Can symbiotic fungi protect plants from insect pests? A simple mathematical model
Funding information: COST Action: FA 1405 - ``Food and Agriculture: Using three-way interactions between plants, microbes and arthropods to enhance crop protection and production'', ---; TUBITAK (The Scientific and Technological Research Council of Turkey), TOVAG-110O160, TOVAG-110O160
Abstract
A mathematical model is proposed for simulating and understanding the relationship between the potato plant and its most significant pest, the Colorado potato beetle, when mediated by mycorrhiza, a symbiotic plant-fungus association. From its analysis it is seen that, quite counterintuitively, an overabundant use of mycorrhiza for enhancing productivity is rather detrimental, in that it triggers persistent oscillations in the foliage and the population of the beetle, giving an instance of the paradox of enrichment. Some indications for biological pest control arise from the investigation of the bifurcations that occur in the model ecosystem.
1 INTRODUCTION
The Colorado potato beetle (CPB) originated from the south-west of North America, where it fed on plants of the genus Solanum in the family Solanaceae. It has now spread through most of North America, Europe, and Asia (especially Russia and China), feeding on the potato, Solanum tuberosum. The potato plant is native to the central Andes in South America, but is now an important and widely cultivated food crop. CPB is one of its most destructive and devastating insect pests. Chemical control has proven not to provide a long-term solution to the problems created by CPB, as the beetle has become resistant to a wide variety of chemicals. Biological control may represent a viable alternative. In this context, the use of mycorrhiza, a symbiotic association between the root of the plant and a fungus, has been advocated, as it shows interesting features. Mycorrhiza is indeed known to affect mineral composition of plants.1 Mycorrhiza symbiosis influences several aspects of plant physiology: mineral nutrition uptake, plant growth, and plant resistance.2-4 Among these we mention improved rooting and plant establishment, enhanced uptake of low mobile ions, improved nutrient cycling, enhanced plant tolerance to biotic and abiotic stress factors, enhanced quality of soil structure, improved plant community diversity.
The beneficial effects of mycorrhiza on plants decrease if mycorrhizal dependency levels are reduced due to various factors such as root exudates, in particular carbon, physical texture of soil, and competition with other soil-borne beneficial and disease-causing microorganisms. The development of mycorrhiza in the rhizosphere depends on root exudates, especially carbon allocated to roots and all the other factors mentioned above.
In this article we propose a model for the interactions of the three agents described above, the potato plant, the CPB, and mycorrhiza. The article is structured as follows. Section 2 contains the biological background, the following one describes the mathematical model for the mutual interactions, whose analysis for equilibria feasibility and their local stability is performed in Section 4. This section contains also the study of global stability for some of these equilibria, and of the bifurcations that relate them to each other. A final section consisting of interpretation and discussion of the model concludes the paper.
2 BIOLOGICAL BACKGROUND
The view that the likelihood of pest outbreaks is reduced with organic farming practices, including the establishment and maintenance of “healthy soil,” has long been promoted by proponents of alternative agricultural methods.5, 6 On the other hand, organic farmers still need to employ natural pest controls, for example, biological control, enhancing plant growth and resistance, plant varieties with pest control properties.7 In organic farming, a variety of practices were used to enhance soil fertility that contribute to plant resistance of insects and diseases through creating optimal physical, chemical, and, perhaps most importantly, biological properties of soil.8 It was reported that plants grown on organic farms were less favorable to herbivorous insects than those grown in soils treated with synthetic fertilizers9-11 and that pest densities in organic farms are lower.9-13
The Colorado potato beetle, Leptinotarsa decemlineata, is one of the most destructive and devastating insect pests of potato. Uncontrolled CPB populations can completely defoliate potato plants and can cause a complete loss of tuber production.14 In Reference 15 it is claimed that the control techniques against this pest developed in the past provided only limited protection of potato crops. High fecundity, flexible life history, and development of pesticide resistance are the main tasks in the control of the CPB.16 Chemical control has not yet provided long-term solutions to the problems created by CPB, so searching for alternative approaches is inevitable. If soil management could decrease beetle damage, this might provide a valuable addition to the CPB chemical control.17 In Reference 17 it was discovered that CPB populations were almost universally lower in plots receiving manure soil amendments in combination with reduced amounts of synthetic fertilizers compared with plots receiving full rates of synthetic fertilizers, but no manure. Unlike beetle abundance, plant height and canopy cover were comparable between plots receiving manure and synthetic fertilizer. Furthermore, tuber yields were higher in manure-amended plots. Thus, the difference in beetle density was unlikely to be explained simply by poor plant vigor in the absence of synthetic fertilizers. Subsequent field-cage and laboratory experiments18 confirmed that potato plants grown in manure-amended soil were indeed inferior CPB hosts compared with plants grown in synthetically fertilized soil. The observed negative effects were broad in scope. Female fecundity was lower in field cages set up on manure-amended plots early in the season, although it later became comparable between the treatments. Fewer larvae survived past the first instar, and development of immature stages was slowed down on manure-amended plots.18 Plant susceptibility to phytophagous insects is determined by behavioral responses (host finding and acceptance), developmental responses (efficiency of food utilization, developmental rate, and survivorship), or by their combination.10 Mineral composition has been shown to affect both behavioral and developmental responses of pests.10, 11, 18-21 Effects of soil fertility management on pest resistance can be mediated through changes in nutritional content of crops.8 Soil microorganisms are an important factor in the soil fertility and the health of plants. Symbiotic arbuscular mycorrhizal fungi (AMF) form a key component of the microbial populations influencing plant growth and uptake of nutrient.22 The function of AMF depends on the ability of the fungal symbiont in the absorption of nutrients available in inorganic and/or organic forms in soil. In most mycorrhizal types, organic carbon derived by photosynthesis is also transferred from the plant to the fungus followed by translocation to the growing margins of the extra radical mycelium and to the developing spores and fruit bodies.1 The fungus, in fact, becomes an integral part of the root system. The symbiosis is considered the most metabolically active component of the absorbing organs of the autotrophic host plant, which, in addition to furnishing the heterotrophic fungal associate with organic nutrients, is an ecologically protected habitat for the fungus.3, 4 The physiology of the plant is highly affected by the presence of the fungal symbionts.1 The key effects of AMF symbiosis can be summarized as follows: (1) improved rooting and plant establishment; (2) enhanced uptake of low mobile ions; (3) improved nutrient cycling; (4) enhanced plant tolerance to biotic and abiotic stress factors; (5) enhanced quality of soil structure; (6) improved plant community diversity.2-4 Thus, AMF symbiosis influences a variety of aspects of plant physiology, such as mineral nutrition uptake, plant growth, and plant resistance. The natural roles of the organisms in the mycorrhizosphere (the zone influenced by both the root and the mycorrhizal fungus) may have been marginalized in intensive agriculture, since microbial communities in conventional farming systems have been modified due to tillage, and high inputs of inorganic fertilizers, herbicides, and pesticides.23 Microbial diversity in these systems has been reduced.24 As a consequence, AMF fungi seem to be only of minor importance under conventional agricultural conditions, whereas the AMF symbiosis is regarded as a key component of sustainable agriculture.
We formulate the following hypothesis: soil amendment using arbuscular mycorrhiza may be helpful to achieve an optimal nutrient condition that results in both good plant health and resistance to herbivory in ecologically sound crop management systems. However, the effect of soil amendment on the ecology of pests needs to be closely monitored and studied for a sustainable organic farming system.
- To determine the impacts of AMF on the development, survival, and fecundity, that is, the population parameters of the Colorado potato beetle.
- To determine the impacts of AMF on feeding capacity (or the consumption rate and the damage potential) of the Colorado potato beetle.
While these objectives pertain to field and laboratory experiments, their outputs will be needed for the simulations of the mathematical model describing the interactions of the three populations.
3 THE MODEL
We consider the following time-dependent populations: B represents the Colorado potato beetle population, L denotes the potato leaf area, and M is the symbiotic association of fungus with the potato plant, that is, the mycorrhiza.
In the equation for the plant leaf area L, the first term assumes that the potato plant has enough resources so that it can grow, that is, the leaves can extend their surface at rate s. Furthermore, they are subject to losses, modeled in the next two terms. They may experience natural mortality at rate m and also competition for resources, at rate c. This occurs primarily for light, when the lower leaves are somewhat overshadowed by the uppermost ones. In addition, we consider the interactions that are described in what follows. There is a direct beneficial influence that the leaves receive from the mycorrhiza, modeled by the function g(M), which occurs only if leaves are present and disappears if L = 0, as is given by the fourth term in the second equation. Note also that we assume that only a fraction p of the mycorrhiza is used for this task. Moreover, there is a loss of leaves due to the predation exerted by the grazing beetles, modeled in the last term. The function L(1 + hL)−1 expresses the beetles' feeding saturation. This harvest function is mitigated by the influence of the mycorrhiza, via the function a(M). Note that the function a(M) is taken to be a decreasing function of M, so that the larger M is, the more effective the reduction by the mycorrhiza of the damage incurred.
The beetle population, modeled in the first equation, is assumed to grow as long as leaves are available, with a feeding saturation feature, modeled via a Holling type II functional response of the resource L, where the maximal intake of food per unit of time is h−1 and with conversion coefficient e. Furthermore, as remarked above, its growth rate is also hindered by the presence of the mycorrhiza and is expressed by the decreasing function a(M). In addition, beetles have a natural mortality rate n, second term, and compete with each other for grazing on the leaves, at intraspecific competition rate b, third term.
Table 1 lists all the model parameters with their meaning.
| Parameter | Interpretation |
|---|---|
| n | Mortality rate of B |
| m | Mortality rate of L |
| d | Decreased growth rate of M |
| b | Intraspecific competition rate among B |
| c | Intraspecific competition rate among L |
| f | Intraspecific competition rate among M |
| e | Conversion factor |
| a(M) | Direct mycorrhiza-induced inhibition on beetle growth rate |
| a0 | Basic (maximal) beetles harvesting rate |
| a1 | Minimal beetles harvesting rate due to mycorrhiza presence |
| h−1 | Beetle feeding saturation level |
| g(M) | Direct enhancement of M on the growth rate of L |
| g1 | Mycorrhiza enhancement rate on leaves growth |
| p | Portion of mycorrhiza used for leaves growth enhancement |
| 1 − p | Portion of mycorrhiza used for mycorrhiza's own benefit |
| s | Growth rate of L |
| r | Growth rate of M |
4 ANALYSIS
4.1 Boundedness
Any initial conditions are in DK for K sufficiently large, and the trajectory from these initial conditions remains in DK for all t ≥ 0, so all trajectories are bounded.
4.2 Equilibria and their feasibility
Note that M2 = M4 = M6, since the M equation is uncoupled from the system.
4.3 Stability of equilibria
For the local stability of the equilibria, we find the following results.
The second condition is always satisfied if E3 is feasible with L3 = (s − m)/c > 0. The third condition is satisfied (with L3 = (s − m)/c > 0) if either (a) ea0 < nh (or equivalently L5 < 0) or (b) ea0 > nh (or equivalently L5 > 0) and s − m < nc/(ea0 − nh) (or equivalently s − m < cL5 or B5 < 0). In other words, the third condition is satisfied if E5 is not feasible.
If L4 > 0 and M4 > 0 the first two conditions are automatically satisfied. Noting that M4 = M6, the third is satisfied if either (a) ea(M6) < nh (or equivalently L6 < 0) or (b) ea(M6) > nh (or equivalently L6 > 0) and g1(r − d) + f(s − m) < ncf/(ea(M6) − nh) (or equivalently s − m + g1M6 < cL6 or B6 < 0). In other words, the third condition is satisfied if E6 is not feasible.
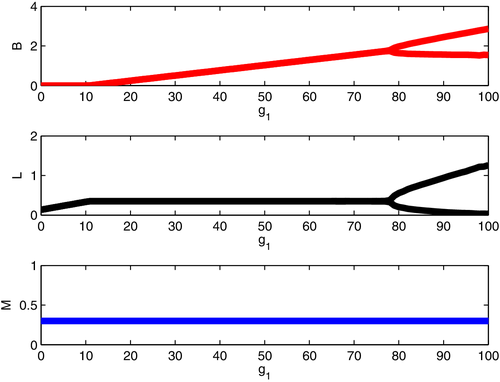
Remark. Note that, whenever the trace vanishes, a Hopf bifurcation arises, in view of the fact that the determinant is positive. A discussion of this occurrence can be found below, and an illustration through a simulation is reported in Figure 2.
In Table 2 we summarize the possible equilibria of system (3) and in Table 3 their salient features.
| Point | Population values |
|---|---|
| E1 = (0, 0, 0) | (0, 0, 0) |
| E2 = (0, 0, M2) | |
| E3 = (0, L3, 0) | |
| E4 = (0, L4, M4) | |
| E5 = (B5, L5, 0) | |
| E6 = (B6, L6, M6) |
| Feasibility | Stability (if feasible) | |
|---|---|---|
| E1 | Always feasible | r < d, s < m |
| E2 | r ≥ d | g1(r − d) + f(s − m) < 0 |
| E3 | s ≥ m | r < d, (ea0 − nh)(s − m) < nc |
| E4 | r ≥ d, g1(r − d) + f(s − m) ≥ 0 | (ea(M6) − nh)(g1(r − d) + f(s − m)) < ncf |
| E5 | ea0 > nh, s − m ≥ cL5 | r < d, s − m < 2cL5 + c/h |
| E6 | r ≥ d, ea(M6) > nh, | f(s − m) + g1(r − d) < 2fcL6 + fc/h |
| g1(r − d) + f(s − m) ≥ fcL6 |
- Note: The stability conditions are given assuming the feasibility conditions hold with strict inequality.
4.4 Global stability issues
In this section we consider some of the equilibria and formally show, for mathematical sake, their global stability. For the remaining more complicated ones, we discuss here briefly some of their features.
Note that E2, E3, and E4 are not globally stable when they are stable, because trajectories starting at the origin do not tend to them, but they are globally stable for initial conditions in the interior of the positive octant.
For the remaining nontrivial equilibria E5 and E6 note that for r < d, all trajectories tend to the plane M = 0, as seen above in Subsection 4.1. Bounded trajectories in the plane M = 0 can only tend to either an equilibrium or a periodic solution surrounding an equilibrium. If E5 is not feasible there is no equilibrium to surround, so the trajectory tends to an equilibrium in that plane, that is, E3. A similar situation occurs for r > d if E6 is not feasible, with the plane M = M6, in this case with possible omega points E2 or E4.
4.4.1 E1
On the assumption that the origin E1 is stable, namely (11) hold, we show that the ecosystem collapse follows.
4.4.2 E2
4.4.3 E3
4.4.4 E4
4.5 Bifurcations
The bifurcations that occur in the system are shown in Figure 1. The bifurcation curves (or lines) are marked with the bifurcation that occurs as they are crossed. Curves marked with two numbers XY represent a transcritical bifurcation between EX and EY. For example, the negative s − m axis in panel (i) is a bifurcation curve marked 12, and E2 passes through E1 and becomes stable through a transcritical bifurcation as r increases through d and the curve is crossed from left to right. Dashed curves are also bifurcation curves marked in the same way, but there is no interchange of stability. The curves marked H5 and H6 represent Hopf bifurcations, so, for example, E6 loses stability through a Hopf bifurcation leading to a periodic solution (which we call H(E6)) as H6 is crossed from below to above in the right-hand half plane. Again, there is no transfer of stability on the dashed parts of these curves. Finally, the curve H56 represents a bifurcation between H(E5) and H(E6), with H(E6) passing through H(E5) and becoming stable as the curve is crossed from left to right. Note that H(E5) is a periodic solution in the M = M5 = 0 plane, while H(E6) is a periodic solution in the M = M6 = (r − d)/f plane, and they coincide when M6 = 0, that is, when r = d.
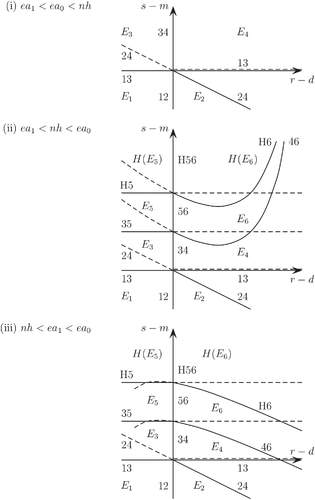
There is no bistability in the system, so that its asymptotic behavior may be marked unambiguously in each region bounded by the bifurcation curves. There is no change in asymptotic behavior on crossing a dashed curve.
The s − m axis is unusual in that (because M2 = M4 = M6 = 0) several bifurcations occur simultaneously as it is crossed, namely 12 and 34, and 56 and H56 when the parameters are such that E5 and E6 exist. The one that we have marked on each part of the axis in the figure is the one where an interchange of stability occurs. For example, E2 passes through E1 with an interchange of stability on crossing 12; E4 also passes through E3 but does not become feasible or stable, since L3 = L4 < 0 here.
4.5.1 Case (i): ea1 < ea0 < nh
This case is the simplest, since both L5 and L6 are negative (whatever the value of M6 = (r − d)/f), so neither E5 nor E6 can be a feasible equilibrium. The only bifurcation curves are 12, 34, 13, and 24. The equilibrium E1 is stable between 12 and 13, E2 between 12 and 24, E3 between 13 and 34, and E4 between 24 and 34, as shown, always disregarding the dashed lines when seeking a change of asymptotic behavior.
4.5.2 Case (ii): ea1 < nh < ea0
In this case L5 > 0, so E5 is a feasible equilibrium as long as B5 > 0. L6 > 0 if ea(M6) > nh, or M6 = (r − d)/f < Mcrit = a−1(nh/e). Both L6 > 0 and M6 > 0 if (r − d) is sufficiently small and positive, and then E6 is feasible if B6 > 0. Three additional transcritical bifurcation curves appear in the figure: line 35, where s − m = cL5, or B5 = 0; line 46, where s − m + g1M6 = cL6, or B6 = 0; and line 56, where M6 = 0. Two Hopf bifurcation curves also appear: H5, where s − m = 2cL5 + c/h, or ; and H6, where s − m + g1M6 = 2cL6 + c/h, or . Finally H56 appears, the bifurcation with an interchange of stability between the two periodic Hopf solutions H(E5) and H(E6). The s − m axis is divided into 12, 34, 56, and H56, as shown.
4.5.3 Case (iii): nh < ea1 < ea0
In this case, both L5 and L6 are positive wherever we are in the (r − d, s − m) plane, but there are still conditions for B5 > 0 and B6 > 0. The lines 46 and H6 look rather different (because their vertical asymptotes are now in the left half plane), but the bifurcations we see are essentially the same as in case (ii).
4.5.4 Bifurcation parameters g1 and a1
We have discussed bifurcations with the parameters r − d and s − m, and sketched the bifurcation curves in (r − d, s − m) space.
Armed with that information, it is possible to deduce the bifurcations that occur with other parameters. Biologically important parameters are g1, a measure of the direct benefit of the mycorrhiza on the plant, and (a0 − a1), the maximum mycorrhizal inhibition of herbivory.
So let us fix r > d (otherwise the mycorrhiza cannot survive) and s > m (otherwise the plant cannot survive in the absence of mycorrhiza) and decrease a1 from a0, or increase the mycorrhizal inhibition of herbivory from zero. If ea0 < nh there is essentially no effect, as herbivory is too small even without mycorrhiza to allow the beetle to survive. But if ea0 > nh then we move from case (iii) to case (ii), as the lines 46 and H6 acquire a vertical asymptote (at a value of r − d defined by ea((r − d)/f) = nh). It is easy to show that the lines 46 and H6 move upward as a1 decreases, so that the sequence of bifurcations is H(E6) to E6 to E4, at least for r − d and s − m sufficiently large. The beetle is driven to extinction, and we are left with the plant and the symbiotic mycorrhiza.
Now consider the effect of increasing g1, with nh < ea1 < ea0. The lines f(s − m) + g1(r − d) = 0, f(s − m) + g1(r − d) = fcL6, and f(s − m) + g1(r − d) = 2fcL6 + fc/h become steeper, and a typical progression is from E4 to E6 to H(E6). This destabilization of the system, allowing the beetle to invade, is detrimental to the plant. It is an example of the paradox of enrichment,26 where an increase in the resources available to a prey species leads to destabilization of a prey-predator system.
A numerical simulation to this effect relates this behavior, see Figure 2.
5 INTERPRETATION AND DISCUSSION
The model introduced here contains a combination of predator-prey and symbiotic mechanisms. CPB harvests the plants leaves, but the latter receive benefit from and provide benefit to mycorrhizal fungi. In this three populations interactions, CPB still negatively affects the plant, but with some mitigation provided by mycorrhiza, which also directly enhances plant growth.
The model indicates that for increasing values of the benefit that the fungus exerts on the leaves, expressed by the parameter g1 on the horizontal axis of Figure 2, at first the beetles are absent, see the top frame, for low values of the mycorrhiza enhancement rate on the growth of the leaves. Then past the value g1 = 10, CPBs invade the plant attaining larger and larger numbers, the higher g1 becomes. Correspondingly, in the middle frame the leaves have an initial benefit in the absence of the beetles, and grow linearly with g1. When the beetles are present, the leaves are kept at a constant level, without being able to increase even by increasing the mycorrhiza enhancement rate, g1. Then at a value near 80 the beetle and leaves populations start to persistently oscillate. In so doing, the leaves drop to alarmingly low levels, which most likely indicates the plant death. Furthermore, this destabilization of the system through higher mycorrhizal input rates induces persistent oscillations that favor beetle invasion, which in turn hinders plant thriving. The phenomenon is known as the paradox of enrichment.26 Although we might have expected that increasing g1 and decreasing a1 would both be beneficial to the plant, in fact the paradox of enrichment implies that increasing g1 beyond a certain threshold is instead detrimental, while decreasing a1 is indeed beneficial.
Looking at the possible ecosystems outcomes, see Table 2, the equilibrium that should absolutely be avoided is E2. To achieve this goal, it should be rendered either unfeasible or unstable. The feasibility issue entails that mycorrhiza mortality should exceed its reproduction rate. This is quite unexpected, but it is linked to the above remarks on the paradox of enrichment. Alternatively, the stability condition could be violated. Note that if feasible, for stability to hold, the requirement s < m is necessary, which means that the plant dies off, as is implied by the population values of the equilibrium itself. But to reverse the inequality and obtain fs > fm − g1(r − d), a condition that ensures instability, is probably biologically not possible. How indeed to enhance the growth rate of a plant under attack by its predators in practice?
Also possibly to be avoided is E5, because there mycorrhiza vanishes. There are four conditions among feasibility and stability. The occurrence of this equilibrium could be prevented by ensuring that the mycorrhiza net growth rate s − m is kept at low values, lower than cL5. Maybe a way of doing it is by pruning the leaves.
Coexistence, E6, implies that the CPB persists in the environment. In such case, perhaps external interventions could be aimed at keeping low the beetle population, while enhancing the foliage L6. This can be obtained by reducing r − d, thereby externally incrementing the mycorrhiza death rate d to levels close to its reproduction rate r, or by incrementing its intraspecific competition rate f. Again, this is counterintuitive, but related to the paradox of enrichment. To increase the foliage instead entails increasing the CPB mortality rate n, possibly, again with external measures, or reducing the gap between ea(M6) and nh, which could be obtained by reducing h, which means increasing the beetles' feeding saturation level. This may not be a viable biological measure, however.
The CPB-free equilibria are E1, E3, E4, but the first one is not acceptable as the ecosystem collapses. To attain the latter two beneficial equilibria, the necessary feasibility conditions require s > m and r < d, while for E4, r > d. Moreover, additional more complicated inequalities are needed. For E3 one must impose either ea0 < nh or an upper bound on s − m. At E4, s − m could also be negative, provided that g1(r − d) + f(s − m) is positive. But in addition, it should either be bounded above, or ea(M6) < nh, to achieve stability. Also, in view of the transcritical bifurcation between them, the two system's outcomes E3 and E4 cannot occur simultaneously.
Alternative control measures as mentioned could consider both the feasibility and stability conditions of the above relevant equilibria. However, more is needed as in such case, the transcritical bifurcation analysis of Subsection 4.5 proves to be instrumental, in that the possible measures taken should have in mind their outcomes, so as not to destabilize a configuration and then ending up in a worse one. For instance, in case the system is settling toward E5, where mycorrhiza disappears, the beetles and plant thriving together, we are in case (ii) or (iii) of Figure 2, as ea0 > nh at this point. From the bifurcation diagrams, an increase in the net mycorrhiza reproduction rate r − d to make it positive would push the system toward coexistence, which does not help if the goal is beetles eradication. Only a further increase of r − d to higher positive values could make the system attain the best possible beetle-free equilibrium E4. Instead, an increase of s − m past the value 2cL5 + c/h triggers oscillations, without altering the presence of the populations thriving already at E5. One should strive instead to reduce s − m so as to achieve the desirable but mycorrhiza-free equilibrium E3. From it, moving toward positive values of r − d would yield the optimal ecosystem outcome, E4. Whether biological means of achieving these transitions are not only available, but also implementable in practice is still debatable.
The proposed system is a first attempt to model these three populations interactions. It still has some drawbacks, but nevertheless its analysis sheds some light on its possible outcomes. Among the shortcomings of this model, we observe that the mycorrhizal equation is almost essentially uncoupled from the rest of the system. There is indeed no direct influence of leaves on mycorrhiza. Perhaps a better formulation should account for the whole plant as a dependent variable, in place of just the leaves. We shall leave this for a subsequent investigation.
Biographies

Remzi Atlihan received Bachelor's degree (1990): Yuzuncu Yil University, Faculty of Agriculture, Department of Plant Protection. PhD degree (1997): Çukurova University, Institute of Science, Department of Entomology, Adana, Turkey. Postdoctoral: 2004: Visiting scientist at the University of Maine, Orono, USA via NATO Science Fellowship (NATO B2). 2011: Visiting scientist at University of Newcastle (UK) via Scholarship by The Council of Higher Education, Turkey. Research interests: Insect ecology, pest management, interactions between plants, microbes, and arthropods.

Nicholas F. Britton was for many years director of the Centre for Mathematical Biology at the University of Bath, UK, where he is now Professor Emeritus. His research interests are in mathematical biology, mainly in ecology and epidemiology.

Semra Demir received Bachelor's degree (1991): Yuzuncu Yil University, Faculty of Agriculture, Department of Plant Protection. PhD degree (1999): Ege University, Institute of Science, Department of Phytopathology, İzmir, Turkey. Postdoctoral (2003): Visiting Scientist at the University of Bari, Italy via project of The Scientific and Technological Research Council of Turkey (Turkey-Italy Cooperation). Research interests: Mycology, arbuscular mycorhizae plant pathology, physiology of plant diseases, biological control of plant diseases.

Annalisa Papasidero is currently finishing her Master degree in Mathematics.

Mehmet Ramazan Risvanli received Bachelor's degree (2012): Kahramanmaraş Sütçü İmam University, Faculty of Agriculture, Department of Plant Protection, Kahramanmaraş, Turkey. MSc degree (2015): Kahramanmaraş Sütçü İmam University, Institute of Science, Department of Entomology, Kahramanmaraş, Turkey. Current status: research assistant, PhD student. Research interests: Insect ecology, interactions between plants, microbes, and arthropods

Manuela Seminara is currently finishing her Master degree in Mathematics.

Ezio Venturino received his PhD degree in Applied Mathematics from SUNY at Stony Brook in 1984. He is currently Professor of Mathematics at the Dipartimento di Matematica, University of Torino, Italy. He has visited several international institutions worldwide and has a wide scientific collaboration network. His research earlier focused on numerical analysis, mainly methods for integral equations, and currently on nonlinear models for biological and ecological applications.




