ++ | ++ |
| Retrorubral part | + | ++ | ++ |
| Peripeduncular n. | +++ | ++ | ++ |
| Pedunculopontine n. | +++ | +++ | ++ |
| Cuneiform n. | ++ | +++ | + |
| Pontine reticular | + | ++ | ++ |
| Linear n. medulla | + | ++ | ++ |
| Parvicellular ret. field | + | + | ++ |
| Gigantocellular ret. field | + | + | ++ |
| Lateral paragigantocellular | ++ | ++ | ++ |
| Paramedian reticular n. | + | ++ | ++ |
| D. Pre- and postcerebellar | |||
| 1 Pontine gray | + | ++ | +++ |
| 2 Tegmental reticular n. | + | ++ | +++ |
| 3 Inferior olive | +++ | ++ | + |
| 4 Lateral reticular n. | + | ++ | +++ |
| 5 Red nucleus | + | +++ | + |
| 6 N. Roller | + | ++ | ++ |
| 7 N. Prepositus | + | +++ | +++ |
| III. Cerebellum | |||
| A. Deep nuclei | + | ++ | ++ |
| B. Cortex | |||
| Molecular layer | + | +++ | ++++ |
| Purkinje layer | + | +++++ | + |
| Granular layer | ++++ | ++ | ++ |
| IV. Spinal cord | |||
| A. Marginal zone (laminae I) | +++ | +++ | ++ |
| B. Substantia gelatinosa (laminae II) | +++ | +++ | ++ |
| C. Intermediate gray | + | ++ | ++ |
| D. Ventral horn | + | ++ | +++ |
| E. Central gray | ++ | ++++ | ++ |
| V. Dorsal root ganglia | +++ | +++ | +++ |
Distribution of cells containing α2δ-1 mRNA
Cerebrum (telencephalon).
The most extensive labeling of α2δ-1 mRNA was observed in the cortical regions, with the heaviest labeling over neurons in areas involved in olfaction, learning and memory, such as the piriform and the entorhinal cortices, postpiriform transition area, and the hippocampal formation (Figs. 1A–G, 4A,B; Table 1). A low (layers IV–VI) to moderate (layers II–IV) density of labeled neurons was found in each field of the isocortex, but a higher density of labeling appeared to be over neurons in layer II of the piriform cortex. The entorhinal cortex, one of the major regions that have strong connections with the hippocampal formation, contained a high to very high density of labeled neurons in layers II and III. In the hippocampus the pyramidal layer of each subdivision of Ammon's horn (CA1–CA3) was highly labeled, but only a low level of labeling was seen in the dorsal and ventral subiculum. Moreover, the granular layer of the dentate gyrus contained heavily labeled neurons. A low level of signals was also found in the polymorph layer of the dentate gyrus (Fig. 4B). The main olfactory bulb and the anterior olfactory nucleus contained a high density of α2δ-1 mRNA-containing cells (Fig. 1A), although only a low level of the signal was detected in the endopiriform nucleus. Likewise, a high density of α2δ-1 mRNA-containing cells was found in the dorsal part of the taenia tecta (Fig. 1B), a structure possibly differentiated from the adjacent anterior olfactory nucleus (Swanson,1998).
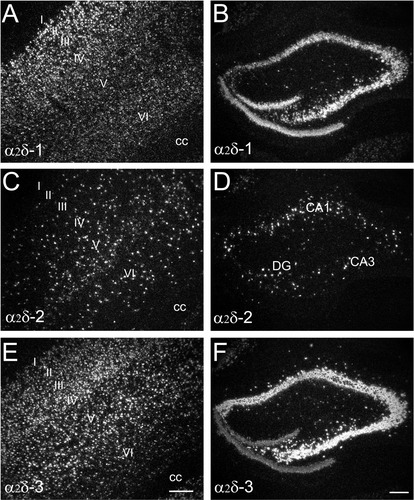
Comparison of labeling for the α2δ-1 (A,B), the α2δ-2 (C,D), and the α2δ-3 (E,F) mRNAs over cells in adjacent sections within the isocortex (A,C,E) and the hippocampus (B,D,F). The α2δ-1 and α2δ-3 mRNAs are highly expressed throughout the isocortex and the hippocampus. The α2δ-2 expression shows a typical sporadic pattern in both of the cortex and the hippocampus. I–IV represents layers of the isocortex; CA, Ammon's horn; cc, corpus callosum; DG, dentate gyrus. Scale bars = 200 μm in E (applies to A,C,E), F (applies to B,D,F).
Overall, the amygdala contained many labeled neurons, with the heaviest labeling observed in regions that receive cortical or hippocampal inputs, such as the amygdalohippocampal area, the cortical nucleus, and the basolateral and basomedial nuclei (Fig. 1E–G; Table 1). We also observed a moderate density of labeled neurons in the intercalated nucleus that receives intra-amygdalar inputs. Only a low level of α2δ-1 mRNA signal was seen in the medial and the central nuclei of the amygdala.
Except for low to moderate levels of α2δ-1 mRNA expression in the bed nucleus of the anterior commissure and the triangular nucleus of the septum, the septal region, including the lateral and medial septal nuclei, and the bed nuclei of the stria terminalis expressed very low levels of α2δ-1 mRNA (Fig. 1C–E; Table 1).
The most prominent labeling in the basal ganglia was found in the subthalamic nucleus (Fig. 1H; Table 1). The caudoputamen and the fundus of the striatum expressed a low level of α2δ-1 mRNA (Figs. 1B–G, 5A; Table 1), while the nucleus accumbens appeared to have a moderate level of the labeled cells (Fig. 1C). Except for the low level of labeled neurons in the magnocellular preoptic nucleus that highly expressed acetylcholine in the basal forebrain, the other parts of the globus pallidus including the entopeduncular nucleus, the internal segment, and the substantia innominata contained only a few sporadic, if any, α2δ-1 mRNA-containing neurons. Similar results were also observed in the pars reticularis of the substantia nigra (Fig. 1E–G; Table 1).
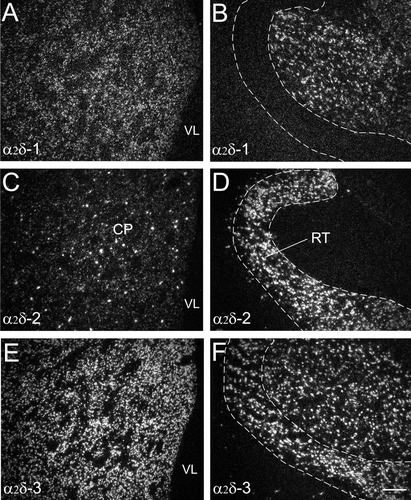
Comparison of labeling for α2δ-1 (A,B), α2δ-2 (C,D), and α2δ-3 (E,F) mRNAs over cells in adjacent sections within the rostral caudoputamen (CP) (A,C,E) and the reticular nucleus of the thalamus (B,D,F). Both α2δ-1 and α2δ-3 mRNAs are highly expressed throughout the rostral caudoputamen, while the high expression of α2δ-2 mRNA is only seen in sporadic cells. Both α2δ-2 and α2δ-3 mRNAs are highly expressed in the reticular nucleus of the thalamus, but the reticular nucleus does not express the α2δ-1 mRNA. RT, reticular nucleus of the thalamus; VL, lateral ventricle. Scale bar = 200 μm in F (applies to A–F).
Hypothalamus.
A high to very high density of labeled neurons were found in three nuclei in the periventricular zone of the hypothalamus; these were the median preoptic nucleus, the supraoptic nucleus, and the paraventricular nucleus, including its autonomic, parvicellular, and magnocellular parts (Figs. 1D–H, 6A; Table 1). Low to moderate levels of labeling were observed in the periventricular zone including the anteroventral periventricular nucleus and the arcuate nucleus (Fig. 6B; Table 1).
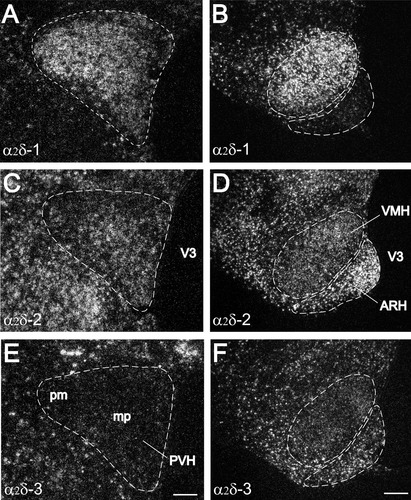
Comparison of labeling for α2δ-1 (A,B), α2δ-2 (C,D), and α2δ-3 (E,F) mRNAs over cells in adjacent sections within the paraventricular nucleus (PVH), ventromedial nucleus (VMH), and the arcuate nucleus (ARH) of the hypothalamus. The α2δ-1 mRNA is highly expressed in both the posterior magnocellular and medial parvicellular parts of the PVH, while the α2δ-2 mRNA is largely expressed in the medial parvicellular part. Very low levels of the α2δ-3 mRNA expression are found in the PVH. The α2δ-1 mRNA is also highly expressed in the VMH but a very low level of expression is found in the ARH. Both α2δ-2 and α2δ-3 mRNAs are expressed in the VMH and the ARH. Expression of the α2δ-2 mRNA in the ventromedial part of the ARH is higher than that in the rest parts of the ARH. ARH, arcuate nucleus; mp, medial parvicellular; pm, posterior magnocellular; PVH, paraventricular nucleus of the hypothalamus; V3, third ventricle; VMH, ventromedial nucleus of the hypothalamus. Scale bars = 100 μm in E (applies to A,C,E); 200 μm in F (applies to B,D,F).
The greatest density of labeling in the hypothalamus was found in the ventromedial nucleus in the medial zone (Figs. 1G, 6B; Table 1). Strongly labeled neurons were also found in the dorsomedial, tuberomammillary, and pre- and supramammillary nuclei. The medial preoptic nucleus, the anterior hypothalamic nucleus, and the lateral and medial mammillary nuclei appeared to express low to moderate levels of α2δ-1 mRNA (Fig. 1E–H; Table 1).
Only a few labeled neurons were found in the lateral hypothalamic and posterior hypothalamic areas in the lateral zone. Sporadically labeled cells were found in the lateral preoptic area.
Thalamus.
In general, low levels of expression of α2δ-1 mRNA were detected throughout almost all of the thalamic cell groups (Fig. 1F–H; Table 1). Areas that were not labeled included the medial habenula and the reticular nucleus. A few scattered labeled cells were observed in the zona incerta.
Brainstem.
α2δ-1 mRNA-containing cells were observed throughout the structures in the brainstem including the sensory, motor, and reticular components. Among brainstem sensory components, the heaviest labeling of α2δ-1 mRNA was found in the structures involved in visceral sensory functions, including the nucleus of the solitary tract and the parabrachial nucleus (Fig. 1K–M; Table 1). Moreover, moderate labeling was found over two circumventricular organs, the area postrema in the brainstem and the subfornical organ in the forebrain. Moderate levels of α2δ-1 mRNA expression were also found in the visual components such as areas in the superior colliculus and the pretectal region. Labeling over regions involved in somatosensory, auditory, vestibular, and gustatory sensory functions in the brainstem appeared to be light (Table 1).
Consistent with the expression in the visceral sensory structures, α2δ-1 mRNA appeared to be highly expressed in the visceral motor neurons, including those in the dorsal motor nucleus of the vagus nerve and the nucleus ambiguus. A low level of labeling was also found in the hypoglossal nucleus. Only a few sporadically labeled cells were observed over other cranial nuclei, including the third, the fourth, the fifth, the sixth, and the seventh (Fig. 1I–M; Table 1).
The greatest density of heavily labeled neurons in the reticular core of the brainstem was found in the dorsal raphe and the locus coeruleus (Fig. 1J,K; Table 1). Moderate labeling was detected over regions of Barrington's nucleus, the peripeduncular nucleus, as well as the pedunculopontine nucleus. The remaining structures of the reticular core appeared to express none to low levels of α2δ-1 mRNA (Fig. 1I–M; Table 1).
The inferior olive appeared to be the only structure within which moderate levels of labeling were found among pre- and postcerebellar structures in the brainstem. α2δ-1 mRNA in the remaining pre- and postcerebellar regions was expressed at a very low level.
Cerebellum.
A high density of labeled neurons was found over regions of the granular cell layer. Sporadically labeled cells were observed in the fastigial nucleus and the parvicelluar part of the interposed nucleus. Signals over regions of the molecular layer and Purkinje layer, as well as the rest of the interposed nucleus and the dentate nucleus, were not detectable (Fig. 7A; Table 1).
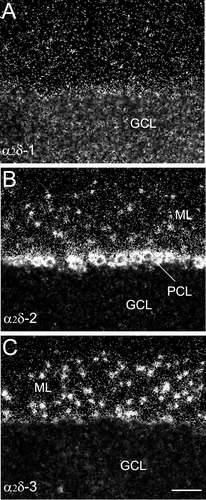
Comparison of labeling for α2δ-1 (A), α2δ-2 (B), and α2δ-3 (C) mRNA over cells in the cerebellum. The α2δ-1 subunit mRNA is highly expressed in the granular cell layer (GCL) (A). A high level of α2δ-2 mRNA is expressed in the Purkinje cell layer (PCL) (B), and the α2δ-3 mRNA is most abundant in the molecular cell layer (MCL) (C). Scale bar = 60 μm C (applies to A–C).
Spinal cord and DRG.
In the spinal cord a moderate level of labeling was found in the lamina I and the lamina II of the dorsal horn. However, the emulsion-coated autoradiograms also showed labeled cells scattered in the remaining laminae of the spinal gray, while only a few motor neurons in the ventral horn appeared to be labeled (Fig. 8A,B; Table 1).
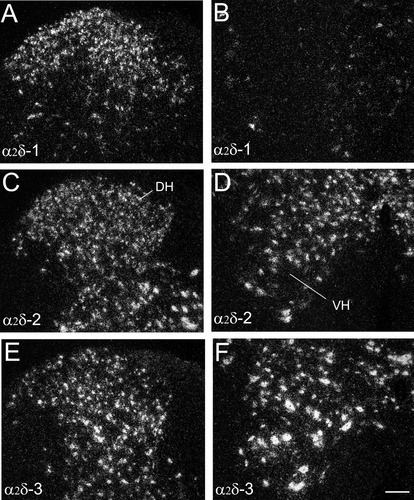
Comparison of labeling for α2δ-1 (A,B), α2δ-2 (C,D), and α2δ-3 (E,F) mRNA over cells in sections within the dorsal horn (A,C,E) and the ventral horn of the lumbar spinal cord (B,D,F). All three subunits are expressed in the dorsal horn cells but α2δ-1 mRNA expression is rarely seen in the ventral horn neurons. VH, ventral horn; DH, dorsal horn. Scale bar = 100 μm in F (applies to A–F).
Nearly all neurons with different diameters in the DRG were found to express α2δ-1 mRNA, but the highest levels of expression appeared to be in the large-diameter neurons (Fig. 9A).
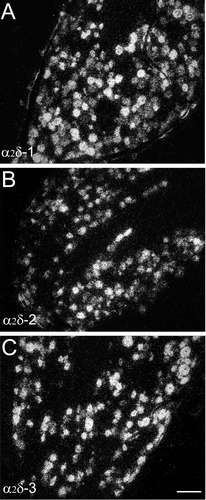
Comparison of labeling for α2δ-1 (A), α2δ-2 (B), and α2δ-3 (C) mRNA over cells in the L5 dorsal root ganglia (DRG). α2δ-1, α2δ-2, and α2δ-3 mRNAs are expressed in small and large diameter neurons in the DRG. The abundance of α2δ-2 mRNA expression in the large-diameter neurons is lower, relative to that of α2δ-1 and α2δ-3 mRNAs. Scale bar = 100 μm in C (applies to A–C).
Distribution of cells containing α2δ-2 mRNA
Cerebrum (telencephalon).
In general, the density of α2δ-2 mRNA-containing cells in most of the regions of the CNS was significantly lower than that of α2δ-1 mRNA-containing neurons. However, it is important to point out that in several brain regions where close-to-background α2δ-2 mRNA signals (+) were detected on film, scattered strongly positive labeled α2δ-2 mRNA-containing cells were often found on emulsion-coated autoradiograms in these regions. This distribution pattern suggests that high levels of α2δ-2 mRNA are expressed in interneurons in specific brain regions. In the isocortex, low levels of α2δ-2 mRNA-containing cells were found throughout all layers, with strongest labeling in neurons of layers IV–VI, which differs from the strongest labeling of α2δ-1 mRNA-containing cells in layers II–IV (Figs. 2A–G, 4C; Table 1). Close-to-background levels of α2δ-2 mRNA-containing cells were also found in the entorhinal cortex and the hippocampal formation and regions involved in olfaction including the main olfactory bulb (Fig. 2A,G), the anterior olfactory nucleus, the piriform cortex, and the endopiriform nucleus (Fig. 2B–G). In the hippocampus, strongly labeled cells were found scattered in the pyramidal layer of each subdivision of Ammon's horn (CA1–CA3) and the granular layer of the dentate gyrus (Figs. 2G, 4D; Table 1). The dorsal part of the taenia tecta expressed close-to-background levels of α2δ-2 mRNA, but high levels of α2δ-2 mRNA were found in the ventral part of the nucleus (Fig. 2B).
The distribution of α2δ-2 mRNA-containing cells in the amygdala was largely opposite or complementary to that of α2δ-1 mRNA-containing cells, suggesting that α2δ-2 mRNA and α2δ-1 mRNA may be expressed in different cell populations (Fig. 2F,G; Table 1). For example, significantly higher levels of labeled α2δ-2 mRNA-containing cells were found in the nucleus of the lateral olfactory tract, the central nucleus, the medial nucleus, and the anterior amygdaloid area. Areas that contained high levels of α2δ-1 mRNA-containing cells, such as the amygdalohippocampal area, the cortical nucleus, as well as the basolateral and basomedial nuclei, only appeared to express close-to-background level of the α2δ-2 mRNA signals.
Heavily labeled α2δ-2 mRNA-containing cells were observed in the septum, in sharp contrast to the very low level of labeling of α2δ-1 mRNA in this region (Fig. 2C–E; Table 1). The greatest density of α2δ-2 mRNA labeled cells was found in the lateral and medial septal nuclei, the nucleus of the diagonal band, the septofimbrial nucleus, and the triangular nucleus. α2δ-2 mRNA was also detected in high density in the islands of Calleja of the olfactory tubercle (Fig. 2B). Only low to moderate levels of labeled α2δ-2 mRNA-containing cells were found in the subnuclei of the bed nuclei of the stria terminalis, but a high level of α2δ-2 mRNA was observed in the anterodorsal preoptic nucleus and the parastrial nucleus (Fig. 2D).
In the basal ganglia, like the distribution of α2δ-1 mRNA, a high level of α2δ-2 mRNA-containing cells was found in the caudal subthalamic nucleus. The heaviest labeling was observed in the cholinergic magnocellular preoptic nucleus and the neighboring substantia innominata, which is consistent with the above-mentioned expression in the medial septum and the nucleus of the diagonal band, the other important basal forebrain cholinergic structures. Low to moderate levels of α2δ-2 mRNA expression were detected in the lateral segment of the globus pallidus, the caudoputamen, and the substantia nigra (Figs. 2D–H, 5; Table 1).
Hypothalamus.
The distribution of α2δ-2 mRNA-containing cells in the hypothalamus showed considerable overlap with that of α2δ-1 mRNA-containing cells, but abundance differences were clearly noticed in some nuclei. In the periventricular zone, the arcuate and the median preoptic nuclei contained the greatest density of α2δ-2 mRNA-labeled cells, relative to that of α2δ-1 mRNA-containing cells. In contrast, the supraoptic and the paraventricular nuclei contained less abundant α2δ-2 labeled cells, relative to α2δ-1 mRNA-containing cells (Figs. 2D,G, 6C; Table 1). Expression of α2δ-2 mRNA was predominantly observed in the ventromedial part of the ARH, but it was also present at low levels in the dorsomedial and ventrolateral parts (Fig. 6D).
In the medial zone of the hypothalamus, the greatest density of α2δ-2 mRNA-containing cells was found in the central and posterior parts of the anterior hypothalamic nucleus, as well as in the dorsal and the ventral parts of the premammillary nucleus (Fig. 2F,H). Higher levels of α2δ-2 mRNA-containing cells were also found in the medial mammillary nucleus, but α2δ-2 mRNA labeled cells were less abundant in the ventromedial, dorsomedial, tuberomammillary, and the supramammillary nuclei, relative to α2δ-1 mRNA-containing cells (Table 1).
Much higher levels of α2δ-2 labeled cells were found in the lateral preoptic and the lateral hypothalamic areas, relative to α2δ-1 mRNA-containing cells.
Thalamus.
In contrast to the near-homogeneous distribution of α2δ-1 mRNA-containing cells in the thalamic nuclei, the distribution of α2δ-2 mRNA-containing cells in the thalamus was quite segregated. The greatest level of α2δ-2 mRNA was expressed in the medial habenula, the reticular nucleus, the zona incerta, as well as the anterodorsal nucleus (Figs. 2F–H, 5D; Table 1). Low levels of expression were found in the lateral habenula, the paraventricular nucleus, the nucleus reunions, the anteroventral nucleus, the central medial nucleus, the interanterodorsal nucleus, the lateral dorsal nucleus, the medial part of the medial geniculate nucleus, the parafascicular nucleus, and the nuclear fields of Forel. The remaining nuclei of the thalamus did not appear to express α2δ-2 mRNA (Fig. 2F–H; Table 1).
Brainstem.
In general, the α2δ-2 mRNA expression in the brainstem appeared to be more abundant than that of α2δ-1 mRNA. Similar to the distribution of α2δ-1 mRNA-containing cells, a high density of α2δ-2 mRNA-containing neurons was found in visceral sensory structures such as the nucleus of the solitary tract and two of the circumventricular organs—the area postrema in the brainstem and the subfornical organ in the forebrain. Labeling of α2δ-2 mRNA in the lateral and medial parabrachial nuclei was less abundant, while a higher density of α2δ-2 mRNA-containing cells was found in the Kolliker-Fuse nucleus, relative to the density of α2δ-1 mRNA-containing cells (Fig. 2E,K–M; Table 1). In addition, compared to α2δ-1, labeling of α2δ-2 mRNA-containing cells was much more abundant in the superior colliculus, the parabigeminal nucleus, the pretectal region, the medial terminal nucleus, and the medial vestibular nucleus (Fig. 2I; Table 1). The inferior colliculus also expressed high levels of α2δ-2 mRNA (Fig. 2J).
The α2δ-2 mRNA appeared to be more abundant in the motor nuclei in the brainstem, relative to α2δ-1 mRNA expression. Like α2δ-1 mRNA labeling, the heaviest labeling of α2δ-2 mRNA was found in the dorsal motor nucleus of the vagus nerve (Fig. 2L,M). Low to moderate levels of α2δ-2 mRNA appeared to be expressed in the rest of the cranial motor nuclei (Table 1).
A high density of α2δ-2 labeled neurons was found in the dorsal raphe and the locus coeruleus in the reticular core. When compared with α2δ-1 mRNA-labeled cells, much stronger α2δ-2 mRNA labeling was seen over structures in the region near the periaqueductal gray area, including the Darkschewitsch nucleus, the dorsal tegmental nucleus, the ventral tegmental nucleus, the nucleus incertus, and the laterodorsal tegmental nucleus (Fig. 2I–K). Heavily labeled α2δ-2 mRNA-containing cells were also found in the interpeduncular nucleus, where signals for α2δ-1 mRNA were not detected. Low to moderate levels of α2δ-2 mRNA appeared to be expressed in other raphe nuclei and most of the structures in the reticular formation (Table 1).
As stated above, α2δ-1 mRNA was expressed most prominently in the inferior olive among all the pre- and postcerebellar structures. In contrast, no specific pattern of distribution of α2δ-2 mRNA-containing cells was observed. Low to moderate levels of α2δ-2 mRNA labeling were found in all pre- and postcerebellar structures, including the pontine gray, the tegmental reticular nucleus, the inferior olive, the lateral reticular nucleus, the red nucleus, the nucleus of Roller, and the nucleus prepositus (Table 1).
Cerebellum.
The most intense labeling of α2δ-2 mRNA in the cerebellum was found in Purkinje cells. Moderate levels of α2δ-2 mRNA-containing cells were found in the molecular layer. A low level of α2δ-2 mRNA expression was also found in the granular layer and the deep cerebellar nuclei (Fig. 7B; Table 1).
Spinal cord and DRG.
Similar to the brainstem, much higher α2δ-2 mRNA expression was found in the spinal cord relative to α2δ-1 mRNA expression. While a similar density of α2δ-2 mRNA-containing cells was seen in the superficial laminae of the dorsal horn compared to α2δ-1 mRNA-containing cells, a much higher expression of α2δ-2 mRNA was found throughout the deep laminae of the spinal gray, especially over the central gray regions and the motor neurons of the ventral horn (Fig. 8C,D).
Similar to the pattern of distribution of α2δ-1 mRNA-containing cells, α2δ-2 mRNA was found to be present in nearly all neurons in the DRG, irrespective of neuronal sizes. However, the abundance of α2δ-2 mRNA in large-diameter neurons was lower relative to that of α2δ-1 mRNA (Fig. 9B).
Distribution of cells containing α2δ-3 mRNA
Cerebrum (telencephalon).
In most of the regions of the cerebrum, the distribution of α2δ-3 mRNA-containing neurons overlapped considerably with that of α2δ-1 and/or α2δ-2 mRNA-containing neurons. The isocortex appeared to express moderate to high levels of α2δ-3 mRNA throughout all layers, with the highest density in layer IV of the primary somatosensory cortex (Figs. 3B–G, 4A,C,E; Table 1). However, the density of labeling of α2δ-3 mRNA-containing cells in the piriform cortex and the entorhinal area did not appear to be as high as α2δ-1 mRNA-containing cells in the same regions. The main olfactory bulb, the anterior olfactory nucleus, and the endopiriform nucleus expressed low levels of α2δ-3 mRNA (Fig. 3A–G), but high levels of α2δ-3 mRNA-containing cells were found in the dorsal part of the taenia tecta (Fig. 3B). The distribution of α2δ-3 mRNA-containing cells in the hippocampus resembled that of α2δ-1 mRNA-containing cells, with highest density of labeled cells found in the pyramidal layer of the Ammon's horn (CA1–CA3) and the granular layer of the dentate gyrus. Yet some differences between the patterns of α2δ-1 and α2δ-3 mRNA expression were observed. First, CA3 neurons showed stronger α2δ-3 mRNA signals relative to α2δ-1 mRNA. Second, the polymorph layer of the dentate gyrus also expressed a higher level of α2δ-3 mRNA. Lastly, a low level of α2δ-3 mRNA was observed in the stratum oriens of the CA1, an area in which α2δ-1 mRNA was not detectable (Fig. 4B,F).
α2δ-3 mRNA-containing cells were found throughout the amygdala (Fig. 3E–G; Table 1). The distribution pattern shared features of both α2δ-1 and α2δ-2 mRNA, suggesting α2δ-3 mRNA may coexpress with α2δ-1 mRNA or α2δ-2 mRNA in different nuclei of the amygdala. For example, a high density of α2δ-3 mRNA-containing and α2δ-1 mRNA-containing cells was found in the amygdalohippocampal area. A moderate density of α2δ-3 mRNA-containing cells and a moderate density of α2δ-2 mRNA-containing cells were found in the central nucleus. Overall, a high level of α2δ-3 mRNA expression was found in the medial, the central, and the intercalated nuclei, as well as in the nucleus of the lateral olfactory tract and the anterior amygdaloid area, whereas the lateral, basolateral, and basomedial nuclei only expressed low levels of α2δ-3 mRNA.
Except for the high expression of α2δ-3 mRNA in some regions of the bed nuclei of the stria terminalis, the distribution pattern of α2δ-3 mRNA in the septum is largely similar to that of α2δ-1 mRNA. In the bed nuclei of the stria terminalis, the greatest density of heavily labeled α2δ-3 mRNA-containing cells was found throughout the anterior–posterior extent of the nuclear complex, especially in the rostrolateral and posterodorsal regions. A high level of α2δ-3 mRNA was also observed in the nucleus accumbens. A low level of α2δ-3 mRNA was found in the medial septal nucleus, the triangular nucleus, and the nucleus of the diagonal band. The remaining structures of the septum such as the entire lateral septal nucleus did not appear to express α2δ-3 mRNA (Fig. 3B–D; Table 1).
The caudoputamen expressed the greatest density of α2δ-3 mRNA among basal ganglia structures. A heavy density of labeled α2δ-3 mRNA-containing cells was also found in the olfactory tubercle, but the islands of Calleja were unlabeled, in contrast to the distribution of α2δ-2 mRNA. In the globus pallidus a low level of α2δ-3 mRNA was expressed in the cholinergic magnocellular preoptic nucleus and the neighboring substantia innominata, while the entopeduncular nucleus and the internal segment appeared to lack α2δ-3 mRNA expression. A low level of α2δ-3 mRNA was observed in the subthalamic nucleus, similar to the levels in the pars compacta of the substantia nigra and the ventral tegmental area (Fig. 3B–I; Table 1).
Hypothalamus.
In general, α2δ-3 mRNA-containing neurons were widely distributed in the hypothalamus but at lower levels relative to α2δ-1 and α2δ-2 mRNA-containing cells (Figs. 3D–H, 6E,F; Table 1). Almost all of the nuclei in the hypothalamus expressed α2δ-3 mRNA except for the median preoptic nucleus, which, in contrast, exhibited high levels of α2δ-1 mRNA and α2δ-2 mRNA expression. The greatest numbers of heavily labeled α2δ-3 cells were localized in the suprachiasmatic nucleus and the posterior part of the dorsomedial nucleus. A moderate level of labeled cells was also found in the anteroventral periventricular nucleus in the periventricular zone, and the central part of the medial preoptic nucleus, the tuberomammillary nucleus, the ventral part of the premammillary nucleus, and the lateral mammillary nucleus in the medial zone. Labeling in the remaining regions of the hypothalamus including the lateral zone was low. In the arcuate nucleus, α2δ-3 mRNA expression in the ventrolateral part was slightly higher (Fig. 6F).
Thalamus.
The overall expression of α2δ-3 mRNA in the thalamus was high (Fig. 3E–H; Table 1), relative to that of α2δ-1 and α2δ-2 mRNA. In addition to a high density of α2δ-3 mRNA-containing cells in the reticular nucleus, which resembled that of α2δ-2 mRNA-containing cells (Fig. 5D,F), the greatest labeling was found in the parataenial and the rhomboid nuclei of the midline group and the parafascicular nucleus of the intralaminar group (Fig. 3F,H). A moderate density of α2δ-3 mRNA-containing neurons was seen in the mediodorsal nucleus, the paraventricular nucleus, the nucleus reuniens, the central median, paracentral, central lateral, and the ventral anterior nuclei. A low level of α2δ-3 mRNA-containing cells was observed throughout the remaining structures of the thalamus, while the medial and lateral habenulae did not appear to express α2δ-3 mRNA.
Brainstem.
Overall, low to moderate levels of α2δ-3 mRNA expression were observed throughout the brainstem (Fig. 3I–M; Table 1), a high density of α2δ-3 mRNA-containing cells was found in some of the regions, including the ventral cochlear nucleus and the nucleus of the trapezoid body that are involved in the process of auditory sensory information. The lateral and the medial parabrachial nuclei appeared to express very low levels of α2δ-3 mRNA, as did Barrington's nucleus, the lateral vestibular nucleus, the nucleus raphe obscurus, the cuneiform nucleus, the inferior olive, and the red nucleus. A low level of α2δ-3 mRNA-containing cells was found in the area postrema, but the subfornical organ appeared to lack α2δ-3 mRNA expression (Fig. 3E,L).
Cerebellum.
The molecular layer of the cerebellar cortex expressed the highest density of α2δ-3 mRNA. A low level of α2δ-3 mRNA was also observed in the granular layer. The Purkinje cells, however, did not appear to express α2δ-3 mRNA. Only a low level of α2δ-3 mRNA expression was found in the deep cerebellar nuclei (Fig. 7C; Table 1).
Spinal cord and DRG.
The distribution pattern of α2δ-3 mRNA-containing cells in the spinal cord resembled that of α2δ-2 mRNA-containing cells. A high level of α2δ-3 mRNA expression was found throughout all the laminae of the gray matter and motor neurons of the ventral horn were also heavily labeled (Fig. 8E,F). Similarly, all neurons in the DRG expressed high levels of α2δ-3 mRNA (Fig. 9C).
Characterization of the chemical identity of α2δ-1 and α2δ-2 mRNA-containing cells
Colocalization of α2δ-1 and α2δ-2 subunits with parvalbumin (PV), a marker for GABAergic neurons, and choline acetyltransferase (ChAT), a marker for cholinergic neurons, were used to assess the neurochemical identity of these cells.
Approximately 75% of PV-positive neurons coexpressed α2δ-2 mRNA in the pyramidal layer of the Ammon's horn in the hippocampus. In contrast, this population of cells did not express α2δ-1 mRNA. Likewise, α2δ-2 mRNA was present in the majority of PV-positive neurons within the cortex, while very little α2δ-1 expression was detected in this cell population (Fig. 10). In addition, all PV-positive Purkinje cells in the cerebellum coexpressed α2δ-2 mRNA. In other brain regions, a high percentage of α2δ-2 and PV coexpression, ranging from 55–95%, was found in the lateral and basolateral nuclei of the amygdala, the lateral and medial septal nuclei, the nucleus of the diagonal band, the magnocellular preoptic nucleus, the reticular nucleus of the thalamus, the substantia innominata, and the intermediate subnucleus of the interpeduncular nucleus. Approximately 40% of the PV-positive cells in the caudoputamen coexpressed α2δ-2 mRNA. In all of these regions, very few, if any, PV-positive cells appeared to coexpress α2δ-1 mRNA.
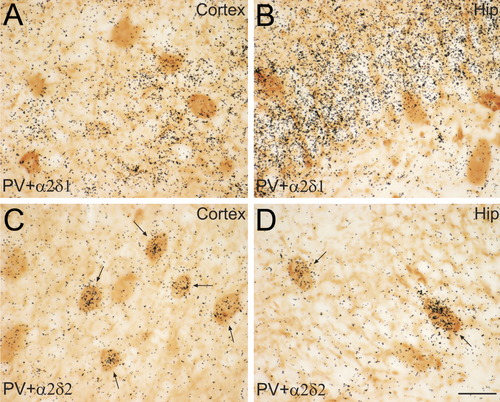
Colocalization of parvalbumin (PV) with α2δ-1 and α2δ-2 mRNA in the hippocampus (Hip) and the cerebral cortex. Colocalization of α2δ-1 mRNA (A,B) and α2δ-2 mRNA (C,D) with PV protein in the cortex (A,C) and the hippocampus (B,D). Brown DAB reaction product represents PV-positive cells. Black grains represent α2δ subunit mRNA autoradiogram signals. Arrows indicate double labeled cells. PV-positive cells in the cortex strongly express α2δ-2 mRNA (C), but not α2δ-1 mRNA (A). Approximately 75% of PV-positive cells in the hippocampus strongly express α2δ-2 (D). PV-positive cells in the hippocampus do not coexpress α2δ1 mRNA (B). Images shown are from the parietal cortex and the dentate gyrus in the hippocampus. Scale bar = 20 μm in D (applies to A–D).
Within the caudoputamen, the vast majority of the ChAT-positive cells, if not all, coexpressed α2δ-2 mRNA, but did not coexpress α2δ-1 mRNA (Fig. 11A,C). However, only a few ChAT-positive cells along the lateral border of the magnocellular preoptic nucleus appeared to coexpress α2δ-2 mRNA, but none of the ChAT-positive cells in this nucleus coexpressed α2δ-1 mRNA (Fig. 11B,D). Similarly, only ∼20–30% of ChAT-positive cells in the medial septal nucleus and the nucleus of the diagonal band coexpressed α2δ-2 mRNA, although α2δ-2 mRNA was generally highly expressed in these regions. However, a high percentage, ranging from 50–90%, of the ChAT-positive cells in the islands of Calleja, the substantia innominata, the medial segment of the globus pallidus, the medial habenula, the laterodorsal tegmental nucleus, and the pedunculopontine nucleus appeared to coexpress α2δ-2 mRNA. Moreover, the majority of the ChAT-positive motor neurons in the third, fifth, seventh, tenth, and twelfth cranial nuclei, as well as those in the nucleus ambiguus and the spinal ventral horn, coexpressed α2δ-2 mRNA. Notably, only a small portion of ChAT-positive cells in the lamina X and the lateral horn of the spinal cord coexpressed α2δ-2 mRNA. In addition, α2δ-2 mRNA did not appear to be coexpressed in the ChAT-positive cells in the intermediate subnucleus of the interpeduncular nucleus.
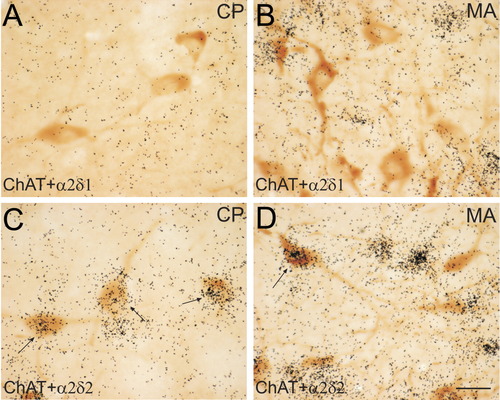
Colocalization of choline acetyltransferase (ChAT) with α2δ-1 (A,B) and α2δ-2 (C,D) mRNAs in the caudoputamen (CP) (A,C) and the magnocellular preoptic nucleus (MA) (B,D). Brown DAB reaction product represents ChAT-positive cells. Black grains represent α2δ subunit mRNA autoradiogram signals. Arrows indicate double-labeled cells. ChAT-positive cells in the striatum strongly express α2δ-2 mRNA (C, arrows), but not α2δ-1 mRNA (A). Only a few of ChAT-positive cells coexpress α2δ-2 mRNA (D, arrows) in the magnocellular preoptic nucleus. Scale bar = 20 mm in D (applies to A–D).
DISCUSSION
The present results provide the first systematic mapping of the VGCC α2δ subunits (1, 2, and 3) throughout the rat CNS. Overall, these three subunits have specific and differential patterns of expression in rat brain and the spinal cord. The in situ hybridization results reported here generally confirm but expand in great detail those of earlier studies on the mapping of these subunits (Klugbauer et al.,1999; Hobom et al.,2000). In addition, our results provide the first evidence that the α2δ-2 subunit is preferentially expressed in GABAergic interneurons in the cerebral cortex and the hippocampus, as well as in cholinergic interneurons in the striatum.
Distribution of α2δ-1, α2δ-2, and α2δ-3 mRNA-containing cells
High-voltage-activated calcium channels are extensively distributed in the CNS. Consistent with this fact, α2δ-1, α2δ-2, and α2δ-3 mRNA-containing cells are widely found throughout the rat brain and the spinal cord. The distribution pattern of the mRNAs encoding the α2δ-1 and α2δ-2 subunits are often observed to be complementary in rat brain regions, suggesting differential regulatory mechanisms underlying the modification of the biophysical and pharmacological properties of the pore-forming α1 subunit in different brain regions. In areas where α2δ-1 expression is high, α2δ-2 expression tends to be low, and vice versa. For example, in regions such as the cortex and in the pyramidal cell layers of the hippocampus, α2δ-1 expression is high and α2δ-2 expression is low. In contrast, regions including the septum, the reticular nucleus of the thalamus, the medial habenula, and the arcuate nucleus show high levels of α2δ-2 mRNA expression and low levels of α2δ-1 mRNA expression. A similar phenomenon is also observed within the amygdala and the cerebellum. However, there are a few brain regions which express high levels of both α2δ-1 and α2δ-2 including the median preoptic nucleus, the subthalamic nucleus and the premammillary nucleus. Expression of α2δ-3 mRNA is observed more ubiquitously, although it can be found in distinct patterns that overlap with either α2δ-1 or α2δ-2 mRNA expression in many brain areas. In some regions all three subunits can be found in the same nucleus. This raises the possibility that different heterooligomeric combinations of the auxiliary subunits exist. Alone, the α2δ-1, α2δ-2, or α2δ-3 subunits have been shown to shift the voltage dependence of channel activation and inactivation in a hyperpolarizing direction and accelerate the kinetics of current inactivation when coexpressed with an α1 subunit such as α1C (Klugbauer et al.,1999; Hobom et al.,2000). However, the function of different combinations of these auxiliary units, especially with regard to binding drugs such as gabapentin (Marais et al.,2001), is yet to be determined. In instances when two or three subunits are found in the same brain region, it is also unknown if the different α2δ subunits exist in the same or different cell populations.
Extensive labeling of α2δ-1 and α2δ-3 mRNA is found in the cortex, the hippocampus, and the amygdala. The α2δ-2 mRNA is considerably less abundant in these regions and appears to be expressed in a specific cell group. Regions involved in somatosensory, visual, auditory, visceral, and motor functions in the cortex, as well as the association cortices all contain a significant amount of α2δ-1 mRNA-containing cells, suggesting that the α2δ-1 subunits may be essential in multiple cortical neuronal functions. Similarly, extensive and heavy labeling of α2δ-1 mRNA is found in the hippocampal formation, including each subdivision of Ammon's horn, the dentate gyrus, and in the perientorhinal region that receives extensive cortical inputs and provides major reciprocal connections with the hippocampus. Because α2δ-1 mRNA does not appear to coexpress with PV in the hippocampus or the cortex, it is likely that most of the above α2δ-1 mRNA-containing neurons are glutamatergic. Although neuronal activity in the hippocampus, mediated by VGCCs containing the α2δ-1 subunit, may play an important role in learning and memory in the normal physiological state, abnormal firing, due to brain trauma, infection, or tumor, of neurons in the hippocampus, cortex, or amygdala has been found to be attributable to the onset of seizures (Nestler,2001; McIntyre et al.,2002). Thus, consistent with our results, gabapentin and its analogs, which specifically bind to α2δ-1 (Gee et al.,1996) and are thought to reduce calcium currents (Stefani et al.,1998; Calabresi et al.,1999; Fink et al.,2000), are efficacious against focal epilepsy in adult humans and in animal models of focal seizures (Dalby and Nielsen,1997; Maj et al.,1999; Lado et al.,2001). Also consistent with our results, radiolabeled gabapentin binds heavily to the rat cortex, the hippocampus, and the amygdala in rats (Gu et al., unpubl. obs.; Hill et al.,1993). The presence of α2δ-2 mRNA in PV-containing cells suggests that VGCCs containing the α2δ-2 subunit may play a role in the activation of GABAergic interneurons in the cortex, the hippocampus, and the amygdala. Although gabapentin and its analogs also bind to the α2δ-2 subunit, the antiepileptic effect of gabapentin does not seem to be mediated through the binding to the α2δ-2 subunit, because gabapentin, especially its analog pregabalin, has much higher affinity for the α2δ-1 than the α2δ-2 subunit (Bryans et al.,1998; Marais et al.,2001). Moreover, reduction of calcium currents through VGCCs containing the α2δ-2 subunit, based on our findings, would be expected to suppress the activities of GABAergic neurons and therefore compromise the antiepileptic effect. Because no specific ligand has been found for the α2δ-3 subunit and gabapentin and its analogs do not seem to bind to this subunit with high affinity, the outcome of suppressing the α2δ-3 subunit in the cortex, the hippocampus, and the amygdala remains unclear. However, the α2δ-3 subunit may serve as a better target for epilepsy because it is only expressed in the brain and, like α2δ-2, the α2δ-3 subunit is involved in the enhancement and modulation of the currents through several different high-voltage-activated (HVA) and low-voltage-activated (LVA) Ca++ channels (Klugbauer et al.,1999; Gao et al.,2000; Hobom et al.,2000; Marais et al.,2001). The low level of expression of the α2δ-1 subunit in the thalamus suggests that the thalamus may not be the primary target for the antiepileptic effect of gabapentin and its analogs, but the high expression of both α2δ-2 and α2δ-3 mRNA in the GABAergic reticular nucleus suggests that VGCCs containing these two subunits may be involved in the control of information outflow from the thalamus.
More evidence has emerged that the binding of gabapentin to the α2δ-1 subunit may be responsible for its efficacy in the treatment of neuropathic pain (Gee et al.,1996; Bramwell,2004), although the mechanism of action has yet to be determined. While some pathological changes have been found in the cortex and the thalamus in neuropathic pain (Apkarian et al.,2004; Weigel and Krauss,2004), it is surprising to find that α2δ-1 and α2δ-2 mRNA expression is low in the somatosensory relay nuclei in the thalamus. Nevertheless, a heavy density of α2δ-1 and α2δ-2 mRNA-containing cells is found in the lateral parabrachial nucleus, an area believed to convey spinal noxious information to the hypothalamus and the amygdala (Craig,2003), and also in the superficial layers of the spinal dorsal horn. The spinal cord results are consistent with the observations in multiple gabapentin binding studies (Gu et al., unpubl. obs.; Thurlow et al.,1996), biochemical and behavioral investigations showing that the α2δ-1 subunit in the spinal cord is significantly altered in the pain states (Luo et al.,2002; Li et al.,2004), and the fact that intrathecal injection of gabapentin is efficacious in suppressing nociception in different animal pain models (Shimoyama et al.,1997; Patel et al.,2001; Kumar,2004). Although α2δ-3 mRNA labeling is low in the thalamic somatosensory relay nuclei and appears to be scant in the lateral parabrachial nucleus, it is highly expressed in the superficial layers of the spinal dorsal horn. In addition to the lateral parabrachial nucleus and the spinal dorsal horn, the dorsal root ganglia may be another important target for gabapentin because all three α2δ subunits are heavily expressed in neurons of all diameters and a high level of gabapentin binding is found in this region (Gu et al., unpubl. obs.).
Consistent with the high expression of α2δ-1 and α2δ-2 mRNA in the visceral sensory areas, heavily labeled α2δ-1 and α2δ-2 mRNA-expressing cells are also found in the visceral motor structure—the dorsal motor nucleus of the vagus nerve. The somatic motor neurons of the spinal cord express a high level of α2δ-3 mRNA. A moderate level of α2δ-2 and low level of α2δ-1 mRNA are also seen in these cells. Nevertheless, the high level of α2δ-2 mRNA in ChAT-positive motor neurons indicates that VGCCs containing the α2δ-2 subunit may play an important role in somatic as well as visceral motor functions. The inferior olive, a structure in the brainstem that sends strong afferents (climbing fibers) to Purkinje cells in the cerebellar cortex, expresses a moderate level of α2δ-1 mRNA. Purkinje cells, however, do not appear to express α2δ-1 mRNA, but rather express high levels of α2δ-2 mRNA. The expression of the three α2δ subunits in the cerebellum appears to be differential. This pattern suggests that different VGCCs that are associated with different α2δ subunits may play roles in motor control and other cerebellar functions at multiple levels (Manto and Padolfo,2002).
An extensive and unique distribution of α2δ-1, α2δ-2, and α2δ-3 mRNA-containing neurons was observed in the periventricular, the medial, and the lateral zones of the hypothalamus, suggesting their broad involvement in neuroendocrine, homeostatic, motivated behavior, and autonomic functions. For example, regions in the hypothalamus known to mediate defensive behavior, such as the dorsomedial part of the ventromedial nucleus and the dorsal part of the premammillary nucleus of the hypothalamus (Canteras,2002), contained a high density of α2δ-1 mRNA-containing neurons. Similarly, the parvicellular part of the paraventricular nucleus that contains corticotropin-releasing hormone (CRH), the autonomic part of the paraventricular nucleus that activates autonomic motor neurons (Swanson and Kuypers,1980), and the dorsomedial nucleus that is associated with behavioral responses to stressors (Inglefield et al.,1994; Thompson and Swanson,1998) also contained a high density of α2δ-1 mRNA-containing neurons. Structures governing fluid homeostasis, such as the median preoptic nucleus, as well as the circumventricular organs—the subfornical organ and the area postrema—express high levels of both α2δ-1 and α2δ-2 mRNA, with a relatively higher density of α2δ-2 mRNA-containing cells. Furthermore, heavily labeled α2δ-2 mRNA-containing neurons were found in the suprachiasmatic nucleus, the arcuate nucleus, the lateral hypothalamic area, the anterior hypothalamic nucleus, and the dorsal part of the premammillary nucleus, suggesting that VGCCs containing the α2δ-2 subunit may play an important role in the regulation of circadian rhythm, ingestion (Sawchenko,1998), and defensive behaviors. Likewise, the high level of expression of α2δ-3 mRNA in the suprachiasmatic nucleus strongly suggests the important role of α2δ-3 in the regulation of circadian rhythm. The expression of α2δ-1, α2δ-2, and α2δ-3 mRNAs in the anteroventral periventricular nucleus, the ventral part of the premammillary nucleus, the medial preoptic nucleus, and the ventrolateral part of the ventromedial nucleus of the hypothalamus suggests the involvement of VGCCs containing these subunits in the control of gonadotropin release and the expression of sexual behavior (Pfaff and Schwartz-Giblin,1988; Simerly and Swanson,1988; Canteras et al.,1992,1994; Gu and Simerly,1997). The supramammillary nucleus relays information to the hippocampus and was recently found to be involved in the genesis and spread of limbic seizures in the hippocampus (Saji et al.,2000). Heavily labeled α2δ-1 mRNA-containing cells were found in this nucleus. This structure could be another possible target for gabapentin in the prevention of seizures.
Both α2δ-1 and α2δ-2 mRNAs are highly expressed in the locus coeruleus and the dorsal raphe in the reticular core of the brainstem. These two nuclei send massive ascending outputs containing norepinephrine and serotonin, respectively, to the cortex and the intralaminar nuclei of the thalamus and are involved in arousal and selective attention. Moderate levels of α2δ-1 and α2δ-2 mRNA are also expressed in the pedunculopontine nucleus, an interface for the basal ganglia to influence sleep and waking (Mena-Segovia et al.,2004). In addition, heavily labeled α2δ-2 mRNA-containing neurons are found in the laterodorsal tegmental nucleus that, like the pedunculopontine nucleus, contains cholinergic neurons and is also involved in the sleep and waking (Kayama and Koyama,2003). Moreover, high expression of α2δ-2 mRNA in the nucleus incertus also supports the involvement of VGCCs containing this subunit in arousal mechanisms (Goto et al.,2001; Olucha-Bordonau et al.,2003). High density of α2δ-2 mRNA-containing neurons are also found in the interpeduncular nucleus. This nucleus is thought to play an important role in the regulation of normal sleep patterns (Haun et al.,1992). The high level of expression of α2δ-2 mRNA in this nucleus and in other sleep/waking structures may be associated with the severe somnolence caused by gabapentin administration.
Functional implications
It is thought that a single α2δ subtype may complex with different α1 subunits (Hobom et al.,2000) since the expression patterns of α2δ subunits do not suggest a selective interaction with known α1 subunits. However, as summarized by Hobom et al. (2000) based on the individual mapping of α2δ subunit and α1 subunit, at least the α2δ-2 subunit could complex with the α1A, α1E, and/or α1G channel in cerebellar Purkinje cells, and the α1E and/or α1I channel in the habenulae. Moreover, α2δ-2 may complex with α1E in the subthalamic nucleus (Trimmer and Rhodes,2004), and the α2δ-2 or α2δ-3 subunit may complex with α1B in the hippocampus (Chung et al.,2000), α1C, D, and E in the motor neurons in the spinal cord (Westenbroek et al.,1998). Indeed, detailed colocalization studies are needed to clarify this issue. Nevertheless, current understanding of the functional role of α2δ subunits is largely based on in vitro coexpression studies with members of the L-type, non-L-type, and T-type calcium channels. Overall, α2δ subunits modulate channel activity by increasing calcium current density, shifting the voltage dependence of activation to more negative potentials, or increasing steady-state inactivation (see Klugbauer et al.,2003, for review).
While the exact in vivo functional significance of the differential distribution of the α2δ subtypes in the CNS remains to be explored, some insight has been gained through the observation of spontaneous mutation mouse models. Mutations in the Cacna2 (α2δ-2) gene have been found to underlie the phenotype in the spontaneously mutant ducky mouse (du and du2j strain) (Barclay et al.,2001; Brodbeck et al.,2002) and the newly identified entla mutant (Brill et al.,2004). These animals exhibit ataxia, seizures, and infertility, suggesting that VGCCs containing α2δ-2 subunit play a pivotal role in neural development and function. The R217A mutation in the α2δ-1 subunit results in the loss of efficacy of gabapentin and pregabalin in a model of neuropathic pain (Bramwell et al.,2004), indicating the involvement of VGCCs containing the α2δ-1 subunit in the mediation of neuropathic pain.
The present study reveals that α2δ-2 is expressed in many neuronal structures that are known to be GABAergic, and thus, VGCCs containing the α2δ-2 subunit may play a specific role at central inhibitory synapses. These structures include the reticular nucleus of the thalamus, the zona incerta, the septum, cerebellar Purkinje cells, as well as hippocampal and cortical PV-positive interneurons. Interestingly, while α2δ-2 expression is highly prominent in GABAergic neurons in the above regions, it is not highly present in the medium-sized spiny GABAergic neurons of the striatum. Only less than half of the PV-positive cells in the striatum coexpresses α2δ-2 mRNA. In this structure, α2δ-2 is highly localized to the large cholinergic interneurons. Because the cholinergic interneurons send their projections to and excite the GABAergic neurons of the striatum (Nestler,2001), activation of VGCCs containing the α2δ-2 subunit on cholinergic interneurons augments the inhibitory output from the striatum.
These results showing selective expression of α2δ-2 in GABAergic structures, may contribute to understanding the neurochemical basis of the ducky (du) mouse phenotype mentioned above. Ducky is a spontaneous autosomal recessive mouse mutation in the α2δ-2 VGCC subunit gene, which results in the expression of a truncated, nonfunctional α2δ-2 protein (Barclay et al.,2001; Brodbeck et al.,2002). Mice carrying homozygous ducky (du) alleles have a phenotype that includes epileptic seizures with features similar to those occurring in human idiopathic generalized epilepsy (Puranam and McNamara,1999), as well as ataxia and paroxysmal dyskinesia (Snell,1955). The loss of α2δ-2 subunit protein on hippocampal and cortical GABAergic terminals could underlie the seizure phenotype. As α2δ-2 is selectively localized on GABAergic interneurons in the hippocampus and cortex, as well as the reticular nucleus of the thalamus, loss of this protein could result in a decrease in GABAergic inhibition, and thus an overly excitable state. The ataxia and paroxysmal dyskinesia associated with the phenotype in ducky (Snell,1955) may result from loss of inhibitory/modulatory influences in motor circuitry, especially from Purkinje cells of the cerebellum. It is well documented that degenerative changes of the cerebellar Purkinje cells caused by CAG repeats lead to spinocerebellar ataxia type-1, and the disturbance of calcium homeostasis has been linked to this autosomal dominant disorder (Vig et al.,2001). In agreement with this observation, the altered morphology of Purkinje cell dendrites has been found to be one of the anatomical correlates of the du phenotype (Brodbeck et al.,2002), and the peak P-type calcium current shows a 35% decrease in Purkinje cells (Barclay et al.,2001).
α2δ-2 mRNA is also highly expressed in several other brain nuclei known to be cholinergic, including the nucleus of the diagonal band, the magnocellular preoptic nucleus, the medial septal nucleus, the nucleus of the diagonal band, the substantia innominata, the pedunculopontine nucleus, the laterodorsal tegmental nucleus, and the motor neurons in the cranial nuclei and spinal cord. However, colocalization studies with ChAT show that α2δ-2 is rarely found in the cholinergic neurons in the magnocellular preoptic nucleus, the medial septal nucleus, and the nucleus of the diagonal band, indicating its expression in other cell groups in these nuclei. Thus, the α2δ-2 subunit is highly expressed by cholinergic interneurons in the striatum, but not in the principle cholinergic neurons in the basal forebrain that provides cholinergic inputs to the cortex. Because cholinergic interneurons in the striatum play an important role in the control of movement, it is not surprising that mutating the α2δ-2 subunit causes dyskinesia. The high expression of α2δ-2 mRNA in almost all motor neurons suggests that the α2δ-2 subunit may be essential in somatic and visceral movements, which may also help explain why dyskinesia occurs when the α2δ-2 subunit is mutated.
CONCLUSIONS
In conclusion, α2δ-1, α2δ-2, and α2δ-3 mRNA-containing cells are extensively distributed throughout the rat CNS and the dorsal root ganglia. Although overlap has been found in some regions, α2δ-1 and α2δ-2 mRNA expression patterns are largely complementary, whereas α2δ-3 mRNA is expressed in distinct patterns in regions that also express α2δ-1 and/or α2δ-2. While α2δ-1 mRNA appears to be expressed in the glutamatergic neurons in the cortex, hippocampus, thalamus, and the cerebellum, α2δ-2 mRNA is expressed in scattered GABAergic interneurons throughout the cortex and hippocampus, and also in the GABAergic reticular nucleus of the thalamus and Purkinje cells of the cerebellum. Moreover, α2δ-2 mRNA is also expressed in cholinergic interneurons in the striatum and in motor neurons in the brain and spinal cord. The expression of α2δ-1, α2δ-2, and α2δ-3 mRNAs in cells in functionally distinct neural systems suggests that these subunits may play an important role in many essential functions. Although the exact mechanism underlying different subunit action remains to be explored, recent observations in α2δ-2 mutant mice indicate that the auxiliary α2δ subunit not only modulates the opening of VGCCs in the normal physiological state, but also plays a role in the development of the nervous system. Additional animal models with spontaneous or transgenic mutations, like R217A for the α2δ-1 subunit, are needed to help elucidate the functions of these important subunits in vivo.
Acknowledgements
We thank Dr. Eugene P. Brandon for helpful comments on the article.




