Pathology of Fibropolycystic Liver Diseases
Abbreviations
-
- ADPKD
-
- autosomal dominant polycystic kidney disease
-
- ARPKD
-
- autosomal recessive polycystic kidney disease
-
- CHF
-
- congenital hepatic fibrosis
-
- H&E
-
- hematoxylin and eosin
-
- PLD
-
- polycystic liver disease
Fibropolycystic disease is an umbrella term that comprises a spectrum of heritable conditions of the intrahepatic bile ducts. All forms are thought to be sequelae of abnormal remodeling or persistence of the ductal plate. Although an in-depth discussion of hepatic development during the embryonal, fetal, and perinatal periods is beyond the scope of this review, there are many excellent reviews on the subject. Suffice to say, the ductal plate that ultimately forms the interlobular bile ducts may demonstrate abnormal development and involution, resulting in what is known as the ductal plate malformation (Fig. 1). A variety of genetic mutations (see later) may cause ductal plate malformation, with a spectrum of clinical and histological findings within the liver and other organs.
The primary syndromes related to ductal plate malformation include autosomal dominant polycystic kidney disease (ADPKD), polycystic liver disease (PLD), autosomal recessive polycystic kidney disease (ARPKD), Caroli disease (CD), and congenital hepatic fibrosis (CHF). Each will be discussed later, with the clinical and pathological findings summarized in Tables 1 and 2, respectively. As noted by Summerfield et al.,1 fibropolycystic disease may result in a space-occupying lesion(s), cholangitis, and/or portal hypertension. It is important to remember that there is considerable overlap, and an individual patient may have more than one type of fibropolycystic disease.1
| Subtype | Gene | Typical Age at Presentation | Clinical Effect in Liver |
|---|---|---|---|
| ADPKD | PKD1 | Adult | Space occupying |
| PKD2 | |||
| PLD | PRKSCH | Adult | Space occupying |
| SEC63 | |||
| LRP5 | |||
| ARPKD | PKHD1 | Neonatal to childhood | Cholangitis, portal hypertension |
| CHF | PKHD1 * | Childhood to adult | Portal hypertension |
| CD | PKHD1 * | Childhood to adult | Cholangitis, abscess |
- * In the setting of ARPKD.
| Gross Findings | Microscopic Findings | |
|---|---|---|
| ADPKD | Enlarged with variably sized cysts | von Meyenberg complexes, cysts lined by cuboidal epithelium |
| PLD | Variably sized cysts | von Meyenberg complexes, cysts lined by cuboidal epithelium |
| ARPKD | Normal to slightly enlarged; no cysts unless associated CD | Inconspicuous—absent normal interlobular bile ducts, prominent ductal plate malformation with proliferating bile duct branches around perimeter of portal areas; may have CHF changes (below) |
| CHF | Slightly enlarged, firm | Ductal plate malformation, fibrous bands with proliferating ducts |
| CD | Segmental and saccular dilation of large bile ducts; bilirubin stones; abscess | Cysts lined by cuboidal epithelium; denudation, bile impregnation, acute inflammation common |
ADPKD
ADPKD was originally referred to as “adult polycystic disease” because it is preferentially seen in the adult population, but it may involve individuals of any age.2-4 It is the most common inherited renal disease, affecting 1 in 1000 live births.3, 5 The majority of cases are secondary to a mutation in the PKD1 gene that encodes the polycystin1 protein.6 A smaller number are related to mutations in the PKD2 gene, which encodes polycystin2.6 Conceptually, ADPKD should be considered a systemic disorder, with involvement of the kidneys, liver, cardiovascular system, and gastrointestinal system.3 Extrarenal involvement includes intracerebral aneurysms, colonic diverticula, cardiac valve issues, and cysts within the liver, ovary, pancreas, spleen, and central nervous system.3
Most patients are identified in the workup of hypertension, via imaging studies that demonstrate cysts in the kidneys, or because of a family history of ADPKD.6 The presence and number of hepatic cysts are age dependent. They are rare before the age of 20 years, but greater than 95% of patients will have them by the age of 35 years.3, 6 There is a gender difference as well, with the size and number of cysts being greater in women than in men.7 The space-occupying nature of the cysts may result in pain, fullness, and reflux.3, 5, 7 Rarely, they may become secondarily infected.8
Hepatic cysts do not communicate with the native biliary tree. They are thought to develop from enlargement and dilation of bile duct hamartomas (von Meyenberg complexes).2 Identification of the cysts is not typically a diagnostic challenge, and the primary role of the pathologist is simply to confirm the diagnosis and exclude occult malignancy, such as cholangiocarcinoma, within the specimen.
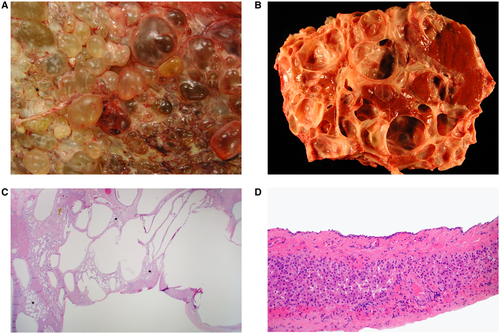
Grossly, livers demonstrate marked enlargement with involvement by cysts of various diameter, which contain clear or yellow fluid (Fig. 2A,B). Microscopically, the cysts are lined by simple, cuboidal-type epithelium overlying a fibrous wall (Fig. 2C,D). With increasing size, the cyst epithelial lining may become attenuated and flattened or completely denuded. The intervening lobular parenchyma is noninflammatory and typically unaffected, unless the cysts produce obstruction of the biliary tree.
PLD
PLD is similar to ADPKD without renal involvement. Like ADPKD, it is inherited in an autosomal dominant fashion, although it is much less common, affecting 1 in 100,000 individuals.5, 9 When identifiable, mutations involve the PRKSCH, SEC63, or LRP5 genes.9 The clinical course is less severe than ADPKD, and the majority of patients are asymptomatic.5
Von Meyenberg Complex
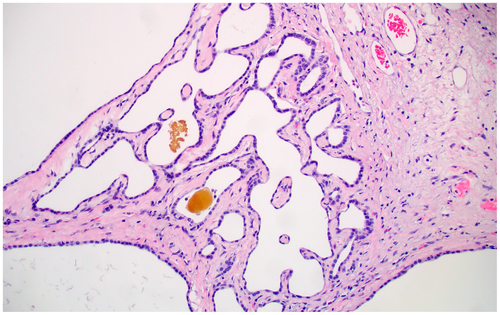
Also known as bile duct hamartoma, the von Meyenberg complex is frequently encountered in otherwise normal livers as an incidental finding. It also occurs in the setting of ADPKD, Caroli syndrome, and CHF.2 Grossly, it appears as small white nodules, often under the liver capsule. Microscopically, they have a characteristic low-power appearance (Fig. 3). There is a relatively circumscribed aggregate of dilated bile ducts set within a fibrous stroma. When they occur in isolation (i.e., not the setting of fibropolycystic disease), the pathologist must take care to not misinterpret a von Meyenberg complex as a well-differentiated cholangiocarcinoma. However, given the characteristic appearance, this is rarely an issue.
ARPKD
ARPKD is primarily a disease of the young, hence its original categorization as “infantile polycystic disease.”2 Much rarer than ADPKD, it affects 1:6,000 to 1:55,000 births.10, 11 ARPKD is caused by a mutation in the PKHD1 gene, which encodes the protein fibrocystin/polyductin.11, 12 Of the fibropolycystic diseases of the liver, ARPKD is the most diverse from both clinical and pathological perspectives. There is a wide spectrum of disease severity, but the kidney and liver are always involved, usually in an inversely proportional manner.10 Patients who present in the perinatal period have severe cystic renal involvement, usually resulting in death from respiratory insufficiency.2, 12 Those who have a milder form of the renal disease and survive the newborn period may present later in life (childhood, adolescence, or early adulthood) with sequelae of hepatic fibrosis (i.e., portal hypertension).11, 12
Grossly, the liver in ARPKD appears normal to only slightly enlarged. Extensive replacement by cysts, characteristic of ADPKD, is not seen.2 Microscopically, normal portal tracts containing an interlobular bile duct adjacent to a hepatic artery branch and portal vein are either severely diminished or absent altogether.2 Instead, what one finds ranges from persistent ductal plates arranged around the perimeter of portal areas (early disease) to enlarged tracts with bridging fibrosis that contains cross sections of proliferating ducts (later disease).2, 12 This latter pattern is identical to that seen in CHF (see later), which many experts consider to be a form of ARPKD.10-12
CHF
Although commonly seen in the setting of ARPKD, CHF may also occur in isolation or in association with other conditions, such as CD (see later), juvenile nephronophthisis, Meckel-Gruber syndrome, ADPKD, and tuberous sclerosis.11 Many cases of CHF are inherited in an autosomal recessive manner, and renal involvement is common in the form of renal tubular ectasia.2, 10, 11 As the name suggests, CHF results in fibrosis within the liver. Portal hypertension is the most common clinical manifestation, although some patients present with cholangitis alone or in combination with portal hypertension.2
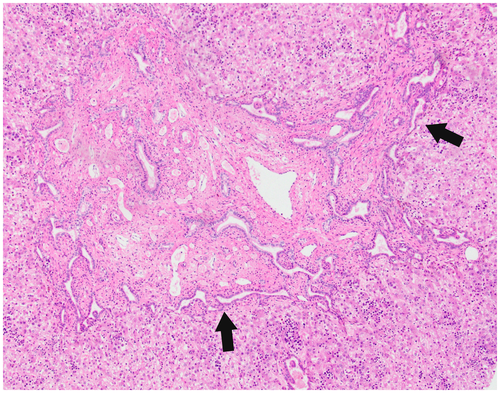
Grossly, livers are mildly enlarged and firm.1 Cysts are seen only in the setting of concurrent CD (see later). Microscopically, portal areas are expanded, with portal-portal bands of fibrosis (Fig. 4). Embedded within the fibrous bands are numerous bile duct structures, which may be “elongated, tortuous, or branched.”1 Portal veins are often absent or difficult to visualize.1, 2 The pattern of scarring is typical of biliary diseases, with an irregular, jigsaw pattern.1
Unlike the cystic diseases, which are diagnosed clinically and by imaging, the pathologist can play a critical role in diagnosing CHF, particularly when patients come to attention at a later age. The absence of cysts and the presence of portal hypertension raise the possibility of cirrhosis of usual etiology (e.g., nonalcoholic fatty liver disease, viral or autoimmune hepatitis). Careful attention to the pattern of fibrosis, paucity of portal veins, and changes of ductal plate malformation help distinguish CHF from cirrhosis.
CD
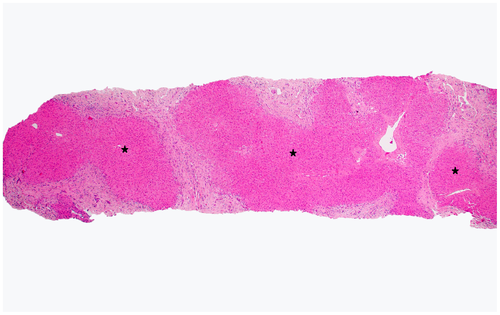
CD is defined as a congenital dilatation of the larger, intrahepatic bile ducts (left and right hepatic, segmental).2 CD can diffusely involve the liver or be localized to a lobe or segment.2 When the duct dilatations occur in the setting of CHF, it is referred to as Caroli syndrome (Fig. 5).2 CD may also occur in the setting of ARPKD and even ADPKD.13 The dilatations in CD may become secondarily infected, resulting in cholangitis or abscess.2 In Caroli syndrome (with associated CHF), patients often present with portal hypertension.2
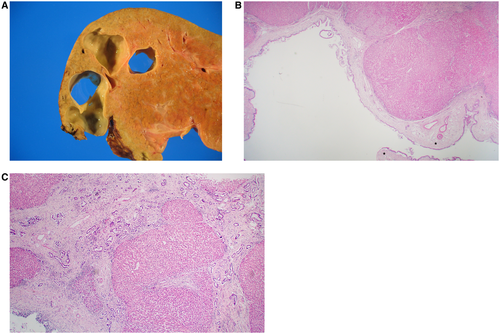
Grossly, CD is characterized by saccular dilatation of the large, segmental ducts that are in continuity with the biliary tree.14 There may be several dilated sections within a given duct, with intervening normal areas. Microscopically, the duct epithelium may be normal in appearance, but it is not uncommon for there to be ulceration with denudation of the epithelium, inflammation, and bile impregnation.




