The actin gene family: Function follows isoform†
Monitoring Editor: Makoto Kinoshita
Abstract
Although actin is often thought of as a single protein, in mammals it actually consists of six different isoforms encoded by separate genes. Each isoform is remarkably similar to every other isoform, with only slight variations in amino acid sequence. Nevertheless, recent work indicates that actin isoforms carry out unique cellular functions. Here, we review evidence drawn from localization studies, mouse models, and biochemical characterization to suggest a model for how in vivo mixing of actin isoforms may influence cytoskeletal function in cells. © 2010 Wiley-Liss, Inc.
Introduction
Actin is essential for a tremendous range of cell functions. A partial list includes cell division, migration, junction formation, chromatin remodeling, transcriptional regulation, vesicle trafficking, and cell shape regulation. How is it possible that one molecule can accomplish such a large diversity of tasks? One answer to this mystery is that actin is not a single entity; rather, actin is composed of several different isoforms. Birds and mammals have six genes, and each encodes one protein isoform. Four isoforms, αskeletal-actin, αcardiac-actin, αsmooth-actin, and γsmooth-actin, are expressed primarily in skeletal, cardiac, and smooth muscle. The remaining two isoforms, βcyto-actin and γcyto-actin are ubiquitously expressed. All of the isoforms possess very similar amino acid sequences, with no isoform sharing less than 93% identity with any other isoform. βcyto-Actin and γcyto-actin, which are exactly conserved from birds to mammals, only differ by four biochemically similar residues (Fig. 1). In an unknown fashion, these family members cooperate to endow the microfilament network with diverse properties. Here, we will describe evidence from mouse models supporting the notion that actin isoforms have specialized cellular functions and discuss possible ways in which actins could exert differential effects in cells.
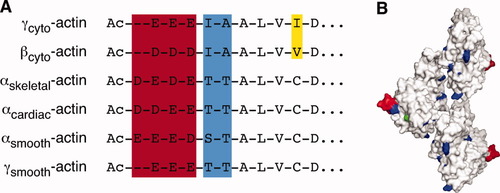
Actin-isoform sequence differences. A: Alignment of the N-terminal ends of the six mammalian actin isoforms. B: Amino acid differences mapped onto F-actin structure. The residues in red exhibit the most variability within and between muscle and cytoplasmic isoforms. Blue residues primarily vary between cytoplasmic and muscle isoforms. Yellow varies between βcyto-actin and γcyto-actin while green indicates substitutions between different muscle isoforms. The F-actin structure is approximate and based on the work of Oda et al. [PDB 2ZWH, Oda et al., 2009].
Experimental Evidence for Actin-Isoform Specific Functions
Studies in model organisms including Drosophila [Wagner et al., 2002] and C. elegans [MacQueen et al., 2005] have provided a rich source of evidence to suggest that actin isoforms have both overlapping and unique cellular functions. In mammalian systems, most evidence has come from mouse models where individual actin-isoform knockouts have distinct phenotypes. In addition, transgenic actin expression studies have demonstrated some overlap and some special properties among actin isoforms.
Muscle Isoforms
All knockout mice reported thus far have distinct phenotypes suggesting that each isoactin has unique and specialized functions (Table I). αcardiac-Actin knockout results in lethality during either embryonic or perinatal development with profound disorganization of cardiac myofibrils [Kumar et al., 1997]. αskeletal-Actin knockout mice appear normal at birth but have weak muscles and all die by 9 days of age [Crawford et al., 2002]. αsmooth-Actin knockout mice are born at Mendelian ratios and are viable. However, these mice have defects in vascular contractility and blood pressure regulation [Schildmeyer et al., 2000]. γsmooth-Actin knockout mice have not yet been reported.
| Protein ablated | Gene | Allele | Transgenic rescue | Phenotype | References |
|---|---|---|---|---|---|
| αskeletal-Actin | Acta1 | Null | Pups die by 9 days of age; exhibit muscle weakness | Crawford et al., 2002 | |
| αcardiac-Actin | Full rescue | Nowak et al., 2009 | |||
| γcyto-Actin | Does not rescue | Jaeger et al., 2009 | |||
| αcardiac-Actin | Actc1 | Null | Embryonic/perinatal death; disorganized myofibrils | Kumar et al., 1997 | |
| αcardiac-Actin | γsmooth-Actin | Partial rescue of lethality; hearts defective | Kumar et al., 1997 | ||
| αsmooth-Actin | Acta2 | Null | Viable; defects in vascular contractility and blood pressure regulation | Schildmeyer et al., 2000 | |
| βcyto-Actin | Actb | Hypomorph | Embryonic lethal | Shawlot et al., 1998; Shmerling et al., 2005 | |
| γcyto-Actin | Actg1 | Null | Reduced viability; small size; progressive deafness | Belyantseva et al., 2009; Bunnell and Ervasti, 2010 | |
| γcyto-Actin | Conditional-skeletal muscle | Progressive centronuclear myopathy | Sonnemann et al., 2006 |
In all of these mouse models, there is compensatory upregulation of a subset of the remaining actin isoforms. However, since each muscle actin is highly expressed the total level of actin in knockout muscle cells remains less than in wild type. Therefore, transgenic overexpression of other isoforms may rescue the knockout phenotype and provide a useful measure of overlapping function between the isoforms. Along these lines, γsmooth-actin was overexpressed in αcardiac-actin knockouts [Kumar et al., 1997] and αcardiac-actin was overexpressed in αskeletal-actin knockouts [Nowak et al., 2009]. Overexpression of γsmooth-actin only partially rescued mice lacking αcardiac-actin. Even when these mice survived to adulthood, their hearts were hypodynamic and hypertrophic, demonstrating that γsmooth-actin and αcardiac-actin each make distinct contributions to cardiac cell function [Kumar et al., 1997].
αcardiac-Actin and αskeletal-actin are 99% identical and have overlapping expression patterns [Vandekerckhove et al., 1986]. These similarities suggest that these isoforms have considerable overlapping functions. Correspondingly, transgenic expression of αcardiac-actin fully rescued the lethality and muscle performance deficits associated with the loss of αskeletal-actin suggesting a potential treatment for human diseases associated with reduced αskeletal-actin [Nowak et al., 2009]. In contrast, overexpression of γcyto-actin did not rescue lethality due to αskeletal-actin deficiency suggesting that muscle and nonmuscle actins are more specialized. Interestingly, transgenic expression of γcyto-actin in wild-type mice resulted in the substitution of 40% of thin filament αskeletal-actin with γcyto-actin [Jaeger et al., 2009]. Therefore, muscle cell function depends on muscle-specific actin isoforms, but can tolerate a surprising amount of contamination by γcyto-actin.
Cytoplasmic Isoforms
βcyto-Actin and γcyto-actin are nearly identical proteins that differ by only four biochemically similar amino acids, all of which are found in the 10 N-terminal residues (Fig. 1A). Mice homozygous for hypomorphic alleles of Actb die during early development of uncharacterized defects [Shawlot et al., 1998; Shmerling et al., 2005]. A conditional knockout has been generated, but not yet described (our unpublished data). In contrast to βcyto-actin-deficient mice, whole-body γcyto-actin knockout mice are viable and can survive to adulthood and beyond [Belyantseva et al., 2009; Bunnell and Ervasti, 2010]. Interestingly, these mice are smaller than wild-type or heterozygous littermates from an early developmental stage, but are born at normal Mendelian ratios. γcyto-Actin confers a clear survival advantage as significant numbers of newborn Actg1−/− mice die as a result of developmental delays and another fraction die stochastically during adulthood [Belyantseva et al., 2009; Bunnell and Ervasti, 2010]. Corresponding to decreased animal survival, primary γcyto-actin-deficient fibroblasts cultured from these mice have decreased viability, impaired growth, and increased apoptosis and necrosis. On the other hand, consistent with the viability of Actg1−/− mice, γcyto-actin null fibroblasts have normal migration in a scratch wound assay [Bunnell and Ervasti, 2010].
Loss of γcyto-actin has similar consequences for cells in vivo. Sensory hair cells in the inner ear depend on stereocilia formed of βcyto-actin and γcyto-actin for proper function. γcyto-Actin-deficient hair cells develop normally but do not maintain stereocilia during aging, which corresponds to progressive hearing loss and deafness [Belyantseva et al., 2009]. Similarly, conditional knockout of γcyto-actin in muscle did not have developmental consequences [Sonnemann et al., 2006] despite in vitro evidence that γcyto-actin is required for sarcomere assembly [Lloyd et al., 2004]. Instead, mice developed a novel progressive centronuclear myopathy. In all cell types from γcyto-actin knockout mice studied thus far, the total cellular concentration of actin remains constant due to compensatory upregulation of the other actin isoforms [Belyantseva et al., 2009; Bunnell and Ervasti, 2010]. Since the actin concentration remains constant, the observed phenotypes can be ascribed to changes in the actin composition.
Mechanisms of Isoform-Specific Functions
There are two commonly held ideas to explain how different isoactins might perform distinct cellular functions. First, a subset of actin-binding proteins could bind specifically to a single isoform and require that interaction for its function. Consistent with this idea, several proteins, including cofilin [De La Cruz, 2005], ezrin [Yao et al., 1996], l-plastin [Namba et al., 1992], βCAP73 [Shuster et al., 1996], Thymosin b4 [Weber et al., 1992], and profilin [Larsson and Lindberg, 1988] have been described that discriminate between muscle and cytoplasmic actin isoforms. In addition, annexin 5a may preferentially bind to γcyto-actin over βcyto-actin [Tzima et al., 2000; Wagner et al., 2002]. The second idea is that actin isoforms are localized to distinct subcellular regions, perhaps as a result of differential interactions with actin-binding proteins or by a mechanism that targets transcripts. Differential localization of actin isoforms has been observed in a variety of cell types, with some discordance between reports as to the precise localization patterns for different actins.
Distinct Localization Patterns
Skeletal muscle provides a seemingly clear example of differential localization of muscle and nonmuscle actin isoforms with αskeletal-actin being confined to the sacromeric thin filaments. In contrast, γcyto-actin, which has the best-described localization pattern in muscle, appears to be absent from thin filaments, but is instead found in other muscle cell structures. γcyto-Actin was initially detected in filamentous structures surrounding mitochondria and adjacent to the sarcolemma [Craig and Pardo, 1983; Pardo et al., 1983]. Subsequent reports found that γcyto-actin is the only actin species detected at costameres [Rybakova et al., 2000], which are structures found between the sarcolemma and the z-disk [reviewed in Ervasti, 2003]. Other recent studies detected γcyto-actin in costameres as well as in a novel zone adjacent to the z-disk [Kee et al., 2004]. Finally, different groups have detected γcyto-actin only in z-disks and not in costameres [Nakata et al., 2001; Papponen et al., 2009].
In other cell types, γcyto-actin seems to be uniformly distributed in all actin-containing structures [Otey et al., 1986]. In contrast, βcyto-actin seems to have a more polarized distribution [Hoock et al., 1991; Bassell et al., 1998]. The differences between βcyto-actin and γcyto-actin localization may be due to a 54-nucleotide ÓzipcodeÓ sequence found in the 3′ UTR of βcyto-actin but not γcyto-actin transcripts. The zipcode sequence binds to zipcode-binding protein (ZBP1, also known as IMP-1), which facilitates targeting of the transcript and regulates translation [reviewed in Condeelis and Singer, 2005]. ZPB1 binds numerous different transcripts [Jonson et al., 2007], perhaps integrating βcyto-actin into a broader cellular program.
ZBP1-mediated regulation of βcyto-actin has important functional consequences in different cell types. In neurons cultured from X. laevis, growth cones exposed to an attractive cue require targeted βcyto-actin transcripts and newly synthesized βcyto-actin protein for normal turning behavior [Leung et al., 2006; Yao et al., 2006]. βcyto-Actin is enriched compared to γcyto-actin on the side of the growth cone exposed to attractant [Yao et al., 2006] and growth cones fail to turn when neurons are treated with antisense oligos to βcyto-actin [Leung et al., 2006]. Together, this evidence strongly predicts that βcyto-actin is essential for normal neuronal development. However, this hypothesis has yet to be tested in an intact mammalian system.
Zbp1-mediated targeting of βcyto-actin transcripts appears to be important in other cell types, including fibroblasts and adenocarcinoma cells, because interference with the ZBP1 activity alters cell morphology and migration [Kislauskis et al., 1994; Shestakova et al., 2001]. Consistent with targeting of βcyto-actin transcripts, several groups report that βcyto-actin is enriched at the leading edge of cultured fibroblasts and myoblasts as compared to stress fibers found in the central region of the cell [Hoock et al., 1991; Hill and Gunning, 1993; Kislauskis et al., 1997; Shestakova et al., 2001]. In contrast, γcyto-actin appears to be uniformly distributed in all actin-containing structures in fibroblasts [Otey et al., 1986].
In a conflicting report, recent work using newly generated isoform-specific antibodies and a different fixation technique suggests that βcyto-actin predominates in stress fibers while γcyto-actin is enriched at the leading edge [Dugina et al., 2009]. Discrepancies in βcyto and γcyto-actin localization have also been noted in auditory hair cell stereocilia. Here, immunofluorescent labeling indicated that γcyto-actin was localized at the periphery of stereocilia [Belyantseva et al., 2009], while a study using immuno-TEM found that βcyto-actin was peripheral and γcyto-actin was more centrally localized [Furness et al., 2005].
Study-to-study differences in observations of cytoplasmic actin localization in cultured cells, stereocilia, and muscle indicate that actin localization presents a formidable challenge. The slight amino acid sequence differences between isoforms require establishing antibody specificity, ideally using knockout tissue. Importantly, antibody binding interactions vary with experimental conditions; for example, the specificity of the anti-acardiac-actin antibody depends on the salt concentration [Franke et al., 1996]. Even with validated antibodies, it is difficult to interpret cases where there is a lack of staining (i.e., costameres, the central regions of stereocilia, stress fibers) because it seems that some actin-based structures may be inherently difficult to immunostain. Tightly bundled actin filaments, such as those found in stereocilia and stress fibers, may resist staining due to epitope masking or limited antibody penetration. Other actin-based structures, such as costameres, are labile and easily lost during fixation.
Actin as an Alloy
Differential localization as a mechanism to explain the distinct functions of actin isoforms is an attractive hypothesis because of its intuitive simplicity. However, it remains unclear how relative enrichment leads to changes in actin function. New biochemical evidence suggests that F-actin properties may vary according to the mix of isoforms in the filament. Bergeron et al. [ 2010] recently characterized purified βcyto and γcyto-actin produced in a recombinant baculovirus system. They observed that under calcium-bound conditions, βcyto-actin exhibited more dynamic behavior than γcyto-actin with faster polymerization and depolymerization rates. Intriguingly, biochemical assays demonstrated that βcyto and γcyto-actin readily copolymerize and that the resulting filaments have polymerization and depolymerization rates that vary according to the ratio of βcyto-actin to γcyto-actin [Bergeron et al., 2010]. In addition to polymerization dynamics, different mixtures of actin isoforms may well have distinct biophysical properties or distinct interactions with stability regulators such as AIP1 or cofilin.
Varying the mixture of actin may be a useful mechanism for adapting the cytoskeleton for a variety of functions. By way of analogy, steel is an alloy composed of numerous different metals. The ratio of each component determines the characteristics of the particular kind of steel, which is engineered to meet the desired compromises between properties such as weight, tensile strength, and cost. It is important that alloys can be manipulated because innumerable different steels are required to meet the varied demands of buildings and machines. Actin is cellular steel. Altering the ratio of actin isoforms within a filament may tune its properties to meet the specific requirements of different cells or subcellular structures.
Acknowledgements
We thank the members of the Ervasti lab for useful discussions and critical reading of this manuscript. This project was supported by NIH grants F32 DC009539 (to B.J.P.) from NIDCD and R01 AR049899 (to J.M.E.) from NIAMS. The content is solely the responsibility of the authors and does not necessarily represent the official views of either funding agency.